Back to Journals » International Journal of Women's Health » Volume 15
A Woman’s Heart: Improving Uptake and Awareness of Cardiovascular Screening for Middle-Aged Populations
Authors Kazzi B, Shankar B, Elder-Odame P , Tokgözoğlu LS, Sierra-Galan LM, Michos ED
Received 1 April 2023
Accepted for publication 11 July 2023
Published 24 July 2023 Volume 2023:15 Pages 1171—1183
DOI https://doi.org/10.2147/IJWH.S328441
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Brigitte Kazzi,1 Bairavi Shankar,1 Petal Elder-Odame,2 Lale S Tokgözoğlu,3 Lilia M Sierra-Galan,4 Erin D Michos2
1Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA; 2Department of Medicine, Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA; 3Department of Cardiology, Hacettepe University Faculty of Medicine, Ankara, Turkey; 4Cardiology Department of the Cardiovascular Division, American British Cowdray Medical Center, Mexico City, Mexico
Correspondence: Erin D Michos, Medicine and Epidemiology Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA, Tel +1 410 502-6813, Email [email protected]
Abstract: Mid-life, the years leading up to and following the menopause transition, in women is accompanied by a change in cardiometabolic risk factors, including increases in body weight, changes in body composition, a more insulin-resistant state, and a shift towards a more atherogenic dyslipidemia pattern. Cardiovascular disease (CVD) risk assessment should be performed continually throughout the lifespan, as risk is not stagnant and can change throughout the life course. However, mid-life is a particularly important time for a woman to be evaluated for CVD risk so that appropriate preventive strategies can be implemented. Along with assessing traditional risk factors, ascertainment of a reproductive history is an integral part of a comprehensive CVD risk assessment to recognize unique female-specific or female-predominant factors that modify a woman’s risk. When there is uncertainty about CVD risk and the net benefit of preventive pharmacotherapy interventions (such as statins), measuring a coronary artery calcium score can help further refine risk and guide shared decision-making. Additionally, there should be heightened sensitivity around identifying signs and symptoms of ischemic heart disease in women, as these may present differently than in men. Ischemia from coronary microvascular disease and/or vasospasm may be present even without obstructive coronary artery disease and is associated with a heightened risk for major cardiovascular events and reduced quality of life. Therefore, correctly identifying CVD in women and implementing preventive and treatment therapies is paramount. Unfortunately, women are underrepresented in cardiovascular clinical trials, and more data are needed about how to best incorporate novel and emerging risk factors into CVD risk assessment. This review outlines an approach to CVD screening and risk assessment in women using several methods, focusing on the middle-aged population.
Keywords: women’s health, mid-life, risk assessment, cardiovascular disease, prevention, menopause
Introduction
Cardiovascular disease (CVD) remains a leading cause of morbidity and mortality for women in the United States (US), as well as internationally, contributing to 48% of deaths among women in the US in 2020.1,2 Furthermore, rates of myocardial infarction (MI) and CVD mortality have been rising in younger and middle-aged women (ages <65) in the past decade.3–5 A recent US analysis showed that ST elevation MI is on the rise even in women <45 years of age over an 11-year time frame.6 Globally, while there has been a decrease in the age-standardized prevalence of CVD in most areas of the world since 1990, with an overall decrease of 4.3%, this reduction has stagnated in recent years. Additionally, some countries have seen increased CVD prevalence, particularly China, India, and Indonesia.7 In Europe, there are large disparities between high and middle-income countries, and for premature mortality (before age 70) due to CVD causes (vs non-CVD), this socioeconomic disparity is even greater for women.8 In women, the proportion of premature mortality due to CVD was 36% in middle income countries compared to 16% in high income countries. For men, the corresponding rates are 36% and 24% in middle and high-income countries, respectively.8
To decrease CVD mortality, a deeper understanding of the contributing risk factors is needed, and individualized, tailored care for each patient is advocated for, particularly for women’s cardiovascular health. Dr. James Herrick’s models of MI, which began circulating in the early 1900s, typically used middle-aged men as the archetype to study CVD.9 Dr. Herrick advocated for individualized, tailored care for each patient. Despite this, an approach focused explicitly on women’s cardiovascular health did not gain prominence until several decades later, in the early 1990s.9
The 1992 National Heart, Lung, and Blood Institute (NHLBI) Conference on Cardiovascular Health and Disease in Women posed an important question: what are the differences in CVD between men and women? Research in the area primarily focused on hormone replacement therapy (HRT) in postmenopausal women and, as a consequence, largely ignored CVD in younger women.9 The Heart and Estrogen/Progestin Replacement Study (HERS) and Women Health Initiative (WHI) studies that came shortly after that showed that HRT was not the universal answer for preventing CVD in women. This finding pivoted discussions about women’s CVD to the modern-day search for differences in traditional risk factors and risk factors unique to women. The 2007 American Heart Association (AHA) Women’s CVD prevention guideline10 and Institute of Medicine (IOM) report11 emphasized the need for more sex-specific research in CVD and shed light on differences between subgroups of women, particularly those who experience a more significant burden of CVD due to social disadvantages because of their race, ethnicity, income or education levels.
The 2020 American College of Cardiology (ACC) Summary of Updated Recommendations for Primary Prevention of CVD in Women further emphasized the prior discrepancies in CVD risk models between men and women.12 It underscored the influence of unique female-specific factors on future CVD risk. These include the age of onset of menarche and menopause, total reproductive years, premenstrual syndrome, severe or persistent vasomotor symptoms (VMS), history of polycystic ovary syndrome (PCOS), infertility, use of assisted reproductive technology (ART), spontaneous pregnancy loss, grand multiparity, lack of breastfeeding, and adverse pregnancy outcomes (APOs) such as gestational diabetes mellitus (GDM), preterm delivery, and hypertensive disorders of pregnancy.13–16 There are also female-predominant conditions, such as autoimmune diseases and depression, that independently increase the risk of CVD. The impact of these non-traditional factors is particularly salient in younger to middle-aged women, who may not present with signs of elevated CVD risk until later in life. Additionally, even among the more traditional risk factors of diabetes, hypertension, smoking, and obesity, there are differences in risk conferred between the sexes, as further discussed below.13
This review outlines an approach to CVD screening and risk assessment in women using several methods, focusing on the middle-aged population. First, we will review the initial approach to risk assessment using equations that estimate 10-year risk for atherosclerotic CVD (ASCVD) as a starting framework. We will then discuss how the ascertainment of female-specific or female-predominant risk factors can be used as “risk-enhancing” or “risk-modifying” factors to modify those estimated risks. Next, we will discuss how the selective use of imaging for subclinical atherosclerosis, specifically the coronary artery calcium (CAC) score, can be useful when there is uncertainty about the risk to refine risk estimation further and then guide shared decision-making about preventive therapies. Finally, we will discuss the impact of menopause, specifically on women’s cardiometabolic risk, and an approach to risk assessment when considering menopausal HRT for managing VMS.
