Back to Journals » Orthopedic Research and Reviews » Volume 15
A Unique Way of Treatment of Giant Cell Tumor of the Distal Femur in a 19-Year-Old Female, a Case Report
Authors Alemayehu ED , Kebede E
Received 22 March 2023
Accepted for publication 1 June 2023
Published 3 June 2023 Volume 2023:15 Pages 119—127
DOI https://doi.org/10.2147/ORR.S402927
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Clark Hung
Elsa Daniel Alemayehu, Eskinder Kebede
Department of Orthopedic Surgery and Trauma, College of Health Science, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Elsa Daniel Alemayehu, Orthopedic Surgery and Trauma Department, Black Lion Hospital, College of Health Science, Addis Ababa University, Addis Ababa, Ethiopia, Email [email protected]; [email protected]
Abstract: Giant Cell tumors (GCT) are benign tumors with aggressive characteristics and the potential to metastasize. These are seldom lethal benign bone tumors but are associated with massive local bony architecture distraction making their treatment difficult, especially if found in peri-articular locations. Several long bone giant cell tumor (GCT) cases have been reported. We report a unique treatment of distal femur GCT in a 19-year-old in a resource-limited setup whose initial presentation was following a pathologic fracture. We used a staged surgical protocol. In the first stage, distal femur resection and implantation of poly methyl methacrylate (PMMA) cement spacer for induced membrane formation was done, followed by SIGN nail and non-vascularized fibula strut graft. There was adequate healing and no recurrence was noted during the two-year follow-up.
Keywords: distal femur, fracture, giant cell tumor, GCT, SIGN nail, peri-articular, polymethyl methacrylate, PMMA, vascularized fibula graft
Introduction
Giant cell tumors are fairly common benign bone tumors that are usually associated with considerable local bony destruction in addition to their tendency to metastasize to the lungs despite being benign. Even though the histogenesis is unclear, microscopy shows the proliferation of mononuclear stromal cells and multiple multi-nucleated giant cells with uniform distribution.1 Although GCT is a benign bone tumor, it remains the most challenging in terms of management. There also remains no single clinical, radiographic, or histologic predictor as to the tendency of recurrence or metastasis.2
Campanacci et al3 classified GCT into three stages/grades based on their radiographic appearance: a stage 1 lesion (latent) has a well-defined margin and an intact cortex; a stage 2 lesion (active) has a relatively well-defined margin but no radiopaque rim, and the cortex is thinned and moderately expanded; and a grade 3 lesion (aggressive) has indistinct borders and cortical destruction (Figure 1).
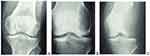 |
Figure 1 Campanacci et al3 grading system for GCT that is based on the radiographic appearance of the tumors. (A) Grade 1 (latent), (B) grade 2 (active), and (C) grade 3 (aggressive). |
Various surgical techniques range from intralesional curettage to wide resection.1 These treatment options include external beam radiation, medical treatment (bisphosphonates and denosumab), extensive curettage, adjuvant treatment, resection, and reconstruction. An important aspect of benign bone tumor treatment is striking a balance between recurrence rate and an acceptable level of surgical morbidity.4
In a study done to evaluate the recurrence rate following curettage and PMMA cement, the initial local recurrence rate was 25% with an average of four years (2–10 years) after the operation. Higher rate of recurrence is recorded in patients who had a pathologic fracture, stage 3 tumor according to Campanacci et al’s classification, and those who did not have adjuvant treatment with either high-speed burr or phenol.5
A comparative study between intralesional excision with adjuvant treatment (PMMA or phenol) and En-bloc resection with or without reconstruction showed that both are excellent oncologic methods of treatment. Intralesional excision was preferred for tumors about the knee unless there was anticipated no bone stock to support weight-bearing after excision and bur. For expandable bone tumors such as fibula and the distal end of the radius, it was preferred to do En-bloc resection. Intralesional procedures were found to be superior to En-bloc excision in properly selected patients in terms of the functional outcome because no matter how sophisticated the reconstruction was following En-bloc resection, it can never be as good as the patient’s joint.6
