Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Abdominal Massage Ameliorates Inguinal Fat Accumulation via Augmentation of PPARγ Signaling in High-Fat Diet-Induced Obese Mice
Authors Zhang J, Wang T, Shi Y, Liu Y, Lu T
Received 25 March 2023
Accepted for publication 26 July 2023
Published 15 August 2023 Volume 2023:16 Pages 2409—2418
DOI https://doi.org/10.2147/DMSO.S412218
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Muthuswamy Balasubramanyam
Jie Zhang,1 Tieshan Wang,2 Yinghui Shi,1 Yansong Liu,1 Tao Lu1
1School of Life Science, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 2Beijing Research Institute of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, People’s Republic of China
Correspondence: Tao Lu, School of Life Science, Beijing University of Chinese Medicine, 11 Beisanhuandong Road, Chaoyang District, Beijing, 100029, People’s Republic of China, Tel +8610 6428 6705, Fax +86 10 6428 6821, Email [email protected]
Purpose: With the increase in prevalence and decrease in age of the obese population, safer weight loss methods have attracted growing attention. While abdominal massage (AM) has been clinically proven for weight loss, the mechanism thereof has yet to be elucidated. We aimed to investigate the effect of AM on abdominal fat in obese mice fed a high-fat diet and explore the possible mechanisms involved.
Materials and Methods: Male C57BL/6J mice were fed a high-fat diet for 16 weeks and then treated with AM for 5 weeks; mice fed a standard diet were used as normal controls. Blood and adipose tissue, including inguinal white adipose tissue (WAT) and epididymal WAT, were collected from the mice after the intervention. We explored the mechanism of weight reduction through inguinal WAT transcriptome sequencing, quantitative real-time polymerase chain reaction (PCR) validation, and Western blot.
Results: The results revealed that AM decreased fat mass, weight, glucose, and serum lipid levels. Meanwhile, AM enhanced the expression of the peroxisome proliferator-activated receptor gamma (PPARγ) and other downstream genes (Fabp4, Acox3, Pck1, and Aqp7) in inguinal WAT. In addition, AM increased the expression of PPARγ protein.
Conclusion: AM may promote fatty acid oxidation, lipid metabolism, and glucose homeostasis by activating the PPARγ signaling pathway in inguinal WAT, thereby exhibiting therapeutic efficacy against obesity, even in the presence of a persistent high-fat diet.
Keywords: abdominal massage, inguinal white adipose tissue, high-fat diet, transcriptome sequencing, PPARγ
Introduction
The prevalence of obesity has gradually increased worldwide,1 and obese adults outnumbered non-obese adults in 2014.2 People around the world are getting heavier in recent years due to dietary and lifestyle changes, such as eating foods with high caloric density and leading a sedentary lifestyle. Although the prevalence of obesity among children and adolescents is lower than that in adults, this should not be ignored.3
Obesity, defined as abnormal fat accumulation, develops over time when energy intake exceeds calories burned.4 Obesity presents a risk to health such as metabolic derangement and metabolic diseases including type 2 diabetes and cardiovascular diseases.5,6 With the continuous increase in the number of people with obesity, weight loss has garnered a high degree of attention.
There has been a recent increase in achievements in the fight against obesity, such as diet restriction,7–9 lifestyle management,10 pharmacotherapy,11,12 and surgical therapy.13 Nevertheless, these interventions cannot meet medical obesity needs on a global scale. Despite regular diet and proper exercise being best for weight loss, these interventions are difficult to maintain. Pharmacotherapy may cause potential discomfort or toxic side effects, and bariatric surgery has limits and risks. Compared with other therapies for weight loss, safe and effective massage therapy has shown great potential, and many clinical reports on massage have verified the effects thereof for various diseases.14 For instance, abdominal massage (AM) therapy has been reported to improve triglyceride levels and reduce abdominal obesity in women.15 Clinical research regarding the role of AM remains important to investigate the mechanisms thereof to reduce obesity.