Initial Approach to Risk Assessment in Women – Risk Estimator Tools
Following a healthy lifestyle across the lifespan is the foundation for all CVD prevention strategies. However, when making decisions about preventive pharmacotherapies, assessment of CVD risk is a central tenet across all preventive guidelines to match the intensity of therapy with the absolute risk of the patient.16–20
The pooled cohort equations (PCE) were developed by the ACC and AHA in 2013 as tools for estimating 10-year risk of ASCVD events [fatal and non-fatal coronary heart disease (CHD) and stroke events] in the primary prevention setting (ie, to be applied to individuals without established ASCVD).21 The PCE derive a risk estimate based on several established cardiovascular risk factors, including age, sex, race, blood pressure, cholesterol, diabetes, and smoking for individuals aged 40–79 years. The PCE are best calibrated for non-Hispanic White and Black adults living in the United States. Even then, the PCE may overestimate or underestimate ASCVD risk in specific populations.22 The PCE may also not estimate risk accurately in other global populations, leading to the need to develop regional-specific risk scores. For example, in their prevention guidelines, the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) use the SCORE2 risk score to estimate 10-year risk, which has modifications for four different risk regions in Europe.16,23
Notably, the PCE do not incorporate nontraditional emerging and female-specific cardiovascular risk factors, limiting their accuracy in specific populations, such as middle-aged women.24,25 A comprehensive cardiovascular risk assessment in middle-aged women requires careful evaluation of their unique risk-enhancing factors. These are especially crucial in women at borderline or intermediate cardiovascular risk, as they may provide insight into their appropriate stratification and counseling. An important aspect is evaluating reproductive history regarding parity, pregnancy (including complications), hormonal therapies, and menopause.
Thus, the PCE are an initial starting framework for risk assessment but not the end of risk assessment. The 2019 ACC/AHA Guideline for the primary prevention of CVD acknowledged the limitation of PCE.17 They further outline a list of “risk-enhancing” factors, which, if present, would place an individual who would otherwise only be estimated to be a borderline or intermediate risk into a higher risk category that would favor the initiation of statin therapy for primary prevention. These risk enhancers include the female-specific factors of early menopause (before age 40) and APOs such as pre-eclampsia, as well as additional risk-enhancing factors such as having a family history of premature ASCVD, chronic kidney disease (CKD), chronic inflammatory conditions, South Asian ethnicity, metabolic syndrome, persistently elevated triglycerides, and if measured, high levels of high-sensitivity C-reactive protein (hsCRP), lipoprotein (a) [Lp(a)], or apolipoprotein B (apoB). We will discuss some of these below, focusing on the female-specific or female-predominant conditions.
Sex Differences in Traditional Risk Factors
When assessing CVD risk in women, it is important to note that multiple traditional risk factors for ischemic heart disease (IHD) have a differential risk between the sexes. For example, hypertension, diabetes mellitus, and smoking confer a greater relative risk for MI in women than men, with odds ratios of 1.5, 1.6, and 1.3, respectively.26
Although diabetes is more prevalent in men at a given age, the presence of diabetes is associated with a significantly greater relative risk for CHD and vascular mortality in women compared to men.27–30 This may be partly because compared to men, women have higher body mass index (BMI) and worse cardiometabolic risk profiles at the time of diabetes diagnosis.27,31 Excess circulating glucose can diminish estrogen-related cardiovascular protection by decreasing vascular and platelet nitric oxide production, thereby increasing vascular tone, platelet aggregation, and vascular proliferation.27
Obesity increases the risk of CVD for both men and women. However, the prevalence of obesity and its impact on cardiovascular risk factors are further influenced by sex. Data from the Framingham study showed that women with obesity had a risk of CHD greater than 64% compared to 46% among men with obesity.32 Menopause increases the prevalence of metabolic syndrome and redistribution of adipose tissue in the visceral cavity.33 Obesity also increases the risk of maternal complications in pregnancy, such as hypertensive disorders of pregnancy, GDM, and heart failure.34
Smoking also confers a greater relative risk in women than in men. Compared with nonsmokers, women who smoke may have a 25% greater relative risk of CHD than male smokers, independent of other cardiovascular risk factors.35 This female vulnerability in the setting of smoking may be related to genes involved in thrombin signaling, while other studies state that it is unclear if related to biological differences or smoking behaviors.35
Hypertension is more prevalent and less well-controlled in older women than in men.36,37 Although, on average, the rise in blood pressure throughout a woman’s lifespan appears linear, consistent with an aging effect rather than an ovarian effect,38 there are differences in the patterns of blood pressure changes during the menopause transition across the population with some women experiencing an acceleration in blood pressure rise later in life.39 Women also have unique considerations throughout their life course that influence blood pressure and/or its treatment. These include pregnancy, PCOS, the use of oral contraceptives or menopausal HRT, and predominant female conditions such as autoimmune disease and fibromuscular dysplasia.
Sleep disorders are well-recognized risk factors for CVD,40 and women have been described as especially sensitive to the negative effects of those at different stages of life.41 The proposed physiological mechanisms include complex interactions that are not fully understood. Sleep disorders include insomnia and short sleep duration (<5 hours), which are highly associated with an increased incidence of MI in a similar proportion to other risk factors.42 A meta-analysis reported that females were at higher risk of MI among people with insomnia. Additionally, it was described that a shorter sleep-onset latency might be a protective factor against stroke and that females experienced a greater reduction in CVD risk from this factor.43
Female-Specific Risk Enhancing Factors
In addition to the sex differences in traditional CVD risk factors, female-specific factors uniquely elevate women’s CVD risk throughout their lifespan, as discussed below.
Menarche, the onset of the 1st menstrual period, hallmarks the entry into a women’s reproductive period of life. In the US, the average age of menarche is approximately 12 years.44 However, both early onset of menarche ≤10 years or late onset menarche ≥17 years have been associated with incident future CVD.45–47
PCOS is the most common endocrine abnormality found in reproductive-age women and is diagnosed by the Rotterdam criteria if at least two of the following three criteria are present: androgen excess, ovarian dysfunction, and cystic morphology of the ovaries.48 Women with PCOS have a worse cardiometabolic risk profile compared to women without PCOS, including insulin resistance, atherogenic dyslipidemia, elevated blood pressure, and elevated BMI.48,49 PCOS has also been associated with an approximate 2-fold increased risk of subclinical CVD50 and 30–50% higher risk of incident CVD events.51,52 Menstrual irregularity is common among women with PCOS due to anovulatory cycles. Growing evidence supports an association between menstrual cycle length and/or cycle irregularity with cardiometabolic risk factors and CVD risk.53,54 Thus, a menstrual history is an essential but underrecognized vital sign of cardiometabolic health. Interestingly, only a tiny proportion of the association between menstrual cycle regularity and length and CVD risk was driven by hypercholesterolemia, chronic hypertension, and type 2 diabetes (T2D),55 suggesting that menstrual cycle dysfunction may be a useful early life marker for future CVD risk.