Many authors recommend an intralesional approach for GCT since it is often found near joints in young adults. This approach aims to preserve the bone’s structure and anatomy instead of removing a portion of it through resection.1 Indications for intralesional procedure include maintained or restorable joint congruity, no extension of the tumor into the soft tissue space, no joint involvement, not a multiply recurrent disease, and sufficient remaining wall to current.7 While doing intralesional curettage, the use of adjuvants with various physical and chemical agents could be utilized to achieve extended curettage to control the microscopic disease remaining in the walls. Liquid nitrogen, phenol, hydrogen peroxide, alcohol, electrocautery, high-speed burring, bone cement, and argon plasma cautery could be utilized as adjuvants.7 The recurrence rate has decreased to 10–20% with the use of mechanized burrs and adjuvant therapy from the historically reported 50–60% recurrence with curettage alone.8 Following this, the defect may be filled with bone, cement, or a combination of both. In cases in which >25% of the articular surface is undermined, subchondral bone grafting is recommended before cementing (ie, a sandwich procedure).7 If residual subchondral bone after extended curettage is less than 5mm a multilayer reconstruction technique is recommended to prevent late articular degeneration. This is done by packing a mixture of morsellized auto and allograft adjacent (around 5–8 mm thick) followed by cement packing of the remaining cavity.8
In a study done on 38 patients with GCT in the knee region, the area of the affected subchondral bone was measured radiographically using plain radiographs, CT and MRI and correlated with the mean Enneking functional score at follow-up. Patients initially treated with wide resection had a mean area of the affected subchondral bone of 68.2 (41–100) %.9 Thus occasionally, resection may be preferable to joint preserving surgery if intralesional approaches would result in a severe mechanical compromise that skeletal integrity is not likely to be maintained or restored after healing, leading to a compromise in ultimate function.8
If wide local excision is selected as the treatment of Campanacci et al stage 3 GCT, one of the best-accepted methods of treatment is En bloc resection and reconstruction with a prosthesis (hinge knee and rotating hinge-knee). Few complications were noted following this treatment method, of which the majority were related to the prosthesis, mainly prosthesis loosening and limb shortening.10
When comparing reconstruction with non-vascularized fibular graft and vascularized fibular graft, it is shown that non-vascularized fibular transfer is a simpler, less expensive, and shorter procedure than its counterpart. It is a biological reconstruction with good long-term results and a relatively low donor site complication rate of 16%. It allows the remodeling of the fibula at the donor site. This study found seven fatigue fractures in six patients, but only two needed treatment.11
A comparative study of vascularized and non-vascularized fibular grafts showed that there was no strong evidence found that clarified grafts longer than 6 cm should be vascularized. Vascularization made no difference to union rate or time to union, instead vascularized grafts were more likely to require surgical revision for wound breakdown, nonunion, graft fracture, or mechanical problems.12
In a study that used SIGN intramedullary nail for knee fusion, the nail was inserted through an anteromedial entry point on the femur, and full weight bearing was permitted immediately. All knees had clinical and radiographic evidence of fusion at the final follow-up and none required further surgery. This study showed that SIGN is both safe and effective for knee fusion in austere environments with limited fluoroscopy and implant options.13
Recently, the clinical observation of retrospectively enrolled patients with GCT and desmoplastic fibroma (DF- A benign bone sarcoma with locally aggressive behavior but no metastatic propensity) showed the first molecular and pharmacological investigation of these bone tumors with the use of transitional and clinical data. The focus of this study was on the molecular biology of GCT and DF showing the upregulation of RANK-L, RANK, OPN, CXCR4, RUNX2, and FLT1 and the downregulation of OPG and CXCL12 genes. The aim was to investigate the activity of bone-targeted therapy and multi-receptor tyrosine kinase inhibitors in mono-regimen or combination for GCT treatment. In vitro, analyses provided evidence for suggesting the combination of denosumab and lenvatinib (LENVA 78% and DENO + LENVA 80%) as a promising therapeutic strategy because these molecules exerted antitumor activity involving the inhibition of cells’ motility. Furthermore, in vivo, analysis through the use of xenotransplanted DF1 in zebrafish embryos validated the obtained data. These preliminary results show the tumor biology of these poorly understood lesions, highlighting the promising role of some biomarkers as potential predictive and druggable targets for these diseases.14
Case
We present the case of a 19 years old female, who was referred to our tertiary specialized hospital with a 06 days duration of deformity, pain over the right knee, and inability to weight-bear following a fall from a standing height. She had a 5-month history of dull aching pain over the right knee with no radiation that does not respond to over-the-counter pain medications, which tend to be worse at night. It was of insidious onset and could get severe at times, leading to an inability to sleep. She also had associated limping for which she did not seek medical attention. The pain and limping were not preceded by any trauma or sports injury. The patient was otherwise well, reporting no history of trauma to other sites during the fall, no known chronic medical illnesses, no history of chronic cough or fever, and no history of excessive alcohol consumption, smoking, or any other substance abuse.