Obesity is a heterogeneous disease regulated by multiple pathways.16 As the understanding of the signaling pathways involved in the initiation and development of obesity improves, obesity can be combated in more specific ways. Peroxisome proliferator-activated receptor gamma (PPARγ) is expressed in mature adipocytes or tissues and is regarded as the master regulator of adipogenesis, playing a critical role in systemic lipid and glucose metabolism.17 Metabolic pathways under PPARγ control include lipid metabolism, adipocyte differentiation, and glucose homeostasis. The increased level of PPARγ is regulated in the control of adipocyte development and function.18
We aimed to clarify the mechanism whereby AM reduces white adipose tissue (WAT) and inhibits weight gain in obesity model mice, to determine whether AM activates the PPARγ signaling pathway and upregulates protein levels of PPARγ. The investigation into this mechanism may offer some support for the use of AM in the prevention and treatment of obesity.
Materials and Methods
Animals
The animal protocol was approved by the Committee of Ethics of Animal Experimentation of the Beijing University of Chinese Medicine (BUCM-4-202110201-4024). All experiments were performed following the Health Guide for the Care and Use of Laboratory Animals, at Beijing University of Chinese Medicine. Male C57BL/6J mice (4 weeks) were purchased from SPF Biotechnology Co., Ltd (Beijing, China). The mice were housed at 20–24°C, 45 ± 5% relative humidity and maintained on a 12 h light/dark cycle during the experiment. After an adaptive feeding period, the mice were randomly divided into a control group and a high-fat diet (HFD) group. The HFD food (Beijing HFK Bioscience, Beijing, China) consisted of 20% sucrose, 1.2% cholesterol, 15% lard, 0.2% sodium cholate, and 63.6% standard chow. After 16 weeks of HFD feeding, mice in the HFD group whose weight reached the requirements were divided into one of two additional groups: a model group and an AM group. The control group did not undergo any changes in diet, while the model and AM groups continued the HFD.
AM
The control and model groups were not treated. The AM group received massage for 5 min, once daily for 5 weeks. AM uses the whorl area of the index fingers to gently rub in a circular motion; the tips of the fingers were pressed firmly against the abdomen of the mouse, and massage was performed in a clockwise direction with the fingers relaxed, at medium speed, with no entrainment of the subcutaneous tissues.
Serum Biochemical Analysis
Glucose (GLU) and four blood lipid components, including total cholesterol (TC), triglycerides (TGs), and low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), were detected in the serum and measured by CX4 Pro automatic biochemistry analyzer (Beckman, USA).
Inguinal WAT RNA Extraction and Sequencing
RNA was extracted from the inguinal WAT using the Total RNA Isolation Kit (TIANGEN, Beijing, China). Sample quantity and quality were determined by Nanodrop 2000 (Thermo Fisher). The RNA integrity values of all samples in the study were > 8.0. An RNA sample was used to prepare DNA nanoballs containing multiple copies of DNA and were sequenced through combinatorial probe-anchor synthesis.
Quantitative Real-Time Polymerase Chain Reaction (PCR)
To verify the transcriptome sequencing data, genes in the PPAR signaling pathway were selected for quantitative real-time PCR (qPCR) analysis. SYBR Green was chosen as the fluorescent dye for the qPCR experiment at a reaction volume of 20 μL. Primers for these genes were designed on NCBI website with the following sequences: PPARγ, forward 5′-CTCCAAGAATACCAAAGTGCGA-3′ and reverse 5′-GCCTGATGCTTTATCCCCACA-3′; Fabp4, forward 5′-ATCATAACCCTAGATGGCGGG-3′ and reverse 5′-TCATAACACATTCCACCACCA-3′; Pck1, forward 5′-TGCAGAACACAAGGGCAAGAT-3′ and reverse 5′-TTTGCCGAAGTTGTAGCCGA-3′; Aqp7, forward 5′-GTCCACCCACAACTCCACAA-3′ and reverse 5′-ACCAAGGCCAAACACCATCA-3′; β-actin, forward 5′-TGGGACGACATGGAGAAGA-3′ and reverse 5′-TGGGGTGTTGAAGGTCTCA-3′. The β-actin gene was used as an internal control and the qPCR data were processed by the 2(-ΔΔCt) method.19
Western Blot Analysis
Western blot analysis was performed on PPARγ protein expression in inguinal WAT. Total protein extracted from inguinal WAT using RIPA buffer. 10 μL of protein samples were loaded onto 10% of gels without TGX staining (TGX Stain-FreeTM FastCastTM Acrylamide Kit, Bio-Rad) and run at a temperature of 80 V to 100 V. Gels were imaged (ChemiDoc XRS+ Gel Imaging System, Bio-Rad) for protein loading prior to blotted onto PVDF sheets (Immun-Blot, Bio-Rad) by using a semi-dry transfer cell (TransBlot Turbo, Bio-Rad). The primary antibody was rabbit anti-mouse (Proteintech, 16643-1-AP), the secondary antibody was HRP goat anti-rabbit IgG (Abcam, ab6721). Blots for PPARγ was semi-quantified using Image Lab (version 6.0, Bio-Rad).