Parity, the number of live births, and gravidity, the number of pregnancies, are important factors in middle-aged women’s cardiovascular risk assessment. A “J”-shaped relationship between parity and future CVD risk has been described.56–59 It should be noted that higher parity is also a CVD risk factor in men, suggesting confounding by cultural and socioeconomic factors.56 However, there is greater risk associated with multiparity seen in women, suggesting additional biological mechanisms such as those potentially mediated by adverse cardiometabolic and adipokine profiles and a more androgen sex hormone profile.60–62
Notably, pregnancy is another critical time in a person’s life that could adversely affect their future cardiovascular outcomes, even decades after an index pregnancy with an APO. Several pregnancy complications are now recognized to confer long-term cardiovascular risk: hypertensive disorders of pregnancy, GDM, preterm births, and small-for-gestational-age newborns.63 The association of preeclampsia with later-life CVD has been thoroughly studied, with several systematic reviews and meta-analyses showing at least a twofold increase in heart failure, CHD, stroke, and cardiovascular death.64–66 Pre-term delivery before 37 weeks is also associated with a 2-fold risk of future CVD.67 In a nationwide Swedish study, preterm or small-for-gestational-age infant delivery was associated with later-life maternal hospitalization or death from CVD.68 Similarly, in a systemic review and meta-analysis, women with GDM had a twice higher risk of cardiovascular events, even without progressing to T2D post-partum.69 In the Coronary Artery Risk Development in Young Adults (CARDIA) Study, women with a history of GDM also have a nearly twofold higher prevalence of subclinical atherosclerosis as detected by the CAC score approximately 15 years after their index pregnancy, even among those who returned to euglycemia after delivery.70
Other pregnancy-related factors have been associated with CVD. A history of infertility has been associated with an increased risk of ASCVD71 and heart failure with preserved ejection fraction, even after accounting for traditional CVD risk factors.72 Spontaneous pregnancy loss has also been associated with incident future ASCVD.73 Breastfeeding, on the other hand, is thought to be a protective factor associated with decreased risk of incident diabetes74 and CVD,75 and well as associated with a lower CVD risk among women with a hypertensive disorder of pregnancy compared to women with a hypertensive disorder of pregnancy who did not breastfeed.76
The premenstrual syndrome is another emerging risk factor in reproductive age women. The premenstrual syndrome is characterized by symptoms that may be physical, behavioral, or affective/psychological and is estimated to occur in up to 80% of women.77 The premenstrual syndrome correlates with fluctuations in arterial stiffness and monthly blood pressures.78 Additionally, moderate to severe premenstrual symptoms may be associated with an increased risk of developing hypertension before age forty.79
The menopause transition hallmarks the end of a woman’s reproductive lifespan. The risk of CVD is higher in women after menopause compared to premenopausal women of the same age.80 The onset of menopause that occurs early (before 45 years) or premature (before 40 years) has also been demonstrated to be a risk factor for adverse cardiovascular outcomes in women.45,81,82 Additionally, shorter total reproductive years (from menarche to menopause) have also been linked to increased CVD risk.83,84 This may be due to significant changes in lipid profiles that occur at the menopause transition, as longer duration since menopause is linked to lower high-density lipoprotein cholesterol (HDL-C), higher low-density lipoprotein cholesterol (LDL-C), higher triglycerides, higher total cholesterol, higher apoB and potentially higher Lp(a) levels.85,86
Vasomotor symptoms (ie, “hot flashes”) are experienced by 70–80% of women transitioning through menopause, but less than 25% of women seek help for them. Many patients and clinicians are unaware of the link between VMS and CVD risk. Very severe, frequent, or persistent VMS are associated with an increased risk of future CVD, even after accounting for traditional CVD risk factors.87,88
Auto-immune diseases, such as rheumatoid arthritis and systemic lupus erythematosus, are also more prevalent in women and have been associated with excess CVD risks beyond traditional risk factors.89–94 The presence of an inflammatory state is emerging to be a separate causal mechanism in atherothrombotic pathogenesis and may be a therapy target in and of itself.95
Finally, social determinants of health should be ascertained as part of comprehensive CVD risk assessment.96 Socioeconomic disparities at each stage of life disproportionately affect a woman’s future cardiovascular health. They encompass multiple factors and include, but are not limited to, family income, education, literacy, language barriers, health care systems (health insurance, quality of care), marital status, social support, physical environment, economic stability, structural racism, culture, sexual orientation and gender identity.96–99
In sum, these studies highlight the uniqueness of CVD risk factors in women, compared to men, and emphasize the importance of taking a comprehensive reproductive history to identify these “red flags” of risk that may not capture through traditional risk factor assessment alone (Figure 1). Many pregnancy-associated factors are harbingers of short- and long-term CVD risks. Additionally, special intensive prevention efforts are needed to address individuals with inflammatory conditions.95 Unfortunately, to date, there has been limited success in changing the C-statistic in risk prediction by trying to incorporate these factors directly into traditional risk assessment tools such as the PCE, likely because the cohorts that these risk calculators were derived were generally older and more data are needed to derive risk equations specific to the younger cohorts to which these equations would be applied. These risk calculators also work better on a population level (ie, reflecting an average risk across a group) but less well on an individual level. Better risk stratification tools and tailored prevention strategies for the individual are sorely needed to figure out how to incorporate these emerging factors, which are currently not captured in traditional 10-year risk estimator tools. In the meantime, as mentioned above, these factors, particularly early menopause and APOs, are considered “risk enhancers” that modify risk estimates generated by the risk equations like the PCE, placing women into a higher category where statin therapy may be considered for prevention.
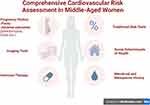 |
Figure 1 Comprehensive Cardiovascular Risk Assessment in Middle-Aged Women. (Figure was created with Biorender.com). |
Subclinical Atherosclerosis Assessment
The 2019 ACC/AHA Guideline on the Primary Prevention of CVD acknowledges that in many cases that even after estimating 10-year risk with the PCE and considering the “risk-enhancing factors” described above, there can still be uncertainty about an individual’s risk and the net benefit for preventive pharmacotherapies, notably statins, and aspirin.17 In these cases of risk uncertainty, the guideline recommends using the CAC score to help refine risk estimation (for ages 40-75 years). CAC scoring is a noninvasive imaging technique widely used in CVD risk stratification and guides shared decision-making regarding statin initiation in patients who would otherwise be considered at low or intermediate risk according to traditional risk factors.100 Women are less likely to have prevalent CAC than men at a given age. However, for a given CAC score, the presence of CAC is associated with a greater risk for future ASCVD in women than in men.101,102 In other words, CAC is equally (if not more so) prognostic in women when it is present.
CVD risk is on a continuum. The detection of subclinical atherosclerosis offers a window of opportunity to detect disease before it has become clinically manifest so that more intensive preventive efforts can be implemented.103 The CAC score has emerged as a superior risk marker for clinical ASCVD events. It predicts risk better than age, LDL-C, or traditional risk score because it directly captures the presence of atherosclerotic disease.100,104 Furthermore, the absence of CAC (ie, CAC=0) is associated with a low risk for ASCVD events in the next 5–10 years and, as such, has the potential to “de-risk” an individual into a lower-risk category where potentially statin therapy could be deferred.100,105
However, the CAC score has limitations and should be applied appropriately, not superseding clinical judgment. A CAC score of 0 is “low risk” but not “no risk”. It is meant to be applied to asymptomatic individuals for risk assessment. Thus, a CAC score of 0 should not deter further cardiac investigation in a person with signs and symptoms of IHD. In patients with symptoms suggestive of IHD, using a CAC of 0 to avoid perfusion stress imaging could miss coronary microvascular dysfunction (CMD) in up to 4 out of 10 patients.106 Also, as it takes time for plaques to become calcified, a CAC score of 0 has reduced prognostic utility in younger individuals (such as men <40 and women <50 years of age). In one study of symptomatic patients <60 years of age, a sizeable proportion still had coronary artery disease (CAD) despite having a CAC score of 0.107
For women with symptoms concerning for CHD (stable angina) whose coronary anatomy is unknown, a coronary CT angiography (CCTA) is a good initial test to determine the presence and extent of CAD burden.108,109 Not only can a CCTA exclude obstructive CAD, but it can also identify the presence of non-obstructive CAD, offering the opportunity to initiate or intensify preventive therapy once identified. The benefit of this approach was highlighted in the SCOT-Heart trial, which enrolled patients with stable chest pain and demonstrated a significant reduction in major adverse cardiovascular events (MACE) at five years in the CCTA-guided arm compared to a usual care arm, with no significant interaction by sex.110 This benefit was likely driven by the 40% greater uptake in preventive therapies such as statins in the CCTA arm.