Upon initial physical examination, she was acutely sick looking in pain. The blood pressure was 120/80 mmHg, the pulse rate was 80 times per minute, and the respiratory rate was 16 times per minute. On the head and neck examination, there was no sign of anemia, cyanosis, icterus, or dyspnea. On the thoracic examination, the heart and lungs were normal. On the abdominal examination, it was soft and moved with respiration, the liver, and spleen were not palpable. On the skin examination, there was no lesion. On the Lymph and glandular examination, there was no palpable lymph node in all accessible areas, and the thyroid was not enlarged.
The pertinent positive finding was on the musculoskeletal system. She was on a long leg posterior gutter with no wound. She had swelling and bruising over the right knee. There was warmth and tenderness over the right distal femur and knee area. There was a right knee joint effusion. Distal Neurovascular structures were intact. The range of motion was not assessed.
The patient was admitted for local and systemic staging. Subsequently, investigations were performed at the hospital.
The laboratory examination results were: Complete blood cell count: Hemoglobin: 12, Hematocrit: 34.8, WBC = 4100, platelet = 376,000; Renal function test: Creatinine = 0.4, Urea = 29; COVID test: Negative.
Imaging results were: Chest x-ray was normal; Right knee x-ray (Figure 2) locally aggressive geographic lytic lesion eccentrically located in the distal femur. It extends from the distal one-third of the femur shaft to the femur subchondral bone. The lesion does not breach the subchondral bone but it is ominously thinned. The lesion has expanded and thinned out the surrounding femur cortex. There was a fracture line through the thinned-out distal femur cortex. There was no extension into the surrounding soft tissue, and no periosteal reaction was noted. The proximal tibia, proximal fibula, and proximal femur have a normal appearance.
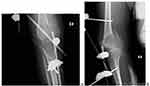 |
Figure 2 (A) Anteroposterior and (B) Lateral x-ray of the right knee. This X-ray was taken post-operatively after external fixation was applied. |
Right knee MRI (Figure 3) shows a distal femur sub articular expansile lesion having heterogeneous increased signal on T2 and isointense signs on T1 with multiple internal areas of low T1 signal changes, lesion also showed mild heterogeneous enhancement of the lesion with peripheral rim enhancement of the T2 bright signal areas which suggest cystic area. There was a breach of the distal femur articular surface centrally over the intercondylar notch. The patella was displaced anterior but had a normal marrow signal. The visualized proximal tibia and fibula had also a normal marrow signal. There was minimal fluid in the joint cavity. The articular cartilage overlying the patella and femoral condyle was unremarkable. The proximal femur shaft that was visualized had normal marrow and cortical signals. No focal lesion proximally.
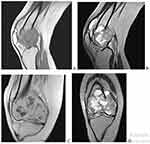 |
Figure 3 Right knee MRI. (A and B) are T1 and T2 weighted sagittal images, respectively. (C and D) are T1 and T2 weighted coronal images, respectively. |
Computerized tomography (CT) scan of the chest and abdomen did not identify any metastatic lesions and a skeletal survey was not done due to limited resources.
