Back to Journals » Infection and Drug Resistance » Volume 16
Abdominal Abscesses Caused by Nocardia farcinica in an Immunocompromised Patient: A Case Report and Literature Review
Authors Wang X, Liang Y, Cheng Q, Nong W, Hu L
Received 12 October 2023
Accepted for publication 28 November 2023
Published 5 December 2023 Volume 2023:16 Pages 7447—7454
DOI https://doi.org/10.2147/IDR.S441117
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xiuri Wang,1,* Yunxiao Liang,1,* Qiuchen Cheng,1 Wei Nong,1 Liuyang Hu2
1Department of Gastroenterology, The People’s Hospital of Guangxi Zhuang Autonomous Region, Guangxi Academy of Medical Sciences, Nanning, 530016, People’s Republic of China; 2Department of Laboratory Medicine, The People’s Hospital of Guangxi Zhuang Autonomous Region, Guangxi Academy of Medical Sciences, Nanning, 530016, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liuyang Hu, Department of Laboratory Medicine, The People’s Hospital of Guangxi Zhuang Autonomous Region, Guangxi Academy of Medical Sciences, Taoyuan Road N 6, Nanning, Guangxi, 530016, People’s Republic of China, Email [email protected]
Abstract: Nocardiosis is mainly an opportunistic infection that affects immunosuppressed individuals, with the most common manifestation being the pulmonary infection and cerebral abscesses. Abdominal abscesses caused by Nocardia is rare in diabetes patients. Here, we report a rare case of abdominal abscesses caused by Nocardia farcinica (N. farcinica) in a 56-year-old man with poorly controlled type 2 diabetes and prolonged use of corticosteroids for the treatment of secondary adrenal insufficiency. Abdominal CT suggested abdominal abscesses, and the culture of the abscess puncture fluid identified it as N. farcinica by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). Treatment with a combination of trimethoprim-sulfamethoxazole (TMP-SMX) and imipenem/cilastatin (IPM/CS), along with surgical drainage and reduction in corticosteroid dosage, achieved successful outcomes in treating disseminated abdominal abscesses. Immunocompromised patients with unexplained fever, abdominal pain, and abdominal abscess should be suspected of Nocardia infection.
Keywords: abdominal nocardiosis, Nocardia farcinica, abdominal abscesses, diabetes, corticosteroid
Introduction
Nocardia is an aerobic Gram positive actinomycete, commonly considered an opportunistic pathogen. Nocardia infections are typically caused by traumatic bacterial invasion or inhalation, with the latter more common in immunocompromised patients, such as those on long-term corticosteroid or HIV/AIDS patients, or organ transplant recipients, and can lead to pulmonary infections.1,2 Nocardia can also cause infections in various extrapulmonary sites, with extrapulmonary infections typically resulting from hematogenous dissemination from primary lung lesions. The central nervous system (CNS) is the second most frequently involved organ (2–26%),2,3 but it can also lead to rare infections in the cornea,4 abdomen,5 heart,6 joints,7 and bones.8 Clinically, Nocardia spp. were proved to differ in virulence. N. farcinica is distinct from others Nocardia spp. by its high degree of antibiotic resistance and virulence. N. farcinica is more prone to cause disseminated infection involving multiple organs, especially the brain abscess.5,9 N. brasiliensis is the more aggressive than N. caviae, and N. asteroides.10 As already reported, the Nocardia species were closely correlated with infection sites, N. farcinica was more likely isolated in blood cultures (54%) and central nervous system (44.2%), respectively.11 Moreover, N. brasiliensis was related predominately to skin and soft tissue infections (72.2%).11 N. nova complex, N cyriacigeorgica, and N. farcinica were the most common clinical isolates in pulmonary nocardiosis.12 Difficulty in early diagnosis and delayed treatment of Nocardia can result in dissemination and high fatality rates, and the overall prognosis of disseminated nocardiosis patients is unsatisfactory.13 Currently, the diagnosis of nocardiosis relies mainly on direct microscopic examination and bacterial culture, although molecular methods have been shown to be more accurate, sensitive, and specific in identifying Nocardia species.14 In this case report, we describe a rare occurrence of abdominal abscesses caused by N. farcinica in a 56-year-old man with poorly controlled type 2 diabetes and prolonged use of corticosteroids for the treatment of secondary adrenal insufficiency.