Statistical Analysis
All the data are presented as means ± standard deviation. SPSS 23.0 software was used for the statistical analysis. For multi-group comparisons, a one- or two-way analysis of variance was applied. P < 0.05 was deemed to be statistically significant.
Results
AM Inhibited Weight Gain and Reduced Adipose Tissue Mass
To determine the effect of AM therapy on the weight of obese mice, the weight changes of obese mice were recorded each week. After high-fat feeding, the mean bodyweight of the mice in the model and AM groups was significantly higher than that of control group (P < 0.01), and the mice were considered obese.
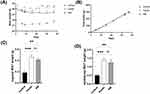
After the intervention, the results showed that the mean weight of the AM group initially decreased and then slightly increased (Figure 1A). There was a general trend toward a slight decrease in weight. Weight inhibition of the AM group was not able to compensate for the weight increase caused by the HFD in the long term, although the AM group had a significantly lower rate of weight growth than the model group. AM was able to inhibit weight gain in obese mice. Furthermore, in terms of dietary intake, the mice in the control, model, and AM groups all increased weight slowly, and there were no significant differences between the groups (Figure 1B), ruling out diet interference on weight gain.
By comparing white fat mass in the groin and epididymis of the mice in each of the groups, our results showed that the model group mass was larger than the AM group, and both the model and AM groups were significantly different than the control (Figure 1C and D). These findings demonstrate that AM therapy can reduce white fat accumulation, and therefore may play a role in the regulation of body weight.
AM Reduced Blood Glucose and Lipid Levels
Glucose was significantly lower in the AM group compared to the model group (Figure 2A), indicating that AM may promote glucose uptake in obese mice. Additionally, the 5-week AM intervention played a role in the regulation of blood lipid level in obese mice. Increased levels of TC, TG, LDL-C, and HDL-C were detected in the serum of the obese mice in the model group. We found a decrease in TC, TG, LDL-C, and HDL-C in the serum of the mice in the AM group (Figure 2B–E), indicating that AM has a regulatory effect on serum TC, TG, LDL-C, and HDL-C.
Differentially Expressed Genes
Gene expression levels were quantified on the basis of transcripts per million values. A total of 1857 differentially expressed genes (DEGs) were found in the model group compared to the control group, among which 1100 were upregulated and 757 were downregulated (Figure 3A). In the AM versus model group comparison, there were 1430 DEGs, 817 of which were upregulated and 613 were downregulated (Figure 3B). There were 3341 DEGs in the AM versus control group comparison, of which 1811 were upregulated and 1530 were downregulated, respectively (Figure 3C). A Venn diagram revealed that 144 DEGs exhibited continuous differential expression across all the groups (Figure 3D).
Gene Ontology (GO) and Analysis
The 144 DEGs were mapped to a GO database for enrichment analysis to understand the detailed functional classification. The DEGs were mapped to the six Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway categories, ie cellular processes, environment information processing, genetic information processing, human diseases, metabolism, and organismal systems (Figure 4A). Gene set enrichment analysis (GSEA) determines whether an a priori defined set of genes shows statistically significant, concordant differences between two biological states. After further KEGG enrichment analysis after GSEA, 11 of the top 20 pathways were related to metabolism and nine to the organism systems (Figure 4B). Among them, the PPAR signal pathway was related to organism systems, and the DEGs involved in the PPAR signal pathway are shown in the Figure 4C.
