Back to Journals » Drug Design, Development and Therapy » Volume 17
ABCG2 Gene Polymorphisms May Affect the Bleeding Risk in Patients on Apixaban and Rivaroxaban
Authors Kim H, Song TJ , Yee J, Kim DH, Park J , Gwak HS
Received 13 April 2023
Accepted for publication 17 August 2023
Published 23 August 2023 Volume 2023:17 Pages 2513—2522
DOI https://doi.org/10.2147/DDDT.S417096
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Hamin Kim,1,* Tae-Jin Song,2,* Jeong Yee,1 Dong-Hyeok Kim,3 Junbeom Park,4 Hye Sun Gwak1
1College of Pharmacy and Graduate School of Pharmaceutical Sciences, Ewha Womans University, Seoul, Korea; 2Department of Neurology, Seoul Hospital, Ewha Womans University College of Medicine, Seoul, Korea; 3Department of Cardiology, Seoul Hospital, Ewha Womans University College of Medicine, Seoul, Korea; 4Department of Cardiology, Mokdong Hospital, Ewha Womans University College of Medicine, Seoul, Korea
*These authors contributed equally to this work
Correspondence: Hye Sun Gwak, College of Pharmacy and Graduate School of Pharmaceutical Sciences, Ewha Womans University, 52 Ewhayeodae-gil, Seodaemun-gu, Seoul, 03760, Korea, Tel +82-2-3277-4376, Email [email protected] Junbeom Park, Department of Cardiology, Mokdong Hospital, Ewha Womans University College of Medicine, 1071, Annyangcheon-Ro, Yangcheon-Gu, Seoul, 07985, Korea, Tel +82-2-2650-5826, Email [email protected]
Purpose: Direct oral anticoagulants (DOACs) are widely used for stroke prevention in atrial fibrillation. However, they have a bleeding complication. Breast cancer resistance protein, encoded by ABCG2, is known to be an efflux transporter of apixaban and rivaroxaban among DOACs. This study aimed to investigate the association between gene variants and bleeding complications during treatment with ABCG2 substrates (apixaban and rivaroxaban).
Patients and Methods: Patients treated with apixaban and rivaroxaban were enrolled from June 2018 to December 2021. Five single nucleotide polymorphisms (SNPs) of ABCG2 were selected. Previously studied genes (ABCB1, CYP3A4, and CYP3A5) were further analyzed as possible confounders. Finally, a total of 16 SNPs were examined in this case–control study. The outcome was defined as major bleeding and clinically relevant non-major bleeding. Two models were constructed using the multivariable analysis.
Results: Among 293 patients, 64 were cases. The mean age of the patients was 68.8 years, and males comprised 62.5% of the study population. Model I revealed that a history of bleeding, concurrent use of proton pump inhibitor (PPI), ABCG2 rs3114018, and ABCB1 rs1045642 were significantly associated with bleeding complications; the AORs (95% CI) were 6.209 (2.210– 17.442), 2.385 (1.064– 5.349), 2.188 (1.156– 4.142), and 3.243 (1.371– 7.671), respectively. Model II showed that modified HAS-BLED score, concurrent use of PPI, ABCG2 rs3114018, and ABCB1 rs1045642 were significantly associated with bleeding complications.
Conclusion: The modified HAS-BLED score, a history of bleeding, concurrent use of PPI, ABCG2 rs3114018, and ABCB1 rs1045642 were significantly associated with the risk of bleeding complications in patients on apixaban and rivaroxaban, after adjusting for other confounders. These findings can be used to develop individualized treatment strategies for patients taking apixaban and rivaroxaban.
Keywords: ABCG2, apixaban, bleeding complications, polymorphism, rivaroxaban
A Letter to the Editor has been published for this article.
Introduction
Anticoagulants are essential for treating and preventing thromboembolism in various fields, including cardiology, neurology, and orthopedics. Previously, vitamin K antagonists (VKAs) were widely used oral anticoagulants, a well-known example being warfarin. However, the need to develop new oral anticoagulants has emerged because VKA therapy requires frequent monitoring of the international normalized ratio and dose adjustment.
Direct oral anticoagulants (DOACs) are more convenient than warfarin because of the fixed-dose administration and the unnecessity of routine blood monitoring. DOACs directly block factor Xa (apixaban, edoxaban, and rivaroxaban) or block thrombin (dabigatran).