Case Presentation
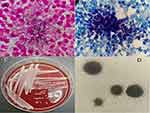
A 56-year-old male patient presented to the emergency department of the People’s Hospital of Guangxi Zhuang Autonomous Region, China, due to cough, fever and abdominal pain. The patient was diagnosed with abdominal infection and received intravenous injection of ceftriaxone for treatment. After 4 days, his condition was steadily deteriorated and he was admitted to the Department of Gastroenterology at our hospital. The patient had a history of poorly controlled type 2 diabetes and abuse of corticosteroids for the treatment of his reiterative gouty arthritis for 10 years, which resulted in secondary adrenal insufficiency. Currently, he takes prednisone acetate 20mg/d for the treatment of secondary adrenal insufficiency. A month ago, he inhaled air particles that had been contaminated during house renovation activities. The laboratory examination revealed leukocytosis (28.56 × 10^9/L), neutrophil ratio of 90.3%, C-reactive protein of >200 mg/L (normal values 0–5 mg/L), procalcitonin of 1.60 ng/mL (normal values 0–0.05 ng/mL), and poor glycemic control (Glu 8.95mmol/L, HbA1c 11.5%). The patient had recurrent fever with a maximum temperature of 39.1°C and a palpable mass in the lower abdomen. Pulmonary CT showed bilateral pneumonia, tracheal diverticulum, multiple bilateral rib old fractures and a small amount of pericardial effusion (Figure 1A). Abdominal CT indicated irregular nodular or slightly low-density shadows with multiple soft tissue densities in the right renal hilum, right retroperitoneal space, and right pelvic extraperitoneal space, with the largest of those locating in front of the right iliac muscle, measuring about 52mm × 38mm, suggesting infectious lesions and encapsulated effusion (Figure 1B). Brain CT demonstrated no abnormalities. Treatment included empirical anti-infection, active blood glucose control, oxygen therapy, and fluid replacement. Due to the seriousness of the patient’s infection and the uncertainty regarding the presence of multi-drug resistant bacterial infection, imipenem/cilastatin (IPM/CS) was used without determination of a clear infectious etiology. Next day, the patient underwent invasive puncture drainage of the largest abscess located in front of the right iliac muscle under CT-guidance, and punctured milky yellow green pus was sent to microbiological examination. Meanwhile, blood cultures were taken. Gram staining of the puncture fluid showed elongated, branching Gram-positive bacteria, with beaded filamentous structures (Figure 2A), which was suspected actinomycosis. However, the modified acid-fast staining showed acid-fast thin branching bacterium, which highly suspected the presence of Nocardia infection (Figure 2B). Small, dry, wrinkled, pale yellow colonies appeared on the blood agar (Figure 2C) and examination of the colonies under a 10x objective lens showed abundant aerial hyphae on agar medium (Figure 2D), which was further dentified as N. farcinica by MALDI-TOF MS (Bruker, France, reference database version is V10 9607MSPs). The treatment plan was modified to include combination therapy with TMP-SMX (3 pieces every 8 hours, each piece containing 80 mg of trimethoprim and 400mg of sulfamethoxazole) and IPM/CS (1 g, every 8 hours), along with continuous drainage of the abdominal abscesses, and reduction the dosage of glucocorticoids. During hospitalization, no pathogens were found in blood and sputum cultures. After 27 days of treatment, the patient’s temperature returned to normal, inflammatory markers significantly decreased (Figure 3A–D), and the abdominal abscesses were notably absorbed (Figure 3E), indicating improvement in the condition and the patient can be discharged. We advised the patient to continue oral TMP-SMX 3 pieces three times a day for 6–12 months.
