Back to Journals » OncoTargets and Therapy » Volume 7
ABCC4 is required for cell proliferation and tumorigenesis in non-small cell lung cancer
Authors Zhao X , Guo Y, Yue W , Zhang L, Gu M, Wang Y
Received 15 October 2013
Accepted for publication 16 December 2013
Published 21 February 2014 Volume 2014:7 Pages 343—351
DOI https://doi.org/10.2147/OTT.S56029
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Xiaoting Zhao, Yinan Guo, Wentao Yue, Lina Zhang, Meng Gu, Yue Wang
Department of Cellular and Molecular Biology, Beijing TB and Thoracic Tumor Research Institute/Beijing Chest Hospital, Capital Medical University, Beijing, People's Republic of China
Background: Multidrug resistance protein 4 (MRP4), also known as ATP-cassette binding protein 4 (ABCC4), is a member of the MRP/ABCC subfamily of ATP-binding cassette transporters, which are capable of pumping a wide variety of drugs out of the cell. However, little is known about the function of ABCC4 in the proliferation of lung cancer cells.
Methods: ABCC4 mRNA and protein levels in lung cancer cell lines were measured by real-time polymerase chain reaction and Western blot, respectively. A lentivirus-mediated RNA interference technique was used to inhibit ABCC4 mRNA expression in A549 and 801D cells. The function of ABCC4 in cell growth was investigated by MTS and colony formation assays. The role of ABCC4 in cell cycle progression was evaluated by flow cytometry and Western blot analysis. ABCC4 mRNA levels in 30 pairs of tumors and corresponding matched adjacent normal tissues from non-small cell lung cancer patients were detected by real-time polymerase chain reaction.
Results: ABCC4 was highly expressed in lung cancer cell lines. ABCC4 expression was markedly downregulated in A549 and 801D cells using the RNA interference technique. Suppression of ABCC4 expression inhibited cell growth. The percentage of cells in G1 phase was increased when ABCC4 expression was suppressed. Phosphorylation of retinoblastoma protein was weakened, originating in the downregulation of ABCC4. ABCC4 mRNA was highly expressed in lung cancer tissue and lung cancer cell lines.
Conclusion: ABCC4 may play an important role in the control of A549 and 801D cell growth. ABCC4 is a potential target for lung cancer therapy.
Keywords: ABCC4, cell proliferation, lung cancer, cell cycle
Introduction
Lung cancer is becoming the leading cause of cancer deaths worldwide,1 and the 5-year survival rate remains very poor.2 Early detection and targeted therapy is a potential method for prevention and treatment of lung cancer.3 An important task is to find a better molecular target of lung cancer in order to develop more effective therapies.
Multidrug resistance proteins (MRPs) are members of the C family of ATP-binding cassette (ABC) transporters,4 and contains 13 members. MRPs have membrane-spanning domains and are located predominantly in the plasma membrane.5–7 Different MRPs have different membrane localizations (apical or basolateral plasma membrane in polarized cells).8,9 MRPs can transport drugs with different structures and molecular mechanisms.4,10 Many are exogenous organic anions and metabolites, such as conjugated drug metabolites and prostaglandins. Some MRPs have similar substrate specificity, at least in vitro.7 For example, MRP1–MRP9 are the major transporters of extruding anticancer drugs which causes multidrug resistance in tumor cells.4 They mainly transport lipophilic anions and have been found to transport free glutathione, glucuronate or sulfate, or their conjugates.4 Important differences in tissue distribution of MRPs and their membrane localization as well as their different functions are responsible for the unique pharmacological and physiological functions of each MRP.