Although DOACs offer safety advantages over warfarin due to a lower rate of life-threatening and intracranial bleeding,1 they still have bleeding complications, including gastrointestinal bleeding, hemorrhage, hematochezia, hematuria, and epistaxis.2 In 2021, more than 3700 bleeding cases by DOAC use were reported according to the FDA Adverse Events Reporting System. Given the higher risk of stroke and intracranial bleeding in Asians, the issue of individualization of DOACs has received much attention.3,4
Changes in drug exposure might affect the efficacy and safety of DOACs. Several metabolizing enzymes and transporters contribute to the pharmacokinetics of DOACs. Efflux transporters, such as multidrug resistance protein 1 (MDR1, P-gp, ABCB1) and breast cancer resistance proteins (BCRP, ABCG2), affect the pharmacokinetics of DOACs. BCRP is located in the apical membrane in the epithelia of the intestine and reduces drug absorption by extruding chemical compounds from the cell.5 Moreover, BCRP is located in the brain, liver, and kidney and limits brain penetration of substrate drugs in addition to secreting substrates actively into the biliary or renal excretion route.6–8 Among DOACs, apixaban and rivaroxaban are substrates of BCRP.9
Although there have been several pharmacogenetic studies on DOAC pharmacokinetics, there have been few conducted in the area of clinical safety.10 Moreover, little is known about the association between ABCG2 and bleeding complications in patients treated with ABCG2 substrates such as apixaban and rivaroxaban. Therefore, this study aimed to investigate the impact of ABCG2 gene polymorphisms on bleeding complications in apixaban and rivaroxaban users.
Materials and Methods
Patients
For this case–control study, patients had been enrolled in Ewha Womans University Mokdong Hospital, Ewha Womans University Seoul Hospital, Severance Hospital of Yonsei University College of Medicine, and Seoul National University Hospital from June 2018 to December 2021. This study was conducted according to the 1975 Helsinki Declaration and its later amendments approved by the Institutional Review Board (IRB) of each hospital following (IRB number: 2018-04-006, 2019-05-038, 4-2018-0823, and 1811-076-9850, respectively). Written informed consent was obtained from the patients.
Patients treated with apixaban or rivaroxaban for reduction of the risk of stroke and systemic embolism in nonvalvular atrial fibrillation were eligible. Patients who met any of the following criteria were excluded: age under 20 years; experience of thromboembolism or infarction in the follow-up period; experience of bleeding which was minor or not verified by health professionals while on treatment; refused consent; less than three months of treatment in the control group; and insufficient sample for genotyping.
Outcome
Cases were patients who experienced major bleeding or clinically relevant non-major bleeding (CRNMB). The criteria for major bleeding and CRNMB followed the International Society on Thrombosis and Haemostasis guideline.11,12 Major bleeding was defined as fatal bleeding, and/or bleeding in a critical organ (eg, intracranial, intraspinal, intraocular), and/or bleeding causing a fall in hemoglobin level of 20 g L−1 or more, or leading to two or more units of blood transfusion. CRNMB was defined as any bleedings which did not satisfy the criteria for major bleeding, but does meet at least one of the following criteria: i) requiring medical intervention by a healthcare professional, ii) leading to hospitalization or increased level of care, or iii) prompting a face-to-face (ie, not just a telephone or electronic communication) evaluation. Cases were followed up until the bleeding events, while controls were followed up until December 31, 2021.
Data Collection
Patients’ data were collected from electronic medical records. The extracted data were as follows: sex, age, body weight, serum creatinine clearance, systolic blood pressure, diastolic blood pressure, dose of DOAC, alcohol, smoking, comorbidities, history of stroke/transient ischemic attacks (TIA)/thromboembolism, history of bleeding, co-medication, and follow-up period. A modified HAS-BLED (hypertension, abnormal renal or liver function, stroke, bleeding history or predisposition, elderly (age≥65 years), concomitant drug, and alcohol use; range 0–8) score was calculated without liable international normalized ratio (INR) compared to HAS-BLED.13
Single Nucleotide Polymorphism (SNP) Selection
To investigate the relationship between ABCG2 and bleeding complications in patients treated with apixaban and rivaroxaban, SNPs were searched in NCBI (https://www.ncbi.nlm.nih.gov/variation/view/). The SNP was excluded if the minor allele frequency was <0.1 in Asians from the 1000 Genome project.14 Only one SNP was chosen if SNPs were in perfect linkage disequilibrium (r2=1) with others.15 Finally, five SNPs in ABCG2 were selected: ABCG2 rs2231142 (NC_000004.11:g.89052323G>T; NM_004827.3:c.421C>A; missense variant), ABCG2 rs2231137 (NC_000004.11:g.89061114C>T; NM_004827.3:c.34G>A; missense variant), ABCG2 rs2622604 (NC_000004.11:g.89078924T>C; NM_004827.3:c.-20+614A>G; intron variant), ABCG2 rs3114018 (NC_000004.11:g.89064581A>C; NM_004827.3:c.-19-3415T>G; intron variant), and ABCG2 rs1481012 (NC_000004.11:g.89039082A>G; NM_004827.3:c.841+179T>C; intron variant).