The detection of non-obstructive plaque on CCTA is similarly prognostic in women and men. In CONFIRM, a large, multi-national, observational registry of individuals undergoing CCTA, non-obstructive CAD was associated with a similar increased risk of MACE in both women [aHR 1.96 (95% CI, 1.17–3.28)] and men [aHR 1.77 (1.07–2.83)] after adjustment for age and traditional risk factors, compared to no CAD.111 Additionally the finding of non-obstructive plaque in the left main coronary artery conferred an increased risk of MACE in women [aHR 1.48 (1.21–1.75)] but not in men [aHR 0.98 (0.81–1.18)]. High-risk coronary plaque features, which are defined as positive remodeling, low CT attenuation, or napkin-ring sign, detected on CCTA were also associated with a greater risk for MACE in women [aHR 2.41 (1.25–4.64)] than in men [aHR 1.40 (0.81–2.39)].112
Additionally, there can be sex-specific differences in the pathophysiology of IHD, and women can still have ischemia with non-obstructive coronary arteries (INOCA).113 In the setting of angina, non-obstructive CAD is more common in women than men. However, INOCA is not benign but rather is associated with an increased 5-year ASCVD event rate compared to asymptomatic women without angina114 and can have a similarly reduced quality of life compared with patients with ischemia from obstructive CAD.115 Thus, for patients with signs and symptoms of IHD, the workup should not end with the exclusion of obstructive CAD by coronary angiography or CCTA; additional testing for the INOCA phenotypes of CMD and vasospastic angina should be considered.116 In the absence of obstructive CAD, the presence of reduced coronary flow reserve (CFR) <2 hallmarks coronary microvascular impairment; this is the inability of the coronary arteries to dilate properly in the setting of stress or vasodilator.117 The detection of CMD identifies an individual at higher risk for MACE, even in the absence of obstructive CAD.118 A severely impaired CFR (<1.6) conferred an even greater prognostic risk in women than in men.119 In the CorMICA trial of patients with suspected INOCA, invasive coronary function testing to examine for INOCA endotypes and to tailor therapy accordingly was associated with a reduction in angina and improved quality of life compared to a control group with invasive angiography but no coronary function testing.120 Non-invasively, CMD can be evaluated by a stress positron emission tomography (PET) or stress cardiac magnetic resonance imaging (CMR) with an assessment of myocardial blood flow reserve. Invasive coronary functional testing, stress PET, and stress CMR were all given a class IIa recommendation in the US Chest Pain guidelines for additional work-up when INOCA is suspected.116
In sum, in women with symptoms of suspected IHD, focusing on evaluating just for the obstructive disease is not enough. CMD is common and associated with poor outcomes, and making the diagnosis and implementing preventive, and treatment strategies is paramount. While INOCA and angina with non-obstructive coronary arteries (ANOCA) are stable presentations, in the setting of acute coronary syndromes, women are also more likely than men to have a myocardial infarction with non-obstructive coronary arteries (MINOCA).121,122 MINOCA, although usually considered a more benign phenomenon, still carries significant morbidity and mortality compared to the general population. Intravascular imaging such as coronary optical coherence tomography, along with CMR, are essential steps in diagnosing these patients, allowing the proper differential diagnosis, targeted treatment, and prognosis.121,123
Furthermore, women have been historically underrepresented in cardiovascular clinical trials compared to their disease burden in the population, limiting the data on the efficacy of cardiovascular therapies in women compared to men.124–128 Adequate representation of women and diverse populations in future cardiovascular trials is critical to ensure that the results from the trial apply to the broad groups of patients being cared for in clinical practice.
Physiological Changes of Perimenopause and Menopause
When considering CVD risk assessment in mid-life, perhaps the most significant influence that comes to mind is the impact of the menopausal transition. The average age of menopause in the US is approximately 51 years of age. Perimenopause is when physiologic changes relating to a decline in follicle number indicate progress toward a woman’s final menstrual period. Perimenopause, on average, lasts around four years but can be as short as a few months or as long as up to 10 years. Perimenopause begins with the onset of menstrual irregularities and continues until a woman reaches menopause, diagnosed after 12 months of amenorrhea.129,130
Perimenopause can be divided into two stages: the early stage, characterized by occasionally skipped cycles and menstrual cycle lengths varying by seven days or more, and the late stage, defined by more significant menstrual irregularity, with periods of amenorrhea ranging from 60 days to 12 months. The symptoms associated with perimenopause are caused by multiple hormonal changes during this time, resulting from a decline in ovarian function. As the number of ovarian follicles decreases, inhibin B levels decline, leading to a loss of pituitary inhibition and an increase in follicle-stimulating hormone (FSH) secretion.131 In the late menopausal transition, estradiol levels decrease as the number of anovulatory cycles increases. As women approach menopause, anti-Müllerian hormone levels decrease and directly reflect the ovarian follicular reserve.131
Menopause is characterized by the complete or near-complete exhaustion of the ovaries’ follicles, leading to low estrogen (estradiol) levels and significantly increased FSH levels.131 After menopause, the withdrawal of endogenous estrogen levels can worsen traditional CVD risk factors. It can accelerate CVD, including body fat redistribution into the visceral cavity, impairment of glucose tolerance, adverse changes in lipid profile, elevations in blood pressure, endothelial dysfunction, and increased sympathetic tone, all of which have detrimental effects on arterial and cardiovascular function.13
Menopausal Hormone Therapy
The ACC/AHA prevention guidelines do not recommend initiating menopausal HRT for the sole purpose of CVD prevention after the landmark HERS and WHI demonstrated increased CVD risks rather than benefits.17 However, it should be noted that WHI enrolled participants aged 50–70 years, and the mean age was 63 years, indicating that most women were quite far out from their final menstrual period. In addition, many women in the HRT trials were not symptomatic with VMS.132,133 The risks associated with HRT likely depend on the age of initiation of HRT, time since menopause, age at menopause, duration of HRT therapy, type of HRT, a dose of HRT, and route of administration. For women with only the genitourinary symptoms of menopause, topical (vaginal) estrogen is not thought to have much systemic absorption and therefore can be safely used even in women at elevated CVD risk.
For systemic HRT (oral or transdermal preparations), HRT is still indicated for women <60 years or within ten years of menopause who have symptomatic VMS or other menopausal symptoms. For women with early menopause without contraindications, HRT is recommended until at least the average age of natural menopause. Generally, HRT is not recommended if >10 years from menopause or age >65 years. Oral estrogens should be avoided in women with a history of CVD, blood clots, high triglycerides, gallbladder disease, or prior breast or endometrial cancer. A cardiovascular risk assessment should be done before the initiation of HRT.14,134 Generally women at low CVD risk (<5% 10-year ASVCD risk), HRT is acceptable for VMS management but should generally be avoided in women at high CVD (≥20% 10-year risk) or established ASCVD. For those at intermediate estimated ASCVD risk, the use of a CAC score can help determine the presence and burden of coronary atherosclerosis and guide risk-based decisions about HRT safety. Shared decision-making about the benefits and risks of HRT tailored for an individual patient transitioning through menopause should be made between the patient and her clinician.