Verification of RNA-Seq Data by qPCR
Five DEGs in the PPAR signal pathways were verified by qPCR to validate the transcriptome results. Overall, the expression patterns of the six DEGs that were identified via qPCR were similar to those found in the sequencing analysis. PPARγ agonism has been reported to regulate lipid metabolism in brown adipose tissue,20 and the same pattern was observed in inguinal WAT. AM significantly upregulated the expression of PPARγ (P < 0.01). When the lipid metabolism related gene was examined, the expression of Acyl-CoA oxidase 3 (Acox3) that promotes fatty acid oxidation showed a significant difference in the AM versus model groups (P < 0.05). The expression of fatty acid binding protein 4 (Fabp4) associated with adipocyte differentiation also showed a significant difference in the AM versus model groups (P < 0.01). Meanwhile, the expression of Pck1 and Aqp7 related to glucose homeostasis in the AM group and the model group were significant (P < 0.01). In summary, PPARγ, Fabp4, Acox3, Pck1, and Aqp7 were significantly upregulated (P < 0.05) by AM treatment (Figure 5). The hypoglycemic and lipid-lowering effects of AM were partially attributed to the regulation of the PPARγ signaling pathway by increasing the expressions of PPARγ, Fabp4, Acox3, Pck1, and Aqp7.
AM Regulated the Expression of PPARγ Protein
In the Western blot experiments, total protein quantification was employed. The upper portion of Figure 6A depicts the protein expression of PPARγ in each group, while the lower portion represents the total protein content of the respective samples within each group. We investigated the protein levels of PPARγ in the inguinal WAT from different groups. In comparison to the control group, we observed a decrease in the PPARγ protein level in the inguinal WAT in the model group (Figure 6B). This finding suggests that the model group, likely representing an obese or metabolically challenged condition, exhibited a downregulation of PPARγ protein expression in the adipose tissue. When comparing the model group with the AM group, we observed an interesting reversal in the PPARγ protein levels. The PPARγ protein level in the AM group was found to be significantly increased compared to the model group (P < 0.05). The model group displayed a decreased PPARγ protein level, potentially associated with the underlying metabolic dysfunction or obesity. However, the AM group exhibited a recovery or enhancement of PPARγ protein expression, suggesting a potential therapeutic effect of the intervention in restoring or promoting PPARγ activity.
 |
Figure 6 Effects of abdominal massage (AM) on PPARγ protein in obese mice between the control, model, and AM groups (A and B). Data are expressed as mean ± standard deviation. *P < 0.05. |
Discussion
We aimed to determine the effect of AM on HFD-induced abdominal fat accumulation. Our results demonstrate that AM can reduce abdominal fat accumulation in obese mice, including inguinal WAT and epididymis WAT, and reduce blood glucose and lipid levels (TC, TG, LDL-C, and HDL-C). One possible mechanism of action is that AM activates PPARγ, which in turn upregulates the expression of downstream related genes, thereby ameliorating white fat accumulation in the groin. To our knowledge, this is the first investigation of the mechanism of AM’s effect on inguinal WAT.
Most massage studies are clinical studies. Since tissue samples are harder to obtain than blood samples, few clinical studies can study the mechanism of massage on tissues. Bodyweight, blood glucose, and blood lipids are common indicators. In our study, bodyweight in the AM group increased slowly in the later intervention phase, which could be due to AM regulating gastrointestinal motility in the obese mice, promoting gastric emptying and intestinal peristalsis, and enhancing food digestion and absorption.21 Compared with the model group, the weight of the AM group increased slowly, indicating that AM has a regulating effect on weight. AM can promote glucose and lipid metabolism in clinical treatments;22 the same conclusion was obtained in our study. Blood glucose in the model group increased while that in the AM group decreased, which could be due to acceleration of local energy metabolism by massage, therefore affecting the level of glucose in the serum. The abnormal levels of TC, TG, LDL-C, and HDL-C in the serum of the model group mice indicated that the mice suffered from HFD have lipid metabolism disorder. In previous reports, the model group which were fed a continuous HFD did not have significantly reduced HDL-C,21,23 and low levels or very high levels of HDL-C may be detrimental to cardiovascular health.24 AM has a regulatory effect on TC, TG, LDL-C, and HDL-C in serum, which also provides preliminary evidence that AM may improve lipid metabolism in obese mice.