Discussion and Literature Review
Nocardiosis is a neglected tropical disease and a potentially life-threatening infection that requires timely diagnosis and appropriate treatment due to its significant morbidity and mortality. Invasive Nocardia infections primarily affect patients with cellular immunodeficiency (solid organ or hematopoietic stem cell transplant recipients and those receiving corticosteroids or immunosuppressants) because T-cell-mediated immunity and pulmonary macrophages play crucial roles in the local control of Nocardia.15,16 Corticosteroids inhibit the influx of neutrophils, thereby promoting the spread of Nocardia.17 Diabetes, chronic kidney disease and cirrhosis are generally considered as risk factors for invasive nocardiosis.12
Direct microscopic examination and bacterial culture are still the main methods for diagnosing nocardiosis, which rely on close cooperation between clinicians and microbiologists. Gram staining and modified acid-fast staining are helpful for early suspicion of nocardiosis. Gram staining is characterized by elongated, branching Gram- positive bacilli, with beaded filamentous structures, which can also be seen in other actinomycetes, such as Streptomyces. However, the modified acid-fast staining showed a positive result, which can distinguish Nocardia from Streptomyces. Nocardia culture grows slowly, and mixed bacterial infections can further mask the identification results, leading to false negatives. Given these difficulties in culture methods, PCR method, MALDI-TOF, and mNGS have become the promising methods for identifying Nocardia to the species level. Although the PCR method exhibits high sensitivity and specificity in detecting Nocardia, recent studies have shown that PCR detection in respiratory samples may only reflect airway colonization, so positive results should be interpreted with caution.18 mNGS is a new approach to the identification of difficult and atypical infectious diseases. However, the main drawback of mNGS is the expensive cost, which may be a burden for some patients.19 In the past ten years, MALDI-TOF has been widely used for the identification of Nocardia due to its rapid and cost-efficient. Recent studies using improved databases found that MALDI-TOF MS has shown remarkable accuracy in correctly identifying the species in 100% of cases for N. farcinica and between 94% and 100% for N. cyriacigeorgica. However, when it comes to other clinically frequent species such as N. nova and N. abscessus, the ability of MALDI-TOF MS to differentiate between closely related species within their respective complexes is limited.2
We used the Web of Science and PubMed databases to review the existing literature written in English from 2010 to 2023 on abdominal nocardiosis. Search items included “nocardiosis”, “nocardia”, “abdominal”, “retroperitoneal”, “infection”, “abscesses”. There are a total of 21 eligible cases, including 10 cases of disseminated abdominal nocardiosis (Table 1) and 11 cases of isolated abdominal nocardiosis (Table 2). As summarized, the mortality disseminated abdominal nocardiosis significantly was higher than isolated abdominal nocardiosis (60% vs 18.2%). Among these cases, disseminated abdominal nocardiosis was found to invade and usually attack the lungs (9 cases, 90%) and brain (6 cases, 60%). N. farcinica (9 cases) represented the most common isolated species. In terms of underlying diseases, solid organ transplant was the most frequent (4 cases), and other underlying diseases included but not limited to use corticosteroids or/and immunosuppressants (4 cases), hematologic malignancy (2 cases), primary nephrotic syndrome (1case), diabetes mellitus (1case) among others.
 |
Table 1 Clinical Characterizations of Disseminated Abdominal Nocardiosis During 2010–2023 |
 |
Table 2 Clinical Characterizations of Isolated Abdominal Nocardiosis During 2010–2023 |
Abdominal nocardiosis is often misdiagnosed as tuberculosis,20,21 or abdominal malignancies.22,23 This misidentification can occur due to the similarities in symptoms and imaging findings between these conditions and abdominal nocardiosis. Misdiagnosed can lead to incorrect treatment methods, including anti-tuberculous medications or unnecessary extensive surgeries.20,22,23 A published case (Table 2, Case 7) initially suspected primary renal cell carcinoma and underwent a right nephrectomy. However, postoperative biopsy not observed evidence of neoplasia; instead, eventually confirmed a diagnosis of Nocardia renal abscess.22 Another published case (Table 2, Case 11) suspected to have a uterine tumor and invaded the ureter and colon and performed hysterectomy, bilateral adnexectomy, colectomy, and partial left ureter resection. The final pathology showed Xanthogranulomatous inflammation caused by Nocardia.23
Surgical debridement and drainage are crucial for the prognosis of abscess caused by Nocardia, as aspiration or resection can control the source. Some patients with abscesses may require repeated aspiration or resection, due to abscess may reduce the permeability of antibiotics.24 In terms of isolated abdominal nocardiosis, 7 cases (63.6%) emphasized the role of surgical resection and drainage in promoting prognosis.
