Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
A Telemedicine Approach for Monitoring COPD: A Prospective Feasibility and Acceptability Cohort Study
Authors Shinoda M , Hataji O , Miura M, Kinoshita M, Mizoo A, Tobino K, Soutome T, Nishi T , Ishii T, Miller BE , Tal-Singer R , Tomlinson R , Matsuki T, Jones PW , Shibata Y
Received 17 May 2022
Accepted for publication 31 October 2022
Published 17 November 2022 Volume 2022:17 Pages 2931—2944
DOI https://doi.org/10.2147/COPD.S375049
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Masahiro Shinoda,1 Osamu Hataji,2 Motohiko Miura,3 Masaharu Kinoshita,4 Akira Mizoo,5 Kazunori Tobino,6 Toru Soutome,7 Takanobu Nishi,7 Takeo Ishii,7 Bruce E Miller,8 Ruth Tal-Singer,8 Ryan Tomlinson,8 Taizo Matsuki,7 Paul W Jones,9 Yoko Shibata10
1Department of Respiratory Medicine, Tokyo Shinagawa Hospital, Shinagawa, Tokyo, Japan; 2Respiratory Center, Matsusaka Municipal Hospital, Matsusaka, Mie, Japan; 3Department of Respiratory Medicine, Tohoku Rosai Hospital, Sendai, Miyagi, Japan; 4Department of Respiratory Medicine, Nagata Hospital, Yanagawa, Fukuoka, Japan; 5Department of Pulmonary Medicine Japan, Japan Community Healthcare Organization Tokyo Shinjuku Medical Center, Shinjuku, Tokyo, Japan; 6Department of Respiratory Medicine, Iizuka Hospital, Iizuka, Fukuoka, Japan; 7Japan Medical & Development, GSK K.K, Minato-Ku, Tokyo, Japan; 8GSK Pharma R&D, Collegeville, PA, USA; 9General Medicine, GSK, Brentford, Middlesex, UK; 10Department of Pulmonary Medicine, Fukushima Medical University, Fukushima, Japan
Correspondence: Taizo Matsuki, Value Evidence Outcomes, Japan Medical & Development, GSK K.K, Akasaka Intercity AIR 1-8-1 Akasaka, Minato-Ku, Tokyo, 107-0052, Japan, Tel +81-8036928935, Email [email protected]
Background: Telemedicine may help the detection of symptom worsening in patients with chronic obstructive pulmonary disease (COPD), potentially resulting in improved outcomes. This study aimed to determine the feasibility and acceptability of telemedicine among patients with COPD and physicians and facility staff in Japan.
Methods: This was a 52-week multicenter, prospective, single-arm, feasibility and acceptability cohort study of Japanese patients ≥ 40 years of age with COPD or asthma-COPD overlap. Participants underwent training to use YaDoc, a telemedicine smartphone App, which included seven daily symptom questions and weekly COPD Assessment Test (CAT) questions. The primary endpoint was participant compliance for required question completion. The secondary endpoint was participant and physician/facility staff acceptability of YaDoc based on questionnaires completed at Week 52. The impact of the Japanese COVID-19 pandemic state of emergency on results was also assessed.
Results: Of the 84 participants enrolled (mean age: 68.7 years, 88% male), 72 participants completed the study. Completion was high in the first six months but fell after that. Median (interquartile range [IQR]) compliance for daily questionnaire entry was 66.6% (31.0– 91.8) and 81.0% (45.3– 94.3) for weekly CAT entry. Positive participant responses to the exit questionnaire were highest regarding YaDoc ease of use (83.8%), positive impact on managing health (58.8%), and overall satisfaction (53.8%). Of the 26 physicians and facility staff enrolled, 24 completed the study. Of these, the majority (66.7%) responded positively regarding app facilitation of communication between physicians and participants to manage disease. Compliance was similar before and after the first COVID-19 state of emergency in Japan.
Conclusion: Daily telemedicine monitoring is potentially feasible and acceptable to both patients and physicians in the management of COPD. These results may inform potential use of telemedicine in clinical practice and design of future studies.
Clinical Trial Registration: JapicCTI-194916.
Keywords: Japan, patient-reported outcome measure, feasibility, acceptability, telemonitoring, smartphone
Plain Language Summary
Why was this study done?
- In Japan, not all patients with chronic obstructive pulmonary disease (COPD) receive treatment and many do not inform their physicians when they experience an exacerbation.
- Technology including smartphone apps (telemedicine) can allow patients and physicians to communicate disease information at a distance.
- The goal of this study was to assess the feasibility of using telemedicine for Japanese patients with COPD and their physicians to monitor symptoms, and whether this was a satisfactory experience for patients and physicians and facility staff.