Local staging included radiographs and a magnetic resonance imaging (MRI) scan. According to Campanacci et al's3 radiographic staging system, this lesion was classified as stage/grade 3 with fracture. Systemic staging confirmed that the patient was generally well with no co-morbidities and normal nutritional status.
An incisional biopsy was performed through a lateral approach. A longitudinal incision was made over the most prominent lateral swelling. Only the lateral portion of the anterior compartment was exposed. No extension to other compartments was made. Biopsy was taken from the soft tissue and bone adequately. Meticulous hemostasis was applied throughout the procedure. A glove drain was left in place through the incision. Knee-spanning external fixation was applied for fracture stabilization. The histological evaluation described fragments of bony tissue having a proliferation of oval to round cells with open chromatin nucleoli, and eosinophilic cytoplasm. There were abundant multinucleated giant cells where nuclei resemble single cells. No osteoid formation was seen. The conclusion result was a giant cell tumor. Molecular analysis of the tumor was not available in our setup.
The initial stage of the surgery was En-bloc (wide margin) resection and cement spacer application. Under spinal anesthesia external fixation was removed, a tourniquet was applied and a lateral approach was used. Around 12 cm incision was made laterally incorporating the previous biopsy incision. The tumor was dissected cautiously. The distal femur was resected from 2–3 cm proximal to the proximal-most end of the tumor up to the entire distal part of the femur articular surface. A gross specimen of about 10–12 cm was resected and sent to pathology. Neurovascular structures were identified carefully and protected throughout the procedure. The dead space after dissection was filled in with an antibiotic-impregnated cement spacer. Subcutaneous tissue and skin were closed in layers. Drainage was left in place. Pulse was checked and it was intact. Skin traction was applied and the patient was transferred to recovery. Postoperatively, antibiotics were administered for 48 hours, drainage was removed after 48 hours when the output was minimal and anti-pain was administered. The pathology result came back and confirmed the diagnosis of GCT. In addition, it showed that the proximal margin was free of tumor cells.
Due to the placement of external fixation during the primary procedure (biopsy and ex-fix) and in place for more than 2 weeks, the second stage procedure was delayed for two weeks after the first stage to allow a pin holiday period. In the second stage of surgery, the previous incision and approach were opened. Cement was embedded in a formed biomembrane (Figure 4). The biomembrane was incised and the cement was removed. Tibia articular surface was shaved. The tibia was prepared for intramedullary nailing. The entry site for the tibia was made midline on the sagittal plane over the intercondylar notch. Tibia was reamed progressively to accommodate the chosen nail size. Then, the femur was reamed from the distal resected part of the femur shaft retrograde to the proximal part progressively to accommodate the chosen nail size. An intramedullary SIGN nail size of 38×8 cm was inserted first through the tibia down, then aligned to insert into the femur in a retrograde fashion. It was made sure the right limb length was 2–3 cm shorter than the contralateral side to allow clearance during gait. The rotation of the limb was checked before locking the intramedullary nail. A proximal (2) and distal (2) interlocking screw was inserted with the guide of an image intensifier (Figure 5). A posterolateral approach to the leg was used to take a non-vascularized fibular graft. The appropriate size of the remaining gap over the femur was measured and a graft was taken with that precise size (around 10cm) from the fibula. The graft was then placed in the gap from the distal-most resected part of the femur to the tibia (Figure 5). Subcutaneous tissue and skin were closed in layers. A drainage tube was left in place. The patient left the OR with stable vital signs and was transferred to the ward. Postoperatively, prophylactic antibiotics were given for 48 hours, drainage was removed after 48 hours when the output was minimal, anti-pain was given and the patient was discharged home after being told to be non-weight bearing.