In vitro drug sensitivity testing of Nocardia can be used to guide antibacterial therapy, but there are some technical difficulties in vitro drug sensitivity testing, including method selection, bacterial suspension preparation, as well as the reading and interpretation of results. Therefore, microbiological laboratories do not routinely conduct in vitro drug sensitivity tests, and most of them rely on empirical anti-infection treatment. Due to the specific antibiotic sensitivity patterns associated with each species of Nocardia, it is crucial for clinical microbiology laboratories to provide reliable species identification to guide the selection of initial antimicrobial therapy. The most significant difference between Nocardia species is their different susceptibility to β - lactam antibiotics. It is worth noting that if species identification or antibiotic sensitivity testing cannot be conducted, β - lactam antibiotics should not be used as a single therapy for initial antibiotic treatment. A study on the distribution of Nocardia species and drug resistance in China showed that TMP-SMX was the most active drug against Nocardia isolated in China, and it could be used as the primary drug for treating Nocardia infections even without species identification or antibiotic susceptibility testing.41 Linezolid and amikacin are also effective drugs against most Nocardia species, with activity of 99.5% and 96.0%, respectively.41 Imipenem has varying sensitivity to various species of Nocardia, with a good sensitivity rate of 90.1% to N. cyriacigeorgica, while it showed a relatively low sensitivity rate to N. farcinica (45.5%).41 For immunocompetent patients, a 6-month course of antibacterial treatment is generally recommended, whereas immunosuppressed patients or those with disseminated CNS lesions typically require at least 6–12 months of treatment.5 The continuous presence or recurrence of Nocardia infection is often attributed to the use of insensitive antibiotics or inadequate treatment.
Conclusion
Poorly controlled diabetes and prolonged use of corticosteroids are the risk factors for disseminated abdominal abscesses in this patient. Antibiotic therapy, combination with surgical interventions and a gradual dosage reduction of glucocorticoids contribute to the management of N. farcinica disseminated abdominal abscesses.
Abbreviations
MALDI-TOF MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; TMP-SMX, trimethoprim-sulfamethoxazole. IPM/CS, impenem/cilastatin; CNS, central nervous system.
Data Sharing Statement
All the data are fully available without restriction.
Ethics Approval and Patient Consent
Written informed consent was obtained from the patient for the publication of the case details. The study was approved by the medical ethics committee of The People’s Hospital of Guangxi Zhuang Autonomous Region.
Acknowledgments
We would like to acknowledge the reviewers for their helpful comments on this paper.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Guangxi Natural Science Foundation under Grant No. 2023GXNSFBA026068.
Disclosure
The authors declare no conflict of interest in this work.
References
1. Mehrabadi SM, Taraghian M, Pirouzi A, et al. Pulmonary nocardiosis in suspected tuberculosis patients: a systematic review and meta-analysis of cross-sectional studies. Ethiop J Health Sci. 2020;30(2):293–300. doi:10.4314/ejhs.v30i2.17
2. Lafont E, Conan PL, Rodriguez-Nava V, et al. Invasive nocardiosis: disease presentation, diagnosis and treatment - old questions, new answers? Infect Drug Resist. 2020;13:4601–4613. doi:10.2147/IDR.S249761
3. Song J, Dong L, Ding Y, et al. A case report of brain abscess caused by Nocardia farcinica. Eur J Med Res. 2021;26(1):83. doi:10.1186/s40001-021-00562-2
4. Verner A, Durrani A, Kowalski RP, et al. A case of Nocardia farcinica keratitis in a pediatric contact lens wearer. Eye Contact Lens-Sci Clin Pract. 2020;46(2):e11–e12. doi:10.1097/ICL.0000000000000594
5. Pan L, Wang XH, Meng FQ, et al. Membranous nephropathy complicated with disseminated Nocardia farcinica infection: a case report and literature review. Infect Drug Resist. 2021;14:4157–4166. doi:10.2147/IDR.S331737
6. Sirijatuphat R, Niltwat S, Tiangtam O, et al. Purulent pericarditis and cardiac tamponade caused by Nocardia farcinica in a nephrotic syndrome patient. Intern Med. 2013;52(19):2231–2235. doi:10.2169/internalmedicine.52.0453
7. Ishiguro T, Yoshioka H, Kawai S, et al. A case of empyema and septic arthritis due to Nocardia farcinica. Clin Case Rep. 2017;5(12):1976–1979. doi:10.1002/ccr3.1228
8. Tripathi S, Meena DS, Rohila AK, et al. Empyema necessitans with osteomyelitis of fifth rib due to Nocardia farcinica: a case report. BMC Infect Dis. 2021;21(1):745. doi:10.1186/s12879-021-06452-6
9. Djennane S, Zecknini K, Billy C, et al. Abcès cérébral à Nocardia farcinica associé à une embolie pulmonaire chez une patiente immunocompétente [Nocardia farcinica brain abscess associated with a pulmonary embolism in an immunocompetent patient]. Presse Med. 2005;34(7):522–524. French. doi:10.1016/S0755-4982(05)83964-8
10. Gonzalez Ochoa A. Virulence of nocardiae. Can J Microbiol. 1973;19(8):901–904. doi:10.1139/m73-144
11. Wang H, Zhu Y, Cui Q, et al. Epidemiology and antimicrobial resistance profiles of the Nocardia species in China, 2009 to 2021. Microbiol Spectr. 2022;10(2):e01560–21.