MRP4, also known as ATP-cassette binding protein 4 (ABCC4), is a member of the MRP family of transporters. ABCC4 mRNA is expressed in almost all tissues except the bone marrow, cervix, thymus, vascular endothelium, and soft tissue.11 ABCC4 protein is present in the kidneys, liver, erythrocytes, adrenal glands, platelets, brain, and pancreas in humans.11 ABCC4 can be located in the apical membrane as well as in the basolateral membrane depending on cell type.11 ABCC4 has specificity for a broad number of substrates, including bile salt conjugates, conjugated steroids, cyclic nucleotides, nucleoside analogs, eicosanoids, and cardiovascular drugs.11,12 Although ABCC4 is present in many human drug-resistant cancer cell lines, there is no conclusive evidence that ABCC4 is associated with resistance to drugs in solid tumors.11,13
Recently, it was found that ABCC4 is expressed in prostate cancer cell lines and upregulated in prostate cancer.14 ABCC4 overexpression was frequently observed in aggressive primary neuroblastoma. Increased ABCC4 levels have been correlated with tumor prognosis in patients with primary neuroblastoma,15 but little is known about the expression level and function of ABCC4 in lung cancer. In this work, the role of ABCC4 in the progression of lung cancer was investigated. ABCC4 was found to be frequently expressed in lung cancer cell lines. Suppression of ABCC4 mRNA expression led to a decrease in cell proliferation activity by regulating G1/S progression. ABCC4 mRNA was also highly expressed in lung cancer tissues comparing with corresponding adjacent tissues.
Materials and methods
Reagents
Dulbecco’s Modified Eagle’s Medium, Roswell Park Memorial Institute 1640 medium, fetal bovine serum, and TRIzol® reagent were sourced from Invitrogen (Carlsbad, CA, USA); M-MLV reverse transcriptase, a CellTiter 96® aqueous nonradioactive cell proliferation assay kit, oligo-dT, and dNTP were from Promega (Madison, WI, USA); and SYBR Green Master Mixture was from Applied Biosystems (Carlsbad, CA, USA). Anti-retinoblastoma protein (RB) antibody, anti-RB (S780 phosphorylation) antibody, and anti-ABCC4 antibody were from Abcam (Cambridge, UK). Propidium iodide, RNase A, and a protease inhibitor cocktail were obtained from Sigma-Aldrich (St Louis, MO, USA). Anti-actin antibody and secondary antibodies were from Kangchen (Shanghai, People’s Republic of China).
Samples
Thirty non-small cell lung cancer specimens were obtained from patients undergoing surgical resection at Beijing Chest Hospital, Capital Medical University. Primary lung cancer samples and matched adjacent normal tissues were used. All patients gave their informed consent according to a study protocol approved by the Beijing Chest Hospital Human Tissue Committee and Research Ethics Board. The clinical characteristics of the patients are listed in Table 1.
  | Table 1 Clinical characteristics of patients with non-small cell lung cancer |
Cell culture
A2, L, 801D, H446, H460, 95C, H1299, and A549 cells were cultured in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 mg/mL streptomycin at 37°C and in 5% CO2. MRC-5 cells (human embryo lung fibroblasts) were cultured in Dulbecco’s Modified Eagle’s Medium with 10% fetal bovine serum and nonessential amino acids.
Construction of ABCC4 shRNA lentiviral vector and infection into cells
Three RNA interference (RNAi) candidate target sequences were designed based on the human abcc4 mRNA sequence and cloned into a pGCSIL-GFP vector (GeneChem, Shanghai, People’s Republic of China). This vector contains an H1 promoter and an ampicillin-resistant cassette. The recombinant virus was packaged into 293T cells using a Lentivector Expression System (GeneChem). The recombinant virus was packaged by GeneChem. The shRNA most effective at depressing the abcc4 mRNA level was used in the following experiments. Nonsilencing (NS)-shRNA was also cloned into the pGCSIL-GFP vector and used as a control (GeneChem). The shRNA sequences were 5′-GCACTCATTAAATCACAAGAA-3′ for ABCC4 and 5′-TTCTCCGAACGTGTCACGT-3′ for the control.