Tactics to Increase Uptake and Awareness
Screening is vital for the early detection and management of CVD women. Several strategies can be implemented to improve CVD screening in this population. First, increasing CVD awareness through public health campaigns and targeted educational materials is essential. Engaging gynecology and primary care clinicians in the screening process of middle-aged women are also crucial. Furthermore, tailored screening methods and risk assessments should be developed: general screening guidelines are helpful. Still, they do not account for women’s unique risks and disease presentations, so an individualized approach incorporating reproductive history is essential. Finally, the need for dedicated research funding to develop new screening modalities and guidelines cannot be stressed enough.
Conclusion
The road to improving women’s cardiovascular health is long and multifaceted, requiring a combination of education, tailored screening, and risk assessment. A growing body of evidence currently supports the limitations of current cardiovascular risk assessment tools. It encourages the additional incorporation of female-specific or female-predominant risk factors in the cardiovascular evaluation of women, including their history of menstrual cycles, parity, pregnancy complications, hormone therapy, menopause, and/or the presence of autoimmune diseases. Proper identification of CVD in women and implementation of preventive and treatment therapies is paramount, as understanding the uniqueness of how IHD may present in women, with INOCA being more common than in men. More data from future studies are necessary to understand how to best incorporate novel and emerging risk factors into CVD risk assessment. Finally, further efforts are strongly needed to ensure women are adequately represented in cardiovascular clinical trials.
Acknowledgments
No additional acknowledgments.
Funding
Dr. Michos is supported by the Amato Fund in Women’s Cardiovascular Health Research at Johns Hopkins University and American Heart Association Grant 946222.
Disclosure
Dr. Michos has served as a consultant for Amgen, Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, Edwards Life Science, Esperion, Medtronic, Novartis, Novo Nordisk, and Pfizer. Dr. Sierra-Galan has served as a speaker for Pfizer. Dr Lale S Tokgözoğlu reports personal fees from Abbott, Amgen, AstraZeneca, Bayer, Daiichi Sankyo, MSD, Mylan, Novartis, NovoNordisk, Sanofi, Servier, Pfizer, and Recordati, outside the submitted work. The other authors have nothing to disclose for this work.
References
1. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation. 2023;147(8):e93–e621. doi:10.1161/CIR.0000000000001123
2. Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80(25):2361–2371. doi:10.1016/j.jacc.2022.11.005
3. Arora S, Stouffer GA, Kucharska-Newton AM, et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation. 2019;139(8):1047–1056. doi:10.1161/CIRCULATIONAHA.118.037137
4. Curtin SC. Trends in cancer and heart disease death rates among adults aged 45–64: United States, 1999–2017. Natl Vital Stat Rep. 2019;68(5):1–9.
5. Khan SU, Yedlapati SH, Lone AN, et al. A comparative analysis of premature heart disease- and cancer-related mortality in women in the USA, 1999–2018. Eur Heart J Qual Care Clin Outcomes. 2022;8(3):315–323. doi:10.1093/ehjqcco/qcaa099
6. Abe T, Olanipekun T, Adedinsewo D, et al. Trends and outcomes of ST-segment-elevation myocardial infarction among young women in the United States. J Am Heart Assoc. 2023;12:e026811. doi:10.1161/JAHA.122.026811
7. Vogel B, Acevedo M, Appelman Y, et al. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet. 2021;397(10292):2385–2438. doi:10.1016/S0140-6736(21)00684-X
8. Timmis A, Vardas P, Townsend N, et al. European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J. 2022;43(8):716–799. doi:10.1093/eurheartj/ehab892
9. Wenger NK. Women and coronary heart disease: a century after Herrick: understudied, underdiagnosed, and undertreated. Circulation. 2012;126(5):604–611. doi:10.1161/CIRCULATIONAHA.111.086892
10. Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol. 2007;49(11):1230–1250. doi:10.1016/j.jacc.2007.02.020
11. Institute of Medicine (US). Committee on Women’s Health Research. Women’s Health Research: Progress, Pitfalls, and Promise; 2010.
12. Cho L, Davis M, Elgendy I, et al. Summary of updated recommendations for primary prevention of cardiovascular disease in women: JACC State-of-The-Art Review. J Am Coll Cardiol. 2020;75(20):2602–2618. doi:10.1016/j.jacc.2020.03.060
13. Elder P, Sharma G, Gulati M, Michos ED. Identification of female-specific risk enhancers throughout the lifespan of women to improve cardiovascular disease prevention. Am J Prev Cardiol. 2020;2:100028. doi:10.1016/j.ajpc.2020.100028
14. O’Kelly AC, Michos ED, Shufelt CL, et al. Pregnancy and reproductive risk factors for cardiovascular disease in women. Circ Res. 2022;130(4):652–672. doi:10.1161/CIRCRESAHA.121.319895
15. Agarwala A, Michos ED, Samad Z, Ballantyne CM, Virani SS. The use of sex-specific factors in the assessment of women’s cardiovascular risk. Circulation. 2020;141(7):592–599. doi:10.1161/CIRCULATIONAHA.119.043429
16. Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2021;42(34):3227–3337. doi:10.1093/eurheartj/ehab484
17. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. doi:10.1161/cir.0000000000000678
18. Pearson GJ, Thanassoulis G, Anderson TJ, et al. 2021 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37(8):1129–1150. doi:10.1016/j.cjca.2021.03.016
19. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046–e1081. doi:10.1161/CIR.0000000000000624
20. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi:10.1093/eurheartj/ehz455
21. Goff DC
22. Amin NP, Martin SS, Blaha MJ, Nasir K, Blumenthal RS, Michos ED. Headed in the right direction but at risk for miscalculation: a critical appraisal of the 2013 ACC/AHA risk assessment guidelines. J Am Coll Cardiol. 2014;63(25):2789–2794. doi:10.1016/j.jacc.2014.04.010
23. Group Sw, collaboration ESCCr. SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J. 2021;42(25):2439–2454. doi:10.1093/eurheartj/ehab309
24. Michos ED, Nasir K, Braunstein JB, et al. Framingham risk equation underestimates subclinical atherosclerosis risk in asymptomatic women. Atherosclerosis. 2006;184(1):201–206. doi:10.1016/j.atherosclerosis.2005.04.004
25. Michos ED, Vasamreddy CR, Becker DM, et al. Women with a low Framingham risk score and a family history of premature coronary heart disease have a high prevalence of subclinical coronary atherosclerosis. Am Heart J. 2005;150(6):1276–1281. doi:10.1016/j.ahj.2005.02.037
26. Aggarwal NR, Patel HN, Mehta LS, et al. Sex differences in ischemic heart disease: advances, obstacles, and next steps. Circ Cardiovasc Qual Outcomes. 2018;11(2):e004437. doi:10.1161/CIRCOUTCOMES.117.004437
27. Broni EK, Ndumele CE, Echouffo-Tcheugui JB, Kalyani RR, Bennett WL, Michos ED. The diabetes-cardiovascular connection in women: understanding the known risks, outcomes, and implications for care. Curr Diab Rep. 2022;22(1):11–25. doi:10.1007/s11892-021-01444-x
28. Angoulvant D, Ducluzeau PH, Renoult-Pierre P, et al. Impact of gender on relative rates of cardiovascular events in patients with diabetes. Diabetes Metab. 2021;47(5):101226. doi:10.1016/j.diabet.2021.101226
29. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57(8):1542–1551. doi:10.1007/s00125-014-3260-6
30. Prospective Studies C, Asia Pacific Cohort Studies C, Halsey J. Sex-specific relevance of diabetes to occlusive vascular and other mortality: a collaborative meta-analysis of individual data from 980 793 adults from 68 prospective studies. Lancet Diabetes Endocrinol. 2018;6(7):538–546. doi:10.1016/S2213-8587(18)30079-2
31. Al-Salameh A, Chanson P, Bucher S, Ringa V, Becquemont L. Cardiovascular disease in type 2 diabetes: a review of sex-related differences in predisposition and prevention. Mayo Clin Proceed. 2019;94(2):287–308. doi:10.1016/j.mayocp.2018.08.007
32. Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162(16):1867–1872. doi:10.1001/archinte.162.16.1867
33. Pasquali R, Casimirri F, Labate AM, et al. Body weight, fat distribution and the menopausal status in women. The VMH Collaborative Group. Int J Obes Relat Metab Disord. 1994;18(9):614–621.