12. Yang J, Ren HT, Wang J, et al. Clinical characteristics, susceptibility profiles, and treatment of nocardiosis: a multicenter retrospective study in 2015–2021. Int J Infect Dis. 2023;130:136–143. doi:10.1016/j.ijid.2023.02.023
13. Cattaneo C, Antoniazzi F, Caira M, et al. Nocardia spp infections among hematological patients: results of a retrospective multicenter study. Int J Infect Dis. 2013;17(8):e610–e614. doi:10.1016/j.ijid.2013.01.013
14. Baio PV, Ramos JN, Dos Santos LS, et al. Molecular identification of nocardia isolates from clinical samples and an overview of human nocardiosis in Brazil. PLoS Negl Trop Dis. 2013;7(12):e2573. doi:10.1371/journal.pntd.0002573
15. Coussement J, Lebeaux D, Rouzaud C, et al. Nocardia infections in solid organ and hematopoietic stem cell transplant recipients. Curr Opin Infect Dis. 2017;30(6):545–551. doi:10.1097/QCO.0000000000000404
16. Margalit I, Muhsen K, Ben Ari Y, et al. Nocardia colonization in contrast to nocardiosis: a comparison of patients’ clinical characteristics. Eur J Clin Microbiol Infect Dis. 2020;39(4):759–763. doi:10.1007/s10096-019-03796-5
17. Malo de Molina R, Mortensen EM, Restrepo MI, et al. Inhaled corticosteroid use is associated with lower mortality for subjects with COPD and hospitalised with pneumonia. Eur Respir J. 2010;36(4):751–757. doi:10.1183/09031936.00077509
18. Rouzaud C, Rodriguez-Nava V, Catherinot E, et al. Clinical assessment of a Nocardia PCR-based assay for diagnosis of nocardiosis. J Clin Microbiol. 2018;56(6). doi:10.1128/JCM.00002-18
19. Jiao M, Deng X, Yang H, et al. Case report: a severe and multi-site Nocardia farcinica infection rapidly and precisely identified by metagenomic next-generation sequencing. Front Med. 2021;8:669552. doi:10.3389/fmed.2021.669552
20. Singh S, Verma Y, Pandey P, et al. Granulomatous hepatitis by Nocardia species: an unusual case. Int J Infect Dis. 2019;81:97–99. doi:10.1016/j.ijid.2019.01.046
21. John MA, Madiba TE, Mahabeer P, et al. Disseminated nocardiosis masquerading as abdominal tuberculosis. South Afr J Surg. 2004;42(1):17–19.