A549 and 801D cells were cultured at a density of 5,000 cells/well in 96-well culture plates for cell infection. Twenty-four hours later, the cells were cocultured with recombinant virus carrying ABCC4-shRNA or NS-shRNA for 10 hours. The GFP expression level was detected using a fluorescence microscope (Nikon, Tokyo, Japan) 2 days later and used to determine the efficiency of infection. Infection would be repeated if GFP was not expressed in more than 80% of cells. The cells were cultured for an additional 2 weeks prior to use. In this study, pGCSIL-abcc4 shRNA-GFP was infected into A549 and 801D cells to obtain A549 ABCC4 knockdown (KD) cells and 801D ABCC4 KD cells. We also used pGCSIL-NS shRNA-GFP lentivirus to infect A549 and 801D cells as a negative control (A549 NC and 801D NC).
RNA isolation and real-time PCR
Total RNA from the tissues and cells was isolated using TRIzol® reagent according to the manufacturer’s protocol. The total RNA concentration was determined spectrophotometrically at a wavelength of OD 260 nm and stored at –80°C. Total RNA (2 μg) was reverse-transcribed using the M-MLV reverse transcriptase kit according to the manufacturer’s protocol. cDNA (20 ng) was mixed with SYBR Green Master Mix, and real-time polymerase chain reaction (PCR) was done with appropriate primers using a real-time detection system (ABI 7500, Applied Biosystems). Relative expression levels of ABCC4 mRNA were calculated by normalizing to the level of β-actin mRNA. PCR primers were used as follows: ABCC4, forward nucleotide, 5′-GGCAGTGACGCTGTATGG-3′, reverse nucleotide, 5′-CGCCAGGTCTGACAGTAAAG-3′; and β-actin, forward nucleotide, 5′-TTAGTTGCGTTACACCCTTTC-3′, reverse nucleotide, 5′-GCTGTCACCTTCACCGTTC-3′. Relative mRNA levels are presented as 2-ΔCT. Each reaction was performed three times. All the data are shown as the mean ± standard error of the mean.
Cell proliferation assay
Cell growth was assessed using the nonradioactive cell proliferation assay. Briefly, 5,000 cells/well were seeded in 96-well culture plates and cultured for 3 days. Next, 20 μL of MTS were added to each well and incubated at 37°C for 3 hours. Absorbance was recorded at 490 nm using a universal microplate reader (Bio-Rad, Hercules, CA, USA), with measurements carried out every 24 hours. The assay was repeated three times and the data are presented as the mean ± standard error of the mean.
Colony formation assay
Cells were seeded in a 6-well plate (Nunc™, Thermo Fisher Scientific, Waltham, MA, USA) at 300 cells/well and cultured in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal bovine serum. After 8 days of culture, the cells were washed twice with NaCl (0.9%), stained with 2% Gentian violet for 20 minutes, and then washed with water and air-dried. Foci were counted by microscopy. The experiments were repeated three times and the data are presented as the mean ± standard error of the mean.
Cell cycle analysis
A549 and 801D cells were used in the flow cytometry analysis. Briefly, the cells were washed in cold phosphate-buffered saline twice, and fixed in 70% ethanol at –20°C overnight. Cells were washed twice using phosphate-buffered saline. The cells were then resuspended in phosphate-buffered saline containing 50 mg/mL RNase A for one hour at 37°C and loaded with 65 mg/mL propidium iodide for 30 minutes in the dark at 4°C. The percentages of cells in different phases of the cell cycle were measured using a flow cytometer (FACSCalibur™, Becton Dickinson, Franklin Lakes, NJ, USA) at the Beijing Determination of Traditional Chinese Medicine Research Institute. The experiments were repeated three times. The data are shown as the mean ± standard error of the mean.
Western blot
Whole-cell lysates were harvested in lysis buffer (10 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol, 1 mM DTT, 0.1% Triton X-100, 1 × protease inhibitor cocktail) and centrifuged at 12,000 rpm for 20 minutes. Insoluble material was then removed and protein concentrations were determined using a bicinchoninic acid kit. For Western blot analysis, cell lysates (10 mg/well) were loaded per well and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane that was incubated with an anti-ABCC4 antibody, an anti-RB antibody, or an anti-retinoblastoma tumor suppressor protein (pRB) antibody, and secondary antibodies conjugated with horseradish peroxidase were used subsequently. Blots were detected using enhanced chemiluminescence. All the experiments were repeated three times.