Obesity is defined as abnormal or excessive fat accumulation that presents a risk to health. Abdominal obesity is a marker of ectopic fat accumulation.25 Considering that, in the fat distribution of the mouse,26 the proportion of white fat in the abdomen is high, the abdomen is selected as the target area. The idea, developed in 1980, that regional abdominal obesity may contribute to metabolic events and alterations, was the catalyst for this study.25 PPARγ has many roles and can induce adipogenesis and regulate lipid metabolism in mature adipocytes.27 According to our results, AM significantly upregulated the expression of PPARγ and the downstream genes Acox3, Fabp4, Pck1, and Aqp7. Acox3 is related to mitochondrial and fatty acid oxidation.28 AM upregulated the expression of Acox3, suggesting that it may restore mitochondrial homeostasis in adipose tissue. FABP4 is mainly expressed in adipose tissue and is downregulated at the mRNA level in obese patients.29 Although FABP4 expression in our model group did not differ from the control, the expression of Fabp4 was significantly upregulated in the AM group, indicating the role of AM in suppressing obesity. Pck1 underexpression causes lipodystrophy in mice,30 and Aqp7-deficient mice develop adult-onset obesity.31 Aqp7 serves as a glycerol channel in adipose tissue in vivo and glycerol derived from adipose tissue determines the glucose level. AM raises the expression of Pck1 and Aqp7, providing further explanation for decreased blood glucose in obese mice after AM treatment. Therefore, the hypoglycemic and lipid-lowering effects of AM were partially attributed to its regulation on the PPARγ signaling pathway by increasing the expressions of PPARγ, Fabp4, Acox3, Pck1, and Aqp7.
Moreover, Western blot analysis confirmed that AM treatment led to an elevation in PPARγ protein expression in the inguinal WAT. The results of our study revealed an intriguing discrepancy between the expression of the PPARγ gene and the levels of PPARγ protein in the obese model group compared to the normal group of mice. While the expression of the PPARγ gene did not show a significant decrease in the obese model group, we observed a significant reduction in the protein levels of PPARγ. One possible explanation for this disparity is post-transcriptional regulation. Another possible explanation is the existence of different isoforms or variants of PPARγ.
AM has been proven to be an effective and safe therapy since 1889.32 The range of diseases treated with AM is gradually expanding, and treatment programs are constantly be optimized. AM increases SIRT1/NF-κB signaling in rat adipose tissue, indicating regulation of the secretion of proinflammatory cytokines, contributing to improvement of insulin resistance.33 In patients with type 2 diabetes, AM significantly reduces abnormalities in the intestinal microbiota and glucose metabolism.34 As the metabolic and inflammatory states, muscle function, and gut microbiota are all interconnected,35 AM can result in greater fat layer reduction in massaged sites to enhance the clinical outcome after cryolipolysis compared with non-massaged sites.36 The therapeutic effect of AM in clinical settings is significant, while research into the mechanism thereof is relatively weak, requiring further research. The possible mechanism of AM treatment continues to be excavated.
Conclusion
AM can reduce WAT mass and inhibit weight gain, and decrease blood sugar and lipid levels, thereby improving HFD-induced obesity. AM regulates glucose homeostasis, adipocyte differentiation, and lipid metabolism in inguinal WAT to ameliorate inguinal fat accumulation, blood glucose, and lipids by the PPARγ signaling pathway even in the presence of a persistent HFD. The efficacy of AM should not be underestimated, and we hope to promote the development of AM through this in-depth research regarding the mechanism thereof. Noninvasive fat reduction techniques, such as cryolipolysis, have typical side effects, such as erythema, bruising, minor pain, and transient loss of sensation. As a noninvasive treatment with no side effects, AM is more easily favored, and combining AM with other technologies will assist in reducing obesity.