34. Kulie T, Slattengren A, Redmer J, Counts H, Eglash A, Schrager S. Obesity and women’s health: an evidence-based review. J Am Board Fam Med. 2011;24(1):75–85. doi:10.3122/jabfm.2011.01.100076
35. Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378(9799):1297–1305. doi:10.1016/S0140-6736(11)60781-2
36. Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294(4):466–472. doi:10.1001/jama.294.4.466
37. Kovell LC, Harrington CM, Michos ED. Update on blood pressure control among US adults with hypertension. JAMA. 2021;325(6):586–587. doi:10.1001/jama.2020.23982
38. Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll of Cardiol. 2009;54(25):2366–2373. doi:10.1016/j.jacc.2009.10.009
39. Lau ES, Michos ED. Blood pressure trajectories through the menopause transition: different paths, same journey. Circ Res. 2022;130(3):323–325. doi:10.1161/CIRCRESAHA.122.320664
40. Full KM, Huang T, Shah NA, et al. Sleep irregularity and subclinical markers of cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2023;12(4):e027361. doi:10.1161/JAHA.122.027361
41. Daugherty SL, Carter JR, Bourjeily G. Cardiovascular disease in women across the lifespan: the importance of sleep. J Womens Health. 2020;29(3):452–460. doi:10.1089/jwh.2020.8331
42. Dean YE, Shebl MA, Rouzan SS, et al. Association between insomnia and the incidence of myocardial infarction: a systematic review and meta-analysis. Clin Cardiol. 2023;46:376–385. doi:10.1002/clc.23984
43. Kadier K, Qin L, Ainiwaer A, et al. Association of sleep-related disorders with cardiovascular disease among adults in the United States: a cross-sectional study based on national health and nutrition examination survey 2005–2008. Front Cardiovasc Med. 2022;9:954238. doi:10.3389/fcvm.2022.954238
44. Martinez GM. Trends and Patterns in Menarche in the United States: 1995 through 2013–2017. Natl Health Stat Report. 2020;146:1–12.
45. Peters SA, Woodward M. Women’s reproductive factors and incident cardiovascular disease in the UK Biobank. Heart. 2018;104(13):1069–1075. doi:10.1136/heartjnl-2017-312289
46. Canoy D, Beral V, Balkwill A, et al. Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation. 2015;131(3):237–244. doi:10.1161/CIRCULATIONAHA.114.010070
47. Lee JJ, Cook-Wiens G, Johnson BD, et al. Age at menarche and risk of cardiovascular disease outcomes: findings from the national heart lung and blood institute-sponsored women’s Ischemia syndrome evaluation. J Am Heart Assoc. 2019;8(12):e012406. doi:10.1161/jaha.119.012406
48. Guan C, Zahid S, Minhas AS, et al. Polycystic ovary syndrome: a “risk-enhancing” factor for cardiovascular disease. Fertil Steril. 2022;117(5):924–935. doi:10.1016/j.fertnstert.2022.03.009
49. Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends Cardiovasc Med. 2020;30(7):399–404. doi:10.1016/j.tcm.2019.08.010
50. Osibogun O, Ogunmoroti O, Kolade OB, et al. A systematic review and meta-analysis of the association between polycystic ovary syndrome and coronary artery calcification. J Womens Health. 2022;31(6):762–771. doi:10.1089/jwh.2021.0608
51. Okoth K, Chandan JS, Marshall T, et al. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ. 2020;371:m3502. doi:10.1136/bmj.m3502
52. Oliver-Williams C, Vassard D, Pinborg A, Schmidt L. Risk of cardiovascular disease for women with polycystic ovary syndrome: results from a national Danish registry cohort study. Eur J Prev Cardiol. 2020;2047487320939674. doi:10.1177/2047487320939674
53. Wang YX, Arvizu M, Rich-Edwards JW, et al. Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: prospective cohort study. BMJ. 2020;371:m3464. doi:10.1136/bmj.m3464
54. Solomon CG, Hu FB, Dunaif A, et al. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002;87(5):2013–2017. doi:10.1210/jcem.87.5.8471
55. Wang YX, Stuart JJ, Rich-Edwards JW, et al. Menstrual cycle regularity and length across the reproductive lifespan and risk of cardiovascular disease. JAMA Netw Open. 2022;5(10):e2238513. doi:10.1001/jamanetworkopen.2022.38513
56. Lawlor DA, Emberson JR, Ebrahim S, et al. Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child-rearing? Findings from the British women’s heart and health study and the British regional heart study. Circulation. 2003;107(9):1260–1264. doi:10.1161/01.cir.0000053441.43495.1a
57. Peters SA, van der Schouw YT, Wood AM, et al. Parity, breastfeeding and risk of coronary heart disease: a pan-European case-cohort study. Eur J Prev Cardiol. 2016;23(16):1755–1765. doi:10.1177/2047487316658571
58. Li W, Ruan W, Lu Z, Wang D. Parity and risk of maternal cardiovascular disease: a dose-response meta-analysis of cohort studies. Eur J Prev Cardiol. 2019;26(6):592–602. doi:10.1177/2047487318818265
59. Parikh NI, Cnattingius S, Dickman PW, Mittleman MA, Ludvigsson JF, Ingelsson E. Parity and risk of later-life maternal cardiovascular disease. Am Heart J. 2010;159(2):215–221 e6. doi:10.1016/j.ahj.2009.11.017
60. Kazzi B, Ogunmoroti O, Rodriguez CP, et al. Parity history and later life sex hormone levels in the multi-ethnic study of atherosclerosis (Mesa). Can J Cardiol. 2022;38(12):1893–1900. doi:10.1016/j.cjca.2022.09.004
61. Rodriguez CP, Ogunmoroti O, Quispe R, et al. The association between multiparity and adipokine levels: the multi-ethnic study of atherosclerosis. J Womens Health. 2022;31(5):741–749. doi:10.1089/jwh.2021.0091
62. Ogunmoroti O, Osibogun O, Kolade OB, et al. Multiparity is associated with poorer cardiovascular health among women from the Multi-Ethnic Study of Atherosclerosis. Am J Obstet Gynecol. 2019;221(6):631 e1–631 e16. doi:10.1016/j.ajog.2019.07.001
63. Hauspurg A, Ying W, Hubel CA, Michos ED, Ouyang P. Adverse pregnancy outcomes and future maternal cardiovascular disease. Clin Cardiol. 2018;41(2):239–246. doi:10.1002/clc.22887
64. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. doi:10.1136/bmj.39335.385301.BE
65. Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2). doi:10.1161/CIRCOUTCOMES.116.003497
66. Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American heart association. Circulation. 2021;143(18):e902–e916. doi:10.1161/CIR.0000000000000961