What did the researchers do/find?
- In total, 84 participants aged 40 years or older with COPD were trained to use the telemedicine platform YaDoc on their smartphone. Of these, 72 participants completed the one-year study.
- Using the platform, participants completed seven daily questions, regarding COPD symptoms, a median of 67% of the days required. Additionally, they completed the 8-question COPD Assessment Test (CAT) weekly a median of 81% of the weeks requested.
- After one year, 84% of participants rated the platform as easy to use, 59% agreed it had a positive impact on managing their condition, and 54% were satisfied with the platform experience.
- Of the 26 physicians and facility staff who used the platform, 79% intended to use telemedicine in the future to manage patients, 67% agreed that telemedicine simplified patient care, and 67% agreed the platform facilitated communication between physicians and patients.
What do these results mean?
- These results suggest telemedicine is feasible and acceptable to patients and physicians in aiding the management of COPD.
Introduction
Chronic obstructive pulmonary disease (COPD), characterized by airflow limitation and persistent respiratory symptoms is a major cause of morbidity and mortality worldwide.1 As outlined in the 2022 Global initiative for chronic Obstructive Lung Disease (GOLD) strategy document, appropriate COPD treatment is important for achieving disease management goals,1 including reducing symptoms and the risk of exacerbations, and improving health status and exercise tolerance to slow disease progression and reduce mortality.1,2 Frequent exacerbations accelerate disease progression and lung function decline, and are associated with worse quality of life and reduced exercise ability.3 COPD exacerbations in Japan appear to be underreported, with the UPLIFT trial indicating the annual rate of exacerbations per patient is 0.61 compared with 0.85 worldwide.4–6 In fact, previous research has found rates of unreported COPD exacerbations to be as much as five-fold those of reported COPD exacerbations.7
The use of telemedicine for COPD disease management, which includes monitoring of symptoms, may improve the detection of symptom worsening and facilitate identification of exacerbations, resulting in better management and improved outcomes.8 A meta-analysis of three studies (all judged to be at high risk of bias) in Western countries suggested that telemedicine interventions aimed at facilitating, supporting, and sustaining self-management in patients with COPD delivered via smart technology may improve health-related quality of life and levels of activity up to six months, although it is unknown whether improvements are sustained over a longer duration.9
In Japan, the use of remote medical consultation services via online devices such as smartphones has increased following recent deregulation of telemedicine, with the Japanese Ministry of Health, Labour and Welfare (MHLW) published guidance on telemedicine in 2018.10 Significant increases in the use of telemedicine during the COVID-19 pandemic compared with before have been observed in the Japanese general population, including among individuals ≥40 years of age (eg 40‒49 years adjusted rates: 1.8% vs 4.1%), and to a larger extent among younger individuals 18–29 years of age (4.3% vs 10.2%).11 Furthermore, the need for telemedicine has increased during the COVID-19 pandemic among patients with COPD who are at an increased risk of hospitalization for COVID-19.1 The feasibility and acceptability of telemedicine in patients with COPD in Japan requires further investigation, particularly since Japanese patients typically report low scores on measures such as the COPD Assessment Test (CAT) and St George’s Respiratory Questionnaire,12 as well as low reporting of exacerbations in Japan.7 Therefore, specific attention to address this clinical need is warranted. The objective of this study was to assess the feasibility and acceptability, according to compliance and satisfaction, respectively, of the telemedicine platform YaDoc for participants with COPD and their physicians/facility staff in Japan.
Materials and Methods
Design
This was a 52-week multicenter, prospective, single-arm, feasibility cohort study assessing an intervention with a telemedicine platform (GSK study JapicCTI-194916). At the screening visit, potential participants gave informed consent, were screened by the inclusion and exclusion criteria outlined below, and (if these steps were completed successfully) completed an initial entry questionnaire (Supplementary Table 1). Following this, participants underwent training by a physician or facility staff member on the use of the telemedicine platform, YaDoc. This platform provides telemonitoring via a smartphone application installed on the participant’s phone. The application includes a daily log of symptoms including quality of sleep, dyspnea, and fatigue (Supplementary Table 2), online inquiry function, electronic patient-reported outcome (ePRO), including weekly CAT questions, and telecommunication.13 Participants were followed for 52 weeks, and completed an interim questionnaire 24 weeks after study initiation and an exit questionnaire at Week 52. Physicians and facility staff enrolled in the study completed an interim questionnaire 24–30 weeks after participant study initiation and an exit questionnaire at Week 52.