Statistical analysis
For real-time PCR and cell cycle analysis, statistical differences were tested with unpaired Student’s t-test, taking P<0.05 as statistically different and P<0.01 as significantly different.
Results
ABCC4 was highly expressed in most lung cancer cell lines
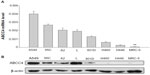
The ABCC4 mRNA expression level was determined by real-time PCR. In most lung cancer cell lines, ABCC4 was highly expressed when compared with MRC-5, a cell line of normal human fetal lung fibroblasts (Figure 1A). The level of ABCC4 protein was detected by Western blot analysis. As shown in Figure 1B, ABCC4 protein was highly expressed in most of the lung cancer cells.
ABCC4 mRNA expression was inhibited in cells infected with ABCC4 shRNA lentivirus
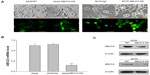
In order to elucidate whether ABCC4 expression has any effect on the growth of lung cancer cells, the RNAi technique was used to suppress ABCC4 mRNA expression. As shown in Figure 1A, of all the cell lines, the ABCC4 mRNA expression level was highest in A549 cells, so this cell line was used in the following study. GFP fluorescence was detected using a fluorescence microscope (Nikon, Tokyo, Japan) 48 hours after infection. Fluorescence images are shown in Figure 2A (left panel); GFP was expressed in most cells infected by pGCSIL-NS shRNA-GFP lentivirus or pGCSIL-abcc4 shRNA-GFP lentivirus. An 801D cell line with lower ABCC4 expression was also used. The GFP fluorescence expressed in 801D cells is shown in Figure 2A (right panel).
The ABCC4 mRNA level in A549 ABCC4 KD was determined by real-time PCR. As indicated in Figure 2B, expression of ABCC4 mRNA was suppressed nearly 80% in A549 ABCC4 KD cells (0.24±0.04) than that in A549 NC cells (1.05±0.07). The level of ABCC4 protein was also detected by Western blot. As shown in Figure 1C, the ABCC4 protein in A549 ABCC4 KD and 801D ABCC4 KD cells was obviously downregulated compared with control cells.
Suppression of ABCC4 expression lead to inhibition of cell proliferation
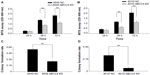
A cell proliferation assay was done to determine whether ABCC4 plays a role in cell growth. Cell proliferation activity was measured using a cell proliferation assay kit every 24 hours. Growth of A549 and 801D cells was obviously suppressed in A549 ABCC4 KD and 801D ABCC4 KD cells (Figure 3A and B). A colony formation assay was done to gain an additional insight into the effect of ABCC4 on cell growth. As shown in Figure 3C and D, the colony-forming efficiency of KD cells was less than half that of NC cells.
ABCC4 expression increased cell growth by promoting cell cycle progression
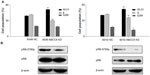
Cell cycle progression in A549 ABCC4 KD, 801D ABCC4 KD, A549 NC, and 801D NC cells was analyzed by flow cytometry to detect the effect of ABCC4 on cell cycle progression. As shown in Figure 4A, the percentage of cells entering S phase was decreased in A549 ABCC4 KD cells and in 801D ABCC4 KD cells along with an increase in the population of cells in G0/G1 phase. This indicates that ABCC4 expression could lead to cell growth by inducing cell cycle progression.
pRB regulates cell proliferation by controlling progression through the restriction point in the G1phase of the cell cycle.16 In this study, phosphorylation of the pRB S780 protein was detected by Western blot analysis. As shown in Figure 4B, phosphorylation of this protein was weakened when ABCC4 expression was inhibited in A549 and 801D cells.