22. Biswas Roy S, Ross MD, Patil PD, et al. Primary Nocardia infection causing a fluorodeoxyglucose-avid right renal mass in a redo lung transplant recipient. Case Rep Transplant. 2018;2018:9752860. doi:10.1155/2018/9752860
23. Shen TA, Hsu YH, Ding DC. Xanthogranulomatous inflammation caused by K. pneumonia and nocardiosis mimicking a uterine tumor and invading the ureter and colon: a case report and review of the literature. Taiwanese J Obstetrics Gynecol. 2022;61(5):889–895. doi:10.1016/j.tjog.2021.12.006
24. Lebeaux D, Coussement J, Bodilsen J, et al. Management dilemmas in Nocardia brain infection. Curr Opin Infect Dis. 2021;34(6):611–618. doi:10.1097/QCO.0000000000000782
25. Al-Tawfiq JA, Al-Khatti AA. Disseminated systemic Nocardia farcinica infection complicating alefacept and infliximab therapy in a patient with severe psoriasis. Int J Infect Dis. 2010;14(2):e153–e157. doi:10.1016/j.ijid.2009.03.017
26. Arora G, Friedman M, Macdermott RP. Disseminated Nocardia nova infection. Southern Med J. 2010;103(12):1269–1271. doi:10.1097/SMJ.0b013e3181faec65
27. Hu Y, Zheng D, Takizawa K, et al. Systemic nocardiosis caused by Nocardia concava in China. Med Mycol. 2011;49(6):662–666. doi:10.3109/13693786.2011.555849
28. de Montmollin E, Corcos O, Noussair L, et al. Retroperitoneal abscesses due to Nocardia farcinica: report of two cases in patients with malnutrition. Infection. 2012;40(1):93–96. doi:10.1007/s15010-011-0176-7
29. Xu J, Yachnis AT, Malaty I. An independent elderly woman with rapid onset of coma. JAMA neurol. 2014;71(8):1043–1047. doi:10.1001/jamaneurol.2014.449
30. Piau C, Kerjouan M, Le Mouel M, et al. First case of disseminated infection with Nocardia cerradoensis in a human. J Clin Microbiol. 2015;53(3):1034–1037. doi:10.1128/JCM.02979-14
31. Jiang Y, Huang A, Fang Q. Disseminated nocardiosis caused by Nocardia otitidiscaviarum in an immunocompetent host: a case report and literature review. Exp Ther Med. 2016;12(5):3339–3346. doi:10.3892/etm.2016.3755
32. Lim MY, Alker AP, Califano S, et al. Concurrent disseminated nocardiosis and GI mucormycosis in a stem-cell transplantation recipient. J Clin Oncol. 2016;34(10):e84–e86. doi:10.1200/JCO.2013.51.4042
33. Senard O, Blanot S, Jouvion G, et al. Fulminant nocardiosis due to a multidrug-resistant isolate in a 12-year-old immunocompetent child. Pediatrics. 2018;141(2). doi:10.1542/peds.2016-3131
34. Schlebusch S, Nimmo G, Carter R. Bowel abscess with Nocardia veterana associated with colon carcinoma. Pathology. 2010;42(3):306–307. doi:10.3109/00313021003633144
35. Kamyab A, Fakhoury JD, Sutkowski R, et al. Fulminant colitis secondary to nocardiosis. Int J Colorect Dis. 2012;27(6):841–842. doi:10.1007/s00384-011-1327-0
36. Naha K, Dasari S, Vivek G, et al. Primary abdominal nocardiosis masquerading as tubercular pelvic inflammatory disease in an immunocompetent individual. BMJ Case Rep. 2013;2013(jan25 1):bcr2012008076. doi:10.1136/bcr-2012-008076
37. Hanchanale P, Jain M, Varghese J, et al. Nocardia liver abscess post liver transplantation—A rare presentation. Transp Infect Dis. 2017;19(2). doi:10.1111/tid.12670
38. Turer D, Gray B, Raghavendran K. Fulminant Nocardia colitis: a case report. Surg Infect Case Rep. 2016;1(1):69–71. doi:10.1089/crsi.2016.0013
39. Acharya R, Amin K, Rajderkar D, et al. Isolated abdominal nocardiosis in a pediatric renal transplant recipient. Pediatr Transplant. 2019;23(4):e13392. doi:10.1111/petr.13392
40. Tramèr L, Mertz KD, Huegli R, et al. Intra-abdominal nocardiosis-case report and review of the literature. J Clin Med. 2020;9(7):2141. doi:10.3390/jcm9072141
41. Wang C, Sun Q, Yan J, et al. The species distribution and antimicrobial resistance profiles of Nocardia species in China: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2023;17(7):e0011432. doi:10.1371/journal.pntd.0011432
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.