67. Wu P, Gulati M, Kwok CS, et al. Preterm delivery and future risk of maternal cardiovascular disease: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7(2). doi:10.1161/JAHA.117.007809
68. Bonamy AK, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: effects of gestational age and fetal growth. Circulation. 2011;124(25):2839–2846. doi:10.1161/CIRCULATIONAHA.111.034884
69. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62(6):905–914. doi:10.1007/s00125-019-4840-2
70. Gunderson EP, Sun B, Catov JM, et al. Gestational diabetes history and glucose tolerance after pregnancy associated with coronary artery calcium in women during midlife: the CARDIA study. Circulation. 2021;143(10):974–987. doi:10.1161/CIRCULATIONAHA.120.047320
71. Murugappan G, Leonard SA, Farland LV, et al. Association of infertility with atherosclerotic cardiovascular disease among postmenopausal participants in the women’s health initiative. Fertil Steril. 2022;117(5):1038–1046. doi:10.1016/j.fertnstert.2022.02.005
72. Lau ES, Wang D, Roberts M, et al. Infertility and risk of heart failure in the women’s health initiative. J Am Coll Cardiol. 2022;79(16):1594–1603. doi:10.1016/j.jacc.2022.02.020
73. Wang YX, Minguez-Alarcon L, Gaskins AJ, et al. Pregnancy loss and risk of cardiovascular disease: the Nurses’ Health Study II. Eur Heart J. 2022;43(3):190–199. doi:10.1093/eurheartj/ehab737
74. Gunderson EP, Lewis CE, Lin Y, et al. Lactation duration and progression to diabetes in women across the childbearing years: the 30-Year CARDIA study. JAMA Intern Med. 2018;178(3):328–337. doi:10.1001/jamainternmed.2017.7978
75. Tschiderer L, Seekircher L, Kunutsor SK, Peters SAE, O’Keeffe LM, Willeit P. Breastfeeding is associated with a reduced maternal cardiovascular risk: systematic review and meta-analysis involving data from 8 studies and 1 192 700 Parous women. J Am Heart Assoc. 2022;11(2):e022746. doi:10.1161/JAHA.121.022746
76. Magnus MC, Wallace MK, Demirci JR, Catov JM, Schmella MJ, Fraser A. Breastfeeding and later-life cardiometabolic health in women with and without hypertensive disorders of pregnancy. J Am Heart Assoc. 2023;e026696. doi:10.1161/JAHA.122.026696
77. Budeiri DJ, Li Wan Po A, Dornan JC. Clinical trials of treatments of premenstrual syndrome: entry criteria and scales for measuring treatment outcomes. Br J Obstet Gynaecol. 1994;101(8):689–695. doi:10.1111/j.1471-0528.1994.tb13186.x
78. Stamatelopoulos KS, Georgiopoulos G, Papaioannou T, et al. Can premenstrual syndrome affect arterial stiffness or blood pressure? Atherosclerosis. 2012;224(1):170–176. doi:10.1016/j.atherosclerosis.2012.05.037
79. Bertone-Johnson ER, Whitcomb BW, Rich-Edwards JW, Hankinson SE, Manson JE. Premenstrual syndrome and subsequent risk of hypertension in a prospective study. Am J Epidemiol. 2015;182(12):1000–1009. doi:10.1093/aje/kwv159
80. Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976;85(4):447–452. doi:10.7326/0003-4819-85-4-447
81. Honigberg MC, Zekavat SM, Aragam K, et al. Association of premature natural and surgical menopause with incident cardiovascular disease. JAMA. 2019;322(24):2411–2421. doi:10.1001/jama.2019.19191
82. Chu JH, Michos ED, Ouyang P, et al. Coronary artery calcium and atherosclerotic cardiovascular disease risk in women with early menopause: the multi-ethnic study of atherosclerosis (Mesa). Am J Prev Cardiol. 2022;11:100362. doi:10.1016/j.ajpc.2022.100362
83. Yang L, Lin L, Kartsonaki C, et al. Menopause characteristics, total reproductive years, and risk of cardiovascular disease among Chinese women. Circ Cardiovasc Qual Outcomes. 2017;10(11):e004235. doi:10.1161/CIRCOUTCOMES.117.004235
84. Mishra SR, Chung HF, Waller M, Mishra GD. Duration of estrogen exposure during reproductive years, age at menarche and age at menopause, and risk of cardiovascular disease events, all-cause and cardiovascular mortality: a systematic review and meta-analysis. BJOG. 2021;128(5):809–821. doi:10.1111/1471-0528.16524
85. Anagnostis P, Bitzer J, Cano A, et al. Menopause symptom management in women with dyslipidemias: an EMAS clinical guide. Maturitas. 2020;135:82–88. doi:10.1016/j.maturitas.2020.03.007
86. Jensen J, Nilas L, Christiansen C. Influence of menopause on serum lipids and lipoproteins. Maturitas. 1990;12(4):321–331. doi:10.1016/0378-5122(90)90012-u
87. Nudy M, Aragaki AK, Jiang X, et al. The severity of individual menopausal symptoms, cardiovascular disease, and all-cause mortality in the women’s health initiative observational cohort. Menopause. 2022;29(12):1365–1374. doi:10.1097/GME.0000000000002089
88. Thurston RC, Aslanidou Vlachos HE, Derby CA, et al. Menopausal vasomotor symptoms and risk of incident cardiovascular disease events in SWAN. J Am Heart Assoc. 2021;10(3):e017416. doi:10.1161/JAHA.120.017416
89. Zeller CB, Appenzeller S. Cardiovascular disease in systemic lupus erythematosus: the role of traditional and lupus related risk factors. Curr Cardiol Rev. 2008;4(2):116–122. doi:10.2174/157340308784245775
90. Avina-Zubieta JA, To F, Vostretsova K, De Vera M, Sayre EC, Esdaile JM. Risk of myocardial infarction and stroke in newly diagnosed systemic lupus erythematosus: a general population-based study. Arthritis Care Res. 2017;69(6):849–856. doi:10.1002/acr.23018
91. Asanuma Y, Oeser A, Shintani AK, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349(25):2407–2415. doi:10.1056/NEJMoa035611
92. Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59(12):1690–1697. doi:10.1002/art.24092
93. Myasoedova E, Chandran A, Ilhan B, et al. The role of rheumatoid arthritis (RA) flare and cumulative burden of RA severity in the risk of cardiovascular disease. Ann Rheum Dis. 2016;75(3):560–565. doi:10.1136/annrheumdis-2014-206411
94. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76(1):17–28. doi:10.1136/annrheumdis-2016-209775
95. Quispe R, Varghese B, Michos ED. Inflammatory Diseases and Risk of Atherosclerotic Cardiovascular Disease: a New Focus on Prevention. In: Shapiro MD, editor. Cardiovascular Risk Assessment in Primary Prevention. Springer International Publishing; 2022:247–270.