Abbreviations
AM, Abdominal massage; DEG, Differentially expressed genes; GO, Gene ontology; GSEA, Gene set enrichment analysis; HFD, High-fat diet; HDL, High-density lipoprotein; LDL, Low-density lipoprotein; KEGG, Kyoto Encyclopedia of Genes and Genomes; PCR, Polymerase chain reaction; PPAR, Peroxisome proliferator-activated receptor; TC, Total cholesterol; TG, Triglycerides; WAT, White adipose tissue.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–298. doi:10.1038/s41574-019-0176-8
2. Di Cesare M, Bentham J, Stevens GA, et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi:10.1016/S0140-6736(16)30054-X
3. Afshin A, Forouzanfar MH, Reitsma MB, et al; GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi:10.1056/NEJMoa1614362
4. Wood LG. Diet, obesity, and asthma. Ann Am Thorac Soc. 2017;14(Supplement_5):S332–S338. doi:10.1513/AnnalsATS.201702-124AW
5. Li Y, Wang D, Ping X, et al. Local hyperthermia therapy induces browning of white fat and treats obesity. Cell. 2022;185(6):949–966. doi:10.1016/j.cell.2022.02.004
6. Wang L, Ye X, Hua Y, Song Y. Berberine alleviates adipose tissue fibrosis by inducing AMP-activated kinase signaling in high-fat diet-induced obese mice. Biomed Pharmacother. 2018;105:121–129. doi:10.1016/j.biopha.2018.05.110
7. Fock KM, Khoo J. Diet and exercise in management of obesity and overweight. J Gastroenterol Hepatol. 2013;28:59–63. doi:10.1111/jgh.12407
8. Hepler C, Weidemann BJ, Waldeck NJ, et al. Time-restricted feeding mitigates obesity through adipocyte thermogenesis. Science. 2022;378(6617):276–284. doi:10.1126/science.abl8007
9. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177(7):930–938. doi:10.1001/jamainternmed.2017.0936
10. Ryan DH, Kahan S. Guideline recommendations for obesity management. Med Clin North Am. 2018;102(1):49–63. doi:10.1016/j.mcna.2017.08.006
11. Idrees Z, Cancarevic I, Huang L. FDA-approved pharmacotherapy for weight loss over the last decade. Cureus. 2022;14:e29262. doi:10.7759/cureus.29262
12. Narayanaswami V, Dwoskin LP. Obesity: current and potential pharmacotherapeutics and targets. Pharmacol Ther. 2017;170:116–147. doi:10.1016/j.pharmthera.2016.10.015
13. Wolfe BM, Kvach E, Eckel RH. Treatment of obesity. Circ Res. 2016;118(11):1844–1855. doi:10.1161/CIRCRESAHA.116.307591
14. Wang G, Zhang Z, Sun J, et al. Abdominal massage: a review of clinical and experimental studies from 1990 to 2021. Complement Ther Med. 2022;70:102861. doi:10.1016/j.ctim.2022.102861
15. Lee KJ, Park JI, Oh SY. The effects of extracorporeal shock wave therapy vs hand massage on serum lipids in overweight and obese women. Ann Med Surg. 2021;63:102057. doi:10.1016/j.amsu.2021.01.005
16. Wen X, Zhang B, Wu B, et al. Signaling pathways in obesity: mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2022;7:298. doi:10.1038/s41392-022-01149-x
17. Stienstra R, Duval C, Müller M, Kersten S. PPARs, obesity, and inflammation. PPAR Res. 2007;2007:1–10. doi:10.1155/2007/95974
18. Lee JH, Woo KJ, Kim MA, et al. Heat-killed enterococcus faecalis prevents adipogenesis and high fat diet-induced obesity by inhibition of lipid accumulation through Inhibiting C/EBP-α and PPAR-γ in the insulin signaling pathway. Nutrients. 2022;14:1308. doi:10.3390/nu14061308
19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi:10.1006/meth.2001.1262
20. Festuccia WT, Blanchard PG, Deshaies Y. Control of brown adipose tissue glucose and lipid metabolism by PPARγ. Front Endocrinol. 2011;2:1–6. doi:10.3389/fendo.2011.00084