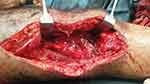 |
Figure 4 Formed bio-membrane after cement removal. |
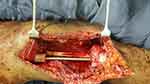 |
Figure 5 Intramedullary nail and non-vascularized fibular graft in place. |
The first appointment was 2 weeks after discharge for a general checkup, wound evaluation, and suture removal. The second appointment was 6 months after the surgery. During this time, the patient was kept non-weight bearing. X-ray showed healing of the graft to the recipient site with callus formation (Figure 6). There was also a radiographic filling of bone noted at the donor site. The patient was told to do a touch-down weight-bearing with the use of crutches. The subsequent follow-up was 1 year after surgery (Figure 7). There was adequate healing on the X-ray at the time and the patient was told to weight-bear fully. There was adequate healing and no recurrence was noted at the two-year follow-up (Figure 8).
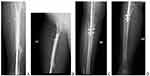 |
Figure 6 AP and Lateral X-ray of the femur (A and B, respectively). AP and Lateral X-ray of the tibia including knee joint (C and D, respectively). |
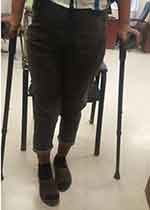 |
Figure 7 Patient weight bearing with crutches at 1 year follow up. |
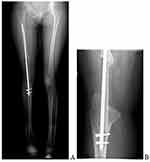 |
Figure 8 Full-length lower extremity radiograph at two years follow up (A) and lateral radiograph (B) showing healing at the proximal graft site at two years follow up. |
Discussion
Once wide resection is the method of treatment chosen in patients with Campanacci et al stage 3 GCT, one of the best-accepted ways is to do En-bloc resection and reconstruction with a prosthesis (hinge knee and rotating hinge knee).2 The lack of reconstruction implants in resource-limited setups makes treatment of stage 3 GCT challenging. This is a plausible option for treatment when knee prosthesis and reconstruction implants are limited.
A non-vascularized fibular graft is a simpler, less expensive, and shorter procedure than the use of vascularized grafts. In addition, it allows remodeling of the fibula at the donor site and a relatively low donor site complication rate of 16% with good long-term results. Nevertheless, the danger of resorption of the graft and lack of biological activity leading to non-union and graft failure is thought to be the disadvantages.11 A study involving 31 patients who had reconstruction with a non-vascularized fibular graft after resection of the bone tumor was done to study the functional and radiographic success of the graft. The functional result according to the Musculoskeletal Tumor Society Score (MSTS) was 77% in the lower limb and 80% in the upper limb. Radiographically, 70% of the patients had hypertrophy of the fibula demonstrating biological activity. Furthermore, the primary union rate at less than 12 months post-op was 89% and radiographic remodeling was seen at the median of 3.6 years after surgery.11 The non-vascularized fibular graft done in this study also showed healing at the recipient site and remodeling of the fibula at the donor site within 2 years of follow-up. Although the functional result was not assessed with MSTS in this case, the patient reports being functional in day-to-day activity with slight limitations as described in the patient perspective section.
In the same study, fatigue fracture was reported in 15% of the patients with the majority occurring after the union of both graft junctions and was asymptomatic (identified retrospectively on follow-up) and hence required no treatment. Only one patient had failed union and was treated by internal fixation. Other reported complications were infection and donor site morbidity (16%) like peroneal nerve damage, ankle joint instability, and knee valgus deformity.11 In our patient, there was no fatigue fracture, no superficial or deep infection, and no donor site morbidity reported within the two years follow-up.
In the largest series done in arthrodesis of the knee using an intramedullary nail, Puranen et al reported 100% fusion in 18 patients within 6 months after arthrodesis (5 failed prior arthrodesis, 5 instability, 2 tuberculosis infection, 3 osteomyelitis, 3 post-traumatic osteoarthritis).15 Reported complications in this series included one patient requiring bone grafting to obtain union by six months and one patient having a broken nail that was exchanged for a larger nail. In a study done on seven knees in Soddo Christian Hospital, Ethiopia, to evaluate the efficacy of using SIGN nails for knee fusion, all knees had clinical and radiographic evidence of fusion at the final follow-up and none required further surgery.13 The SIGN nail was inserted over an anteromedial entry point on the femur and full weight-bearing was permitted immediately. Additionally, there was no bone gap in all the patients in this study. Despite the bone gap in our case report, the patient did not have reported metal failure within two years. However, full weight-bearing was delayed for one year due to fear of implant failure in this patient.