ABCC4 was highly expressed in lung cancer tissues
To confirm the role of ABCC4 in lung cancer, ABCC4 mRNA levels in lung cancer tissue and in matched adjacent tissue were detected to confirm the role of ABCC4 in lung cancer. The clinical characteristics of 30 patients are shown in Table 1. As seen in Figure 5, ABCC4 mRNA was abundantly expressed in tumor tissue compared with adjacent normal tissues. The ABCC4 expression level in lung cancer tissue was higher than that in each matched adjacent normal tissue specimen except in seven cases. In most cases, ABCC4 was expressed mainly in lung cancer tissue. Correlational analysis between ABCC4 levels and tumor/patient characteristics were done, but no correlation was found. However, we believe that the clinical data might be limited due to the small sample size.
Discussion
In this research, the ABCC4 expression level as well as its function was investigated. ABCC4 was highly expressed in lung cancer cell lines and in non-small cell lung cancer tissue. Cell growth was inhibited when ABCC4 mRNA expression was suppressed by the RNAi technique in A549 and 801D cells. Further, ABCC4 accelerated cell cycle progression by regulating the phosphorylation of pRB protein, indicating that ABCC4 may have an oncogenic role in lung cancer.
The ABCC4 transcript is present in almost tissues, except for the bone marrow, cervix, thymus, vascular endothelium, and soft tissue.11,13 It has been reported that ABCC4 is frequently expressed in the blast cells of adult patients with acute myeloid leukemia and childhood acute lymphoblastic leukemia.17,18 ABCC4 expression has also been found to be elevated in malignant prostate tissue when compared with benign prostate tissue.14,19,20 Further, ABCC4 expression is upregulated in docetaxel-resistant MLL cells (a prostate cancer cell line), Y79 cells (a retinoblastoma cell line), melanoma cells, and drug-resistant cancer stem cells (human glioblastoma cell line U138MG).21–24 ABCC4 is also highly expressed in 5-fluorouracil-resistant pancreatic carcinoma cells.25 In head and neck squamous cell carcinoma, abnormal expression of ABCC4 has been detected.26 Our findings show that ABCC4 expression was also stronger in lung cancer cell lines compared with a normal MRC-5 cell line. We then determined the ABCC4 mRNA level in primary lung cancer tissues, and found that ABCC4 mRNA expression was greater in lung cancer tissues than in matched adjacent tissues.
Although ABCC4 is known to transport anticancer drugs, there is no direct evidence linking ABCC4 to drug resistance in human tumors in vivo.11 The physiological importance of ABCC4 remains to be determined. ABCC4 is an ATP-binding cassette transporter, which is capable of pumping a wide variety of nucleoside analogs out of the cell, including cGMP, cAMP, and anticancer drugs.11 Two important components of drugs used to treat acute lymphoblastic leukemia, ie, methotrexate and 6-mercaptopurine, are substrates for ABCC4, and ABCC4 is associated with childhood acute lymphoblastic leukemia.17 Upregulation of ABCC4 has also been shown to play a major role in 6-mercaptopurine resistance in CEM-MP5 cells (a human T-lymphoblastic leukemia cell line).27 In rectal cancer, the ABCC4 level has been found to be related to sensitivity to radiation.28 In lung cancer, the response to cisplatin is related to ABCC4 polymorphism.29 Cell proliferation was inhibited by ABCC4 shRNA in U937 cells (human histiocytic leukemia cell line) and human neuroblastoma cells.18,30 We obtained similar results in lung cancer cells. Cell growth activity was suppressed when ABCC4 mRNA was knocked down in A549 and 801D cells. This indicates that ABCC4 plays an important role in the progression of lung cancer. The mechanism of ABCC4 regulating cell proliferation was still unknown. ABCC4 has the ability to transport molecules involved in cellular signaling.11 In pancreatic acinar cells, cAMP efflux was shown to be regulated by ABCC4.31 ABCC4 could affect human histiocytic leukemia cell growth due to cAMP efflux caused by the ABCC4 transporter.18 In our study, ABCC4 mRNA suppression in A549 and 801D cells lead to increased accumulation in G1 and reduced accumulation in cells in S phase. The major pathway leading to G1/S transition is the phosphorylation of pRB.32 Phosphorylation of pRB results in release of the transcription factor E2F, which induces expression of genes required for progression of the cell cycle.33 Phosphorylation of pRB was reduced while ABCC4 expression was downregulated. This suggests that ABCC4 could regulate cell cycle progression by phosphorylation of pRB S780 protein which could inhibit the activity of pRB and push cells into S phase. ABCC4 may regulate the pRB via transporting cell signal molecules.