96. Mannoh I, Hussien M, Commodore-Mensah Y, Michos ED. Impact of social determinants of health on cardiovascular disease prevention. Curr Opin Cardiol. 2021;36(5):572–579. doi:10.1097/HCO.0000000000000893
97. Lindley KJ, Aggarwal NR, Briller JE, et al. Socioeconomic determinants of health and cardiovascular outcomes in women: JACC review topic of the week. J Am Coll Cardiol. 2021;78(19):1919–1929. doi:10.1016/j.jacc.2021.09.011
98. Churchwell K, Elkind MSV, Benjamin RM, et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory From the American Heart Association. Circulation. 2020;142(24):e454–e468. doi:10.1161/CIR.0000000000000936
99. Caceres BA, Streed CG
100. Michos ED, Blaha MJ, Blumenthal RS. Use of the coronary artery calcium score in discussion of initiation of statin therapy in primary prevention. Mayo Clinic Proceed. 2017;92(12):1831–1841. doi:10.1016/j.mayocp.2017.10.001
101. Shaw LJ, Min JK, Nasir K, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J. 2018;39(41):3727–3735. doi:10.1093/eurheartj/ehy534
102. Wong ND, Cordola Hsu AR, Rozanski A, et al. Sex differences in coronary artery calcium and mortality from coronary heart disease, cardiovascular disease, and all causes in adults with diabetes: the coronary calcium consortium. Diabetes Care. 2020;43(10):2597–2606. doi:10.2337/dc20-0166
103. Blaha MJ, Abdelhamid M, Santilli F, Shi Z, Sibbing D. Advanced subclinical atherosclerosis: a novel category within the cardiovascular risk continuum with distinct treatment implications. Am J Prev Cardiol. 2023;13:100456. doi:10.1016/j.ajpc.2022.100456
104. Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (Mesa). Eur Heart J. 2018;39(25):2401–2408. doi:10.1093/eurheartj/ehy217
105. Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the multi-ethnic study of atherosclerosis (Mesa). Circulation. 2016;133(9):849–858. doi:10.1161/CIRCULATIONAHA.115.018524
106. Patel KK, Peri-Okonny PA, Qarajeh R, et al. Prognostic relationship between coronary artery calcium score, perfusion defects, and myocardial blood flow reserve in patients with suspected coronary artery disease. Circ Cardiovasc Imaging. 2022;15(4):e012599. doi:10.1161/CIRCIMAGING.121.012599
107. Mortensen MB, Gaur S, Frimmer A, et al. Association of age with the diagnostic value of coronary artery calcium score for ruling out coronary stenosis in symptomatic patients. JAMA Cardiol. 2022;7(1):36–44. doi:10.1001/jamacardio.2021.4406
108. Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: executive Summary: a Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144(22):e368–e454. doi:10.1161/CIR.0000000000001030
109. Bullock-Palmer RP, Michos ED, Gaballa D, Blankstein R. The role of imaging in preventive cardiology in women. Curr Cardiol Rep. 2023;25(2):29–40. doi:10.1007/s11886-022-01828-9
110. Investigators S-H, Newby DE, Adamson PD, et al. Coronary CT Angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924–933. doi:10.1056/NEJMoa1805971
111. Leipsic J, Taylor CM, Gransar H, et al. Sex-based prognostic implications of nonobstructive coronary artery disease: results from the international multicenter CONFIRM study. Radiology. 2014;273(2):393–400. doi:10.1148/radiol.14140269
112. Ferencik M, Mayrhofer T, Bittner DO, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol. 2018;3(2):144–152. doi:10.1001/jamacardio.2017.4973
113. Reynolds HR, Bairey Merz CN, Berry C, et al. Coronary arterial function and disease in women with no obstructive coronary arteries. Circ Res. 2022;130(4):529–551. doi:10.1161/CIRCRESAHA.121.319892
114. Bairey Merz CN, Shaw LJ, Reis SE, et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47(3 Suppl):S21–9. doi:10.1016/j.jacc.2004.12.084
115. Reynolds HR, Picard MH, Spertus JA, et al. Natural history of patients with ischemia and no obstructive coronary artery disease: the CIAO-ISCHEMIA Study. Circulation. 2021;144(13):1008–1023. doi:10.1161/CIRCULATIONAHA.120.046791
116. Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: a Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144(22):e368–e454. doi:10.1161/CIR.0000000000001029
117. Ford TJ, Corcoran D, Berry C. Stable coronary syndromes: pathophysiology, diagnostic advances and therapeutic need. Heart. 2018;104(4):284–292. doi:10.1136/heartjnl-2017-311446
118. AlBadri A, Bairey Merz CN, Johnson BD, et al. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J Am Coll Cardiol. 2019;73(6):684–693. doi:10.1016/j.jacc.2018.11.040
119. Taqueti VR, Shaw LJ, Cook NR, et al. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation. 2017;135(6):566–577. doi:10.1161/CIRCULATIONAHA.116.023266
120. Ford TJ, Stanley B, Sidik N, et al. 1-Year Outcomes of Angina Management Guided by Invasive Coronary Function Testing (CorMicA). JACC Cardiovasc Interv. 2020;13(1):33–45. doi:10.1016/j.jcin.2019.11.001
121. Reynolds HR, Maehara A, Kwong RY, et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation. 2021;143(7):624–640. doi:10.1161/CIRCULATIONAHA.120.052008
122. Minissian MB, Mehta PK, Hayes SN, et al. Ischemic heart disease in young women: JACC review topic of the week. J Am Coll Cardiol. 2022;80(10):1014–1022. doi:10.1016/j.jacc.2022.01.057
123. Daneshrad JA, Ordovas K, Sierra-Galan LM, et al. Role of cardiac magnetic resonance imaging in the evaluation of MINOCA. J Clin Med. 2023;12(5):2017. doi:10.3390/jcm12052017
124. Khan MS, Shahid I, Siddiqi TJ, et al. Ten-year trends in enrollment of women and minorities in pivotal trials supporting recent US food and drug administration approval of novel cardiometabolic drugs. J Am Heart Assoc. 2020;9(11):e015594. doi:10.1161/JAHA.119.015594
125. Khan SU, Khan MZ, Raghu Subramanian C, et al. Participation of women and older participants in randomized clinical trials of lipid-lowering therapies: a systematic review. JAMA Netw Open. 2020;3(5):e205202. doi:10.1001/jamanetworkopen.2020.5202
126. Khan SU, Michos ED. Women in stroke trials-A tale of perpetual inequity in cardiovascular research. JAMA Neurol. 2021;78(6):654–656. doi:10.1001/jamaneurol.2021.0624
127. Michos ED, Reddy TK, Gulati M, et al. Improving the enrollment of women and racially/ethnically diverse populations in cardiovascular clinical trials: an ASPC practice statement. Am J Prev Cardiol. 2021;8:100250. doi:10.1016/j.ajpc.2021.100250
128. Thakkar A, Agarwala A, Michos ED. Secondary prevention of cardiovascular disease in women: closing the gap. Eur Cardiol. 2021;16:e41. doi:10.15420/ecr.2021.24
129. Delamater L, Santoro N. Management of the Perimenopause. Clin Obstet Gynecol. 2018;61(3):419–432. doi:10.1097/GRF.0000000000000389
130. Bacon JL. The menopausal transition. Obstet Gynecol Clin North Am. 2017;44(2):285–296. doi:10.1016/j.ogc.2017.02.008
131. Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002;57:257–275. doi:10.1210/rp.57.1.257
132. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi:10.1001/jama.288.3.321
133. Chester RC, Kling JM, Manson JE. What the Women’s Health Initiative has taught us about menopausal hormone therapy. Clin Cardiol. 2018;41(2):247–252. doi:10.1002/clc.22891
134. Lundberg G, Wu P, Wenger N. Menopausal hormone therapy: a comprehensive review. Curr Atheroscler Rep. 2020;22(8):33. doi:10.1007/s11883-020-00854-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