21. Han Y, Lu Z, Chen S, et al. Abdominal massage alleviates skeletal muscle insulin resistance by regulating the AMPK/SIRT1/PGC-1α signaling pathway. Cell Biochem Biophys. 2021;79(4):895–903. doi:10.1007/s12013-021-00983-0
22. Moraska AF, Hickner RC, Rzasa-Lynn R, Shah JP, Hebert JR, Kohrt WM. Increase in lactate without change in nutritive blood flow or glucose at active trigger points following massage: a randomized clinical trial. Arch Phys Med Rehabil. 2018;99(11):2151–2159. doi:10.1016/j.apmr.2018.06.030
23. Joerin L, Kauschka M, Bonnländer B, Pischel I, Benedek B, Butterweck V. Ficus carica leaf extract modulates the lipid profile of rats fed with a high-fat diet through an increase of HDL-C. Phyther Res. 2014;28(2):261–267. doi:10.1002/ptr.4994
24. Sohrabi Y, Schwarz D, Reinecke H. LDL-C augments whereas HDL-C prevents inflammatory innate immune memory. Trends Mol Med. 2022;28(1):1–4. doi:10.1016/j.molmed.2021.11.003
25. Smith U. Abdominal obesity: a marker of ectopic fat accumulation. J Clin Invest. 2015;125(5):1790–1792. doi:10.1172/JCI81507
26. Zhang F, Hao G, Shao M, et al. An adipose tissue atlas: an image-guided identification of human-like BAT and beige depots in rodents. Cell Metab. 2018;27(1):252–262. doi:10.1016/j.cmet.2017.12.004
27. Lehrke M, Lazar MA. The many faces of PPARγ. Cell. 2005;123(6):993–999. doi:10.1016/j.cell.2005.11.026
28. Sandoval V, Femenias A, Martínez-Garza Ú, et al. Lyophilized maqui (Aristotelia chilensis) berry induces browning in the subcutaneous white adipose tissue and ameliorates the insulin resistance in high fat diet-induced obese mice. Antioxidants. 2019;8(9):360. doi:10.3390/antiox8090360
29. Queipo-Ortuño MI, Escoté X, Ceperuelo-Mallafré V, et al. Fabp4 dynamics in obesity: discrepancies in adipose tissue and liver expression regarding circulating plasma levels. PLoS One. 2012;7(11):e48605. doi:10.1371/journal.pone.0048605
30. Beale EG, Hammer RE, Antoine B, Forest C. Disregulated glyceroneogenesis: pck1 as a candidate diabetes and obesity gene. Trends Endocrinol Metab. 2004;15(3):129–135. doi:10.1016/j.tem.2004.02.006
31. Skowronski MT, Lebeck J, Rojek A, et al. Aqp7 is localized in capillaries of adipose tissue, cardiac and striated muscle: implications in glycerol metabolism. Am J Physiol Ren Physiol. 2007;292(3):956–965. doi:10.1152/ajprenal.00314.2006
32. Garry TG. Abdominal massage in constipation and allied conditions. Lancet. 1889;133(3418):422–423. doi:10.1016/S0140-6736(00)30056-3
33. Gao T, Chen S, Han Y, et al. Ameliorating inflammation in insulin-resistant rat adipose tissue with abdominal massage regulates SIRT1/NF-κB signaling. Cell Biochem Biophys. 2022;80(3):579–589. doi:10.1007/s12013-022-01085-1
34. Xie Y, Huan MT, Sang JJ, et al. Clinical effect of abdominal massage therapy on blood glucose and intestinal microbiota in patients with type 2 diabetes. Oxid Med Cell Longev. 2022;2022:2286598. doi:10.1155/2022/2286598
35. Gizard F, Fernandez A, De Vadder F. Interactions between gut microbiota and skeletal muscle. Nutr Metab Insights. 2020;13:1178638820980490. doi:10.1177/1178638820980490
36. Boey GE, Wasilenchuk JL. Enhanced clinical outcome with manual massage following cryolipolysis treatment: a 4-month study of safety and efficacy. Laser Surg Med. 2014;46(1):20–26. doi:10.1002/lsm.22209
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.