In primary neuroblastoma, ABCC4 expression levels have been shown to correlate with tumor prognosis in patients.15 However, we did not find an association between clinical characteristics and ABCC4 expression, and patients with higher expression of ABCC4 protein did not have significantly decreased overall survival (data not shown), but this might be because the samples were not large enough. It is hard to define a prognostic value for ABCC4, although our data show that the ABCC4 level is correlated with cell proliferation, indicating that lung cancer cells expressing ABCC4are more aggressive. Further research is needed to clarify the prognostic role of ABCC4.
In this study, we investigated the role of ABCC4 in lung cancer. ABCC4 is highly expressed in primary lung cancers and cell lines. ABCC4 could promote cell growth by accelerating progression of the cell cycle via phosphorylation of pRB protein. Our results suggest that ABCC4 could be a potential prognostic marker for lung cancer.
Acknowledgment
This research was supported by grants from the Beijing Novel Program (2006B34), the Beijing Research Foundation for Excellent Talents (20061D03), and the Beijing Cultivation Project for Key Technical and Medicine Products (Z101100055610030).
Disclosure
The authors report no actual or potential conflicts of interest in this work.
References
Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: new biological insights and recent therapeutic advances. CA Cancer J Clin. 2011;61(2):91–112. | |
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | |
Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12(2):175–180. | |
Sodani K, Patel A, Kathawala RJ, Chen ZS. Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer. 2012;31(2):58–72. | |
Pattabiraman PP, Pecen PE, Rao PV. MRP4-mediated regulation of intracellular cAMP and cGMP levels in trabecular meshwork cells and homeostasis of intraocular pressure. Invest Ophthalmol Vis Sci. 2013;54(3):1636–1649. | |
Robillard KR, Hoque T, Bendayan R. Expression of ATP-binding cassette membrane transporters in rodent and human Sertoli cells: relevance to the permeability of antiretroviral therapy at the blood-testis barrier. J Pharmacol Exp Ther. 2012;340(1):96–108. | |
Slot AJ, Molinski SV, Cole SP. Mammalian multidrug-resistance proteins (MRPs). Essays Biochem. 2011;50(1):179–207. | |
Zhang Y, Schuetz JD, Elmquist WF, Miller DW. Plasma membrane localization of multidrug resistance-associated protein homologs in brain capillary endothelial cells. J Pharmacol Exp Ther. 2004;311(2):449–455. | |
Westlake CJ, Cole SP, Deeley RG. Role of the NH2-terminal membrane spanning domain of multidrug resistance protein 1/ABCC1 in protein processing and trafficking. Mol Biol Cell. 2005;16(5):2483–2492. | |
Zhou SF, Wang LL, Di YM, et al. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem. 2008;15(20):1981–2039. | |
Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch. 2007;453(5):661–673. | |
Gelhaus SL, Gilad O, Hwang WT, Penning TM, Blair IA. Multidrug resistance protein (MRP) 4 attenuates benzo[a]pyrene-mediated DNA-adduct formation in human bronchoalveolar H358 cells. Toxicol Lett. 2012;209(1):58–66. | |
Kool M, de Haas M, Scheffer GL, et al. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 1997;57(16):3537–3547. | |
Montani M, Herrmanns T, Muntener M, Wild P, Sulser T, Kristiansen G. Multidrug resistance protein 4 (MRP4) expression in prostate cancer is associated with androgen signaling and decreases with tumor progression. Virchows Arch. 2013;462(4):437–443. | |