As apixaban and rivaroxaban are also substrates of ABCB1 and CYP3A,9 we additionally analyzed SNPs in ABCB1, CYP3A4, and CYP3A5 to adjust for their effects. SNPs were selected in the same way as the selection of ABCG2 SNPs: ABCB1 rs1045642 (NC_000007.13:g.87138645A>G; NM_001348946.2:c.3435T>C; missense variant), ABCB1 rs2032582 (NC_000007.13:g.87160618A>C; NM_001348946.2:c.2677T>G; missense variant), ABCB1 rs1128503 (NC_000007.13:g.87179601A>G; NM_001348946.2:c.1236T>C; synonymous variant), ABCB1 rs3789243 (NC_000007.13:g.87220886A>G; NM_001348946.2:c.117+4196T>C; intron variant), ABCB1 rs3213619 (NC_000007.13:g.87230193A>G; NM_001348946.2:c.-129T>C; intron variant), CYP3A4 rs2242480 (NC_000007.13:g.99361466C>T; NM_017460.6:c.1026+12G>A; intron variant), CYP3A4 rs4646437 (NC_000007.13:g.99365083G>A; NM_017460.6:c.671–202C>T; intron variant), CYP3A4 rs12333983 (NC_000007.13:g.99354114T>A; 500B downstream variant), CYP3A5 rs776746 (NC_000007.13:g.99270539C>T; NM_000777.5:c.219–237G>A; splice acceptor variant, CYP3A5*3), CYP3A5 rs15524 (NC_000007.13:g.99245914A>G; NM_000777.5:c.*14T>C; non-coding transcript variant), and CYP3A5 rs4646450 (NC_000007.13:g.99266318G>A; NM_000777.5:c.319–1630C>T; intron variant).
Genotyping
The patient’s genomic DNA was extracted from the blood or saliva samples. Blood samples were used to extract DNA with QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany). Saliva samples were collected by OraGene-600 kits (DNA Genotek, OTT, Canada) and used to extract DNA using PrepIT reagents (DNA Genotek, OTT, Canada).
Extracted DNA sample was genotyped using the TaqMan SNP genotyping assay (Applied Biosystems, Foster City, CA, USA) or SNaPshot Multiplex Kits (Applied Biosystems, Foster City, CA, USA). Genotyping was undertaken by a third party blinded to outcome status.
Statistical Analysis
An unpaired t-test was used for continuous variables. The chi-squared test was used for categorical variables, and Fisher’s exact test was used if more than 20% of cells had expected frequencies of less than five. To evaluate the genetic association, we applied both dominant and recessive models and the most appropriate model was selected based on their effect size and statistical significance. The unadjusted odds ratios (ORs) and adjusted ORs (AORs) with 95% confidence intervals (CIs) were calculated via univariate and multivariable analyses, respectively. In the multivariable logistic regression analysis, factors with a p-value <0.05 in the univariate analysis were put in addition to well-known confounders (sex, age, weight, and creatinine clearance). Next, backward elimination was employed to remove insignificant variables using the probability of the likelihood-ratio statistic based on the maximum partial likelihood estimates.16 Regarding the modified HAS-BLED score and its components, we constructed two separate models: Model I included each component of modified HAS-BLED score, if it satisfied the criteria, and Model II included a modified HAS-BLED score instead of components of HAS-BLED score in the Model I. The predictive model fit was also evaluated by Hosmer–Lemeshow goodness-of-fit test. To determine whether the study was sufficiently powered to detect differences, the post-hoc power analysis was performed using Quanto 1.2.4 (https://pphs.usc.edu/biostatistics-software/#quanto).