We recommend making this a case series to better articulate the outcome.
Patient Perspective
The patient is very thankful for the intervention that was done for her. Due to the involvement of the articular surface, she understands the treatment options available in our setup were either extended curettage which would have been inadequate and led to recurrence, amputation, or this intervention. She is thankful she chose the current intervention.
She has limping because of shortening of the right lower extremity due to the deliberate shortening of 2 cm done for ground clearance during gait. She cannot squat due to the knee fusion in full extension. She also has to straighten her leg when sitting. Otherwise, she reports doing excellent overall.
Ethical Consideration
Institutional approval was not required to publish this case report.
Informed Consent
The patient has given written informed consent for all the surgical interventions that were done. This was witnessed and documented. In addition, she has given verbal informed consent for the publication of this paper which was witnessed by the two authors and two independent personnel.
Disclosure
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the case report.
References
1. Sobti A, Agrawal P, Agarwala S, Agarwal M. Giant cell tumor of bone - an overview. Arch Bone Jt Surg. 2016;4(1):2–9.
2. Eckardt JJ, Grogan TJ. Giant cell tumor of bone. Clin Orthop Relat Res. 1986;204:45–58. doi:10.1097/00003086-198603000-00006
3. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Jt Surg. 1987;69(1):106–114. doi:10.2106/00004623-198769010-00018
4. McGarry SV. Extended curettage for benign bone lesions. Tech Orthop. 2007;22(2):121–126. doi:10.1097/bto.0b013e31811f35d4
5. O’Donnell RJ, Springfield DS, Motwani HK, Ready JE, Gebhardt MC, Mankin HJ. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J Bone Jt Surg. 1994;76(12):1827–1833. doi:10.2106/00004623-199412000-00009
6. Gitelis S, Mallin BA, Piasecki P, Turner F. Intralesional excision compared with en bloc resection for giant-cell tumors of bone. J Bone Jt Surg. 1993;75(11):1648–1655. doi:10.2106/00004623-199311000-00009
7. Gundavda MK, Agarwal MG. Extended curettage for giant cell tumors of bone. JBJS Essent Surg Tech. 2021;11(3). doi:10.2106/jbjs.st.20.00040
8. Puri A, Agarwal M. Treatment of giant cell tumor of bone: current concepts. Indian J Orthop. 2007;41(2):101. doi:10.4103/0019-5413.32039
9. Chen T-H, Su Y-P, Chen W-M. Giant cell tumors of the knee: subchondral bone integrity affects the outcome. Int Orthop. 2005;29(1):30–34. doi:10.1007/s00264-004-0613-7
10. Yu X, Xu M, Song R, Fu Z, Liu X. Long-term outcome of giant cell tumors of bone around the knee treated by en bloc resection of tumor and reconstruction with prosthesis. Orthop Surg. 2010;2(3):211–217. doi:10.1111/j.1757-7861.2010.00089.x
11. Krieg AH, Hefti F. Reconstruction with non-vascularised fibular grafts after resection of bone tumors. J Bone Jt Surg. 2007;89(2):215–221. doi:10.1302/0301-620X.89B2.17686
12. Allsopp BJ, Hunter-Smith DJ, Rozen WM. Vascularized versus nonvascularized bone grafts: what is the evidence? Clin Orthop Relat Res. 2016;474(5):1319–1327. doi:10.1007/s11999-016-4769-4
13. Anderson DR, Anderson LA, Haller JM, Feyissa AC. The SIGN nail for knee fusion: technique and clinical results. SICOT J. 2016;2:6. doi:10.1051/sicotj/2015038
14. De Vita A, Vanni S, Miserocchi G, et al. A rationale for the activity of bone target therapy and tyrosine kinase inhibitor combination in giant cell tumor of bone and desmoplastic fibroma: translational evidences. Biomedicines. 2022;10(2):372. doi:10.3390/biomedicines10020372
15. Puranen J, Kortelainen P, Jalovaara P. Arthrodesis of the knee with intramedullary nail fixation. J Bone Jt Surg. 2017;2017:1.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