Norris MD, Smith J, Tanabe K, et al. Expression of multidrug transporter MRP4/ABCC4 is a marker of poor prognosis in neuroblastoma and confers resistance to irinotecan in vitro. Mol Cancer Ther. 2005;4(4):547–553. | |
Dick FA, Rubin SM. Molecular mechanisms underlying RB protein function. Nat Rev Mol Cell Biol. 2013;14(5):297–306. | |
Ansari M, Sauty G, Labuda M, et al. Polymorphisms in multidrug resistance-associated protein gene 4 is associated with outcome in childhood acute lymphoblastic leukemia. Blood. 2009;114(7):1383–1386. | |
Copsel S, Garcia C, Diez F, et al. Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation. J Biol Chem. 2011;286(9):6979–6988. | |
Ho LL, Kench JG, Handelsman DJ, et al. Androgen regulation of multidrug resistance-associated protein 4 (MRP4/ABCC4) in prostate cancer. Prostate. 2008;68(13):1421–1429. | |
Cai C, Omwancha J, Hsieh CL, Shemshedini L. Androgen induces expression of the multidrug resistance protein gene MRP4 in prostate cancer cells. Prostate Cancer Prostatic Dis. 2007;10(1):39–45. | |
Oprea-Lager DE, Bijnsdorp IV, Van Moorselaar RJ, Van Den Eertwegh AJ, Hoekstra OS, Geldof AA. ABCC4 decreases docetaxel and not cabazitaxel efficacy in prostate cancer cells in vitro. Anticancer Res. 2013;33(2):387–391. | |
Warrier S, Pavanram P, Raina D, Arvind M. Study of chemoresistant CD133+ cancer stem cells from human glioblastoma cell line U138MG using multiple assays. Cell Biol Int. 2012;36(12):1137–1143. | |
Hendig D, Langmann T, Zarbock R, Schmitz G, Kleesiek K, Gotting C. Characterization of the ATP-binding cassette transporter gene expression profile in Y79: a retinoblastoma cell line. Mol Cell Biochem. 2009;328(1–2):85–92. | |
Heimerl S, Bosserhoff AK, Langmann T, Ecker J, Schmitz G. Mapping ATP-binding cassette transporter gene expression profiles in melanocytes and melanoma cells. Melanoma Res. 2007;17(5):265–273. | |
Hagmann W, Jesnowski R, Faissner R, Guo C, Lohr JM. ATP-binding cassette C transporters in human pancreatic carcinoma cell lines. Upregulation in 5-fluorouracil-resistant cells. Pancreatology. 2009; 9(1–2):136–144. | |
Sethi S, Benninger MS, Lu M, Havard S, Worsham MJ. Noninvasive molecular detection of head and neck squamous cell carcinoma: an exploratory analysis. Diagn Mol Pathol. 2009;18(2):81–87. | |
Peng XX, Shi Z, Damaraju VL, et al. Up-regulation of MRP4 and down-regulation of influx transporters in human leukemic cells with acquired resistance to 6-mercaptopurine. Leuk Res. 2008;32(5):799–809. | |
Chai R, Yu ZQ, Fu CG, et al. [Preliminary study on relationship between multi-drug resistance-associated protein 4 and radiosensitivity of rectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2011;14(8):627–630. Chinese. | |
Moyer AM, Sun Z, Batzler AJ, et al. Glutathione pathway genetic polymorphisms and lung cancer survival after platinum-based chemotherapy. Cancer Epidemiol Biomarkers Prev. 2010;19(3):811–821. | |
Henderson MJ, Haber M, Porro A, et al. ABCC multidrug transporters in childhood neuroblastoma: clinical and biological effects independent of cytotoxic drug efflux. J Natl Cancer Inst. 2011;103(16):1236–1251. | |
Rodriguez MR, Diez F, Ventimiglia MS, et al. Atrial natriuretic factor stimulates efflux of cAMP in rat exocrine pancreas via multidrug resistance-associated proteins. Gastroenterology. 2011;140(4):1292–1302. | |
Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25(38):5220–5227. | |
Korenjak M, Brehm A. E2F-Rb complexes regulating transcription of genes important for differentiation and development. Curr Opin Genet Dev. 2005;15(5):520–527. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.