All statistical analyses used SPSS v20.0 (IBM Corp., Armonk, NY, USA), and statistical significance was judged if the p-value was less than 0.05. Hardy-Weinberg equilibrium and missingness tests were conducted using PLINK.17 The SNP would be considered to fail the Hardy-Weinberg equilibrium test if the p-value was <0.001. Missingness was defined as the loss of >10% of data. The outcome of a missingness test was considered a failure when the missingness rate was >0.1 per SNP and per individual.
Results
Among the 351 patients enrolled, 58 patients were excluded for the following reasons: thromboembolism or infarction in the follow-up period (n=24), the experience of bleeding that was minor or not verified by health professionals while on treatment (n=22), less than three months of treatment in the control group (n=8), and overlapping enrollment (n=4). The flow chart of patient selection is shown in Figure 1.
 |
Figure 1 Flowchart of patient selection. |
Among 293 patients selected for analysis, cases comprised 64 patients (21.8%), of whom 26 patients experienced major bleeding and 38 patients experienced CRNMB. The baseline characteristics of the study population are detailed in Table 1. Males comprised 62.5% of all the study population. The mean age of the study population was 68.8 years, 201 patients (68.6%) were apixaban users, and 92 patients (31.4%) were rivaroxaban users. Without the factor of labile INRs, the mean of modified HAS-BLED scores was 2.0. The most common comorbidity was hypertension (71.7%), followed by dyslipidemia (29.0%) and diabetes mellitus (28.3%). Eighteen (6.1%) patients had a history of bleeding, and 115 (39.2%) had a history of stroke/TIA/thromboembolism. The most common co-medications were beta-blockers (74.7%), followed by statins (54.5%). Compared to controls, cases showed higher modified HAS-BLED (p=0.008), had more histories of stroke/TIA/thromboembolism and bleeding (p=0.022 and p<0.001, respectively), and received more proton-pump inhibitor (PPI, p=0.032).
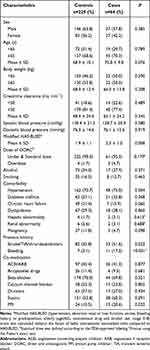 |
Table 1 Baseline Characteristics of the Study Patients |
Genetic differences in candidate SNPs between cases and controls are shown in Table 2. Among 16 SNPs of ABCG2, ABCB1, CYP3A4, and CYP3A5 genotyped, none of the SNPs failed the Hardy-Weinberg equilibrium test or the missingness test. Minor allele frequencies in the selected patients ranged from the smallest 0.07 of CYP3A4 rs4646437 to the largest 0.49 of ABCB1 rs2032582.
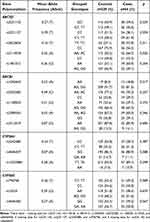 |
Table 2 Genetic Factors Associated with Bleeding Complications in Patients Treated with Apixaban and Rivaroxaban |
The ABCG2 rs2622604 and ABCG2 rs3114018 were significantly associated with the risk of bleeding (rs2622604 T allele carriers 31.1% vs CC genotype carriers 17.7%, p=0.011; rs3114018 A allele carriers 26.8% vs CC genotype carriers 15.6%, p=0.020). In ABCB1, patients with the AA genotype of rs1045642 had a higher bleeding risk than G allele carriers (38.7% vs 19.9%, p=0.017). Assuming a significance level of 0.05, the post hoc power analysis showed that our study had around 65% statistical power for those three SNPs. None of the CYP3A4 and CYP3A5 SNPs were statistically significant.
For the multivariable logistic regression analyses, two models were constructed using variables as follows: female, age ≥ 65 years, weight < 65 kg, creatinine clearance <50 mL min−1, modified HAS-BLED score, history of stroke/TIA/thromboembolism, history of bleeding, concurrent use of PPI, and genetic factors, which were significant in univariate analysis (Table 3). Model I included factors except for the modified HAS-BLED score. Model II included a modified HAS-BLED score instead of components of HAS-BLED score in the Model I. After backward elimination, Model I showed that a history of bleeding, concurrent use of PPI, ABCG2 rs3114018 A allele carriers, and ABCB1 rs1045642 AA genotype carriers were significantly associated with increased risk of bleeding; the AORs (95% CI) were 6.209 (2.210–17.442, p<0.001), 2.385 (1.064–5.349, p=0.035), 2.188 (1.156–4.142, p=0.016), and 3.243 (1.371–7.671, p=0.007), respectively. In the Model II, modified HAS-BLED score, concurrent use of PPI, ABCG2 rs3114018 A allele carriers, and ABCB1 rs1045642 AA genotype carriers were significantly associated with increased risk of bleeding; the AORs (95% CI) were 1.347 (1.018–1.782, p=0.037), 2.359 (1.079–5.156, p=0.031), 2.335 (1.250–4.360, p=0.008), and 3.167 (1.349–7.436, p=0.008). Among the significant SNPs in the univariate analysis, ABCG2 rs2622604 did not remain as a significant predictor in both models. The Hosmer–Lemeshow test showed that both models were a good fit (χ2=5.121 and p=0.163; χ2=4.947 and p=0.763, respectively).