The study was conducted in accordance with the provisions of the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines and received approval from local institutional review boards and independent ethics committees (Supplementary Table 3). Integrity Healthcare Co. Ltd. provide YaDoc, and were responsible for extracting and deidentifying YaDoc data for analyses in accordance with the Personal Information Protection Act of Japan.14 Patient IDs were replaced with study participant ID using a match-up table to generate data in accordance with the data set specification.
Population
Eligible male and female participants were ≥40 years of age at the time of signing the informed consent form with an established clinical history of COPD, according to the American Thoracic Society/European Respiratory Society, which includes symptoms of cough, sputum production, or dyspnea, or history of exposure to risk factors for the disease, and a post-bronchodilator ratio of forced expiratory volume in one second/forced vital capacity ≤0.7.15 The Japanese Respiratory Society has quite strict criteria for diagnosing asthma COPD overlap (ACO),16 but as a large proportion of patients in Japan are diagnosed with ACO on the basis of clinical features of both asthma and COPD,17 patients with a physician’s diagnosis of ACO were permitted to enroll. Eligible participants were included if they had a lung function test result recorded within the prior 12 months to the study, and were able to provide signed informed consent. Additionally, eligible participants were required to be receiving maintenance therapy including inhaled long-acting muscarinic antagonist or long-acting β2-agonist, at screening. Long-acting muscarinic antagonist/ long-acting β2-agonist or inhaled corticosteroid/long-acting bronchodilator combinations were also permitted, as were short-acting bronchodilators, systemic steroids, or antibiotics. Participants were excluded if they were unable or unwilling to use the required telemonitoring device/system, if they were receiving inhaled triple therapy in the three months prior to screening (inclusive), or they received any biologic therapy within six months prior to screening. Women with a current or planned pregnancy or individuals concurrently participating in another clinical trial that involved exposure to an investigational or a non-investigational pharmaceutical product were also excluded. Included physicians and facility staff were selected based on prior involvement in telemonitoring, defined as someone who is regularly monitoring a participant status using the YaDoc platform and interacting with a participant directly by clinical practice, phone or chat.
Endpoints
The primary endpoint was compliance to the platform, based on the proportion of participants who completed the study and the frequency of completion of daily questions and weekly CAT questions over the 52-week study period. The secondary endpoint was the proportion of participants and physicians/facility staff providing positive answers to a questionnaire on their experience of the platform at Week 52 (study end). We focus on a subset of questionnaire items (seven items) that show the responses regarding whether the telemonitoring platform is supportive for aiding communication between patients and doctors, and general questions about the usefulness of the telemedicine platform. An exploratory endpoint, added after study commencement, was the impact of the state of emergency declared for the COVID-19 pandemic in Japan on data entry during the four and eight weeks immediately before and after the pandemic.
Sample Size and Statistical Analysis
An asymptotic method was used to calculate the number of participants required to complete the study to obtain the proportion estimates with a margin of error of less than 10% at 95% confidence level, where the margin of error is half of the confidence interval width. A sample size of 100 was found to achieve a margin of error of 9.8%. Participant demographics and baseline characteristics were collected. For the primary endpoint, data entry compliance was summarized descriptively and in subgroups of participants by sex (male/female), age (<69.5/≥69.5 years), GOLD stage (I, II, ≥III), GOLD group (A, B, C, D), history of exacerbations (yes/no), and history of allergy (yes/no). Kaplan–Meier plots were used to present survival curves for entry completion, with an event defined as the time to the last data entry date of the participant in which data was entered at least once. Spearman’s rank correlation coefficient was performed to assess non-parametric correlations between the compliance in data entry for daily questions measuring symptoms, and the weekly CAT for the following assessment periods: whole study (one year), the first six months, and the second six months. For analyses using weekly CAT scores, the following assessment windows were used: Analysis week (week relative to participant start date) = integer part of ([Study Day - 1] / 7 + 1); Real week (week relative to study start) = integer part of ([Index + Study Day - 1] / 7), where Index = (date of entry for each participant) – (date of entry for the first participant). The secondary endpoint was summarized descriptively. For the exploratory endpoint, the first state of emergency due to the COVID-19 pandemic was declared in Japan approximately mid-way through the study. Since there was a trend over the study for a drop in reporting, a post hoc analysis was carried out to examine the periods four and eight weeks either side of the declaration of the state of emergency and is summarized descriptively.
Results
Participant and Physician/Facility Staff Population
The study was initiated on September 26, 2019, and was completed on June 21, 2021. The first state of emergency in Japan for the COVID-19 pandemic was declared on April 7, 2020, and lasted until May 25, 2020.18 In total, 84 participants with COPD or ACO enrolled in the study (Figure 1A). The mean (SD) participant age was 68.7 (9.17) years and the majority (n=74; 88%) of participants