 |
Table 3 The Results of Logistic Regression Analysis for Bleeding Complications in Patients Treated with Apixaban and Rivaroxaban |
Discussion
In this study, a modified HAS-BLED score, a history of bleeding, concurrent use of PPI, ABCG2 rs3114018, and ABCB1 rs1045642 were significantly associated with the risk of bleeding complications in patients on apixaban and rivaroxaban, after adjusting for other confounders.
The finding that the A carriers of rs3114018 was associated with bleeding complications was supported by research by Custodio.18 Patients harboring the ABCG2 rs3114018 AA genotype had a higher risk of grade 2–3 oxaliplatin-induced peripheral neuropathy compared to the C allele carriers of ABCG2 rs3114018, with marginal significance. Meanwhile, ABCG2 protein located in the renal proximal tubular brush border membranes is known to be involved in the pathogenesis of gout by mediating renal urate secretion.19 The A allele of rs3114018 increased the risk of gout 1.6-fold in the 453 Chinese Han population.20
ABCB1 SNPs are the most commonly documented genetic variants for the alteration of plasma drug levels of apixaban and rivaroxaban.21,22 An in vitro study showed that the ABCB1 mRNA expression in the G allele of rs1045642 was significantly higher than that in the A allele.23 Moreover, A allele carriers in rs1045642 had higher maximum plasma concentration and area under the curve from time 0 to infinity than those of the GG genotype in a meta-analysis involving 535 individuals treated with DOACs.24 Unusual high drug exposure could raise the risk of drug toxicity. Hence, this could also be a possible explanation for the result of this study, which indicated the association of ABCB1 rs1045642 with hemorrhage induced by apixaban and rivaroxaban.
PPI is known as a protective factor in gastrointestinal bleeding.25 Surprisingly, this study found concurrent use of PPI to have a significant relationship with bleeding complications. This result was possibly due to the role of PPI as an ABCG2 inhibitor. A study showed that coadministration of pantoprazole and omeprazole could increase the serum concentrations of methotrexate (ABCG2 substrate), indicating that they inhibited methotrexate transport in Sf9 cells with BCRP vesicles.26 Another study also demonstrated that coadministration of PPIs, including omeprazole, lansoprazole, and rabeprazole, delayed methotrexate elimination by inhibiting BCRP-mediated transport of methotrexate.27 Moreover, PPIs are known as substrates and inhibitors of P-gp.28 Therefore, the inhibition of efflux transporters partly explains the result of this study. However, since our finding showed the association, but did not prove the causality, the possibility remained that patients with higher risk of bleeding may be more likely to be treated with PPI in order to prevent gastrointestinal bleeding.
The history of bleeding showed a 6.3-fold high association with bleeding complications in this study. According to Pisters et al, the percentage of patients with a history of major bleeding was higher in the bleeding group than in the no-bleeding group, from the Euro Heart Survey (17% vs 2%, p<0.001).13 Further, the hazard ratio of bleeding history on major bleeding was 1.73 in 7411 patients from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF).29 Similarly, the history of bleeding was higher in the case group than in the control group in this study.
Among ABCG2 SNPs, rs2231142 (missense variant) has mainly been studied for apixaban and rivaroxaban. However, the results were controversial. In a previous study, the T allele of rs2231142 predicted higher apixaban concentrations in 358 Caucasian atrial fibrillation patients.30 The mean of oral clearance of apixaban was also lower in patients with the TT genotype than in those with the G allele carriers.22 However, no correlations were observed with rs2231142 SNP in the dose-adjusted trough concentrations of rivaroxaban in 86 patients with non-valvular atrial fibrillation.31 No significant increase in the risk of bleeding complications was found in this study. Therefore, additional studies are required to clarify the role of this SNP in DOAC-induced bleeding.
Regarding CYP-mediated metabolism, apixaban and rivaroxaban are metabolized via CYP3A4/5 at ~15% and 18%, respectively.32 One of the noted SNPs among CYP3A-encoding gene variants, CYP3A5 rs776746, is a missense SNP encoding the CYP3A5*3, which is a loss-of-function variant.33 A positive relationship was reported between the CYP3A5*3 allele and apixaban users’ plasma trough concentration/dose ratio in 44 Japanese patients with atrial fibrillation.34 Moreover, according to FDA-approved drug labeling, strong inhibitors and inducers of CYP3A and P-gp should be avoided during treatment with apixaban and rivaroxaban. However, including CYP3A5 rs776746, none of the CYP3A variants had statistical significance in this study. Therefore, further study is needed using populations of various ethnicities.
There are several limitations to this study. First, this study was conducted with a retrospective design, although DNA samples were obtained prospectively. Second, we did not evaluate the pharmacokinetic parameters of DOAC. Moreover, subgroup analysis was not conducted based on the DOAC type (eg, apixaban, rivaroxaban) and bleeding site (eg, brain, gastrointestinal duct) due to the small sample size. Lastly, we did not perform multiple test corrections to avoid the possible loss of the true positives. Therefore, it needs to be validated by further replication studies. However, to the best of our knowledge, this is the first study to discuss the effect of ABCG2-related genetic markers on major bleeding and CRNMB in patients receiving apixaban and rivaroxaban.
Conclusion
In conclusion, this study sought to determine whether demographic and genetic factors had any influence on bleeding in patients treated with apixaban and rivaroxaban. The result of multivariable regression analysis proved the effects of the factors on bleeding complications. The finding of this study, while preliminary, suggested that genotyping could help optimize individualized treatment strategies in patients taking DOACs, especially those who have clinical and genetic risk factors. These results provide wider support for the hypothesis that genotyping could provide patients with effective and safe anticoagulation therapy.
Acknowledgment
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (grant number: NRF-2020R1A2C1008120).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129:S33–40. doi:10.1016/j.amjmed.2016.06.003
2. Hellenbart EL, Faulkenberg KD, Finks SW. Evaluation of bleeding in patients receiving direct oral anticoagulants. Vasc Health Risk Manag. 2017;13:325–342. doi:10.2147/VHRM.S121661
3. Wong KS, Hu DY, Oomman A, et al. Rivaroxaban for stroke prevention in East Asian patients from the ROCKET AF trial. Stroke. 2014;45:1739–1747. doi:10.1161/STROKEAHA.113.002968
4. Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;112:789–797. doi:10.1160/TH13-11-0948
5. Staud F, Pavek P. Breast cancer resistance protein (BCRP/ABCG2). Int J Biochem Cell Biol. 2005;37:720–725. doi:10.1016/j.biocel.2004.11.004
6. Taskar KS, Yang X, Neuhoff S, et al. Clinical Relevance of Hepatic and Renal P‐gp/BCRP Inhibition of Drugs: an International Transporter Consortium Perspective. Clin Pharmacol Ther. 2022;112:573–592. doi:10.1002/cpt.2670
7. Pauli‐Magnus C, Meier PJ. Hepatobiliary transporters and drug‐induced cholestasis. Hepatology. 2006;44:778–787. doi:10.1002/hep.21359
8. Pang KS, Rodrigues AD, Peter RM. Enzyme-and Transporter-Based Drug-Drug Interactions. New York: Springer; 2010:301–307.
9. Gronich N, Stein N, Muszkat M. Association between use of pharmacokinetic‐interacting drugs and effectiveness and safety of direct acting oral anticoagulants: nested case‐control study. Clin Pharmacol Ther. 2021;110:1526–1536. doi:10.1002/cpt.2369
10. Kanuri SH, Kreutz RP. Pharmacogenomics of novel direct oral anticoagulants: newly identified genes and genetic variants. J Pers Med. 2019;9:7. doi:10.3390/jpm9010007
11. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–694. doi:10.1111/j.1538-7836.2005.01204.x
12. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Subcommittee on Control of Anticoagulation. Definition of clinically relevant non‐major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non‐surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119–2126. doi:10.1111/jth.13140
13. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi:10.1378/chest.10-0134
14. 1000 Genomes Project Consortium. An integrated map of genetic variation from 1092 human genomes. Nature. 2012;491:56–65. doi:10.1038/nature11632
15. Ward LD, Kellis M. HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. 2016;44:D877–81. doi:10.1093/nar/gkv1340
16. Field A. Discovering Statistics Using IBM SPSS Statistics.
17. Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi:10.1086/519795
18. Custodio A, Moreno-Rubio J, Aparicio J, et al. Pharmacogenetic predictors of severe peripheral neuropathy in colon cancer patients treated with oxaliplatin-based adjuvant chemotherapy: a GEMCAD group study. Ann Oncol. 2014;25:398–403. doi:10.1093/annonc/mdt546
19. McKnight AJ, Currie D, Maxwell AP. Unravelling the genetic basis of renal diseases; from single gene to multifactorial disorders. J Pathol. 2010;220:198–216. doi:10.1002/path.2639
20. Jiri M, Zhang L, Lan B, et al. Genetic variation in the ABCG2 gene is associated with gout risk in the Chinese Han population. Clin Rheumatol. 2016;35:159–163. doi:10.1007/s10067-015-3105-9
21. Gouin‐Thibault I, Delavenne X, Blanchard A, et al. Interindividual variability in dabigatran and rivaroxaban exposure: contribution of ABCB1 genetic polymorphisms and interaction with clarithromycin. J Thromb Haemost. 2017;15:273–283. doi:10.1111/jth.13577
22. Ueshima S, Hira D, Kimura Y, et al. Population pharmacokinetics and pharmacogenomics of apixaban in Japanese adult patients with atrial fibrillation. Br J Clin Pharmacol. 2018;84:1301–1312. doi:10.1111/bcp.13561
23. Wang D, Johnson AD, Papp AC, et al. Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C> T affects mRNA stability. Pharmacogenet Genomics. 2005;15:693–704. doi:10.1097/01.fpc.0000178311.02878.83
24. Xie Q, Xiang Q, Mu G, et al. Effect of ABCB1 genotypes on the pharmacokinetics and clinical outcomes of new oral anticoagulants: a systematic review and meta-analysis. Curr Pharm Des. 2018;24:3558–3565. doi:10.2174/1381612824666181018153641
25. Lee JY. Risk Factors of Gastrointestinal Bleeding in Patients Receiving New Oral Anticoagulants. Korean J Helicobacter Up Gastrointest Res. 2018;18:219–224. doi:10.7704/kjhugr.2018.18.4.219
26. Breedveld P, Zelcer N, Pluim D, et al. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug-drug interactions. Cancer Res. 2004;64:5804–5811. doi:10.1158/0008-5472.CAN-03-4062
27. Suzuki K, Doki K, Homma M, et al. Co‐administration of proton pump inhibitors delays elimination of plasma methotrexate in high‐dose methotrexate therapy. Br J Clin Pharmacol. 2009;67:44–49. doi:10.1111/j.1365-2125.2008.03303.x
28. Pauli-Magnus C, Rekersbrink S, Klotz U, et al. Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:551–557. doi:10.1007/s00210-001-0489-7
29. O’Brien EC, Simon DN, Thomas LE, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J. 2015;36:3258–3264. doi:10.1093/eurheartj/ehv476
30. Gulilat M, Keller D, Linton B, et al. Drug interactions and pharmacogenetic factors contribute to variation in apixaban concentration in atrial fibrillation patients in routine care. J Thromb Thrombolysis. 2020;49:294–303. doi:10.1007/s11239-019-01962-2
31. Nakagawa J, Kinjo T, Iizuka M, et al. Impact of gene polymorphisms in drug‐metabolizing enzymes and transporters on trough concentrations of rivaroxaban in patients with atrial fibrillation. Basic Clin Pharmacol Toxicol. 2021;128:297–304. doi:10.1111/bcpt.13488
32. Gong IY, Kim RB. Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban. Can J Cardiol. 2013;29:S24–33. doi:10.1016/j.cjca.2013.04.002
33. Schuetz EG, Relling MV, Kishi S, et al. PharmGKB update: II. CYP3A5, cytochrome P450, family 3, subfamily A, polypeptide 5. Pharmacol Rev. 2004;56:159. doi:10.1124/pr.56.2.1
34. Ueshima S, Hira D, Fujii R, et al. Impact of ABCB1, ABCG2, and CYP3A5 polymorphisms on plasma trough concentrations of apixaban in Japanese patients with atrial fibrillation. Pharmacogenet Genomics. 2017;27:329–336. doi:10.1097/FPC.0000000000000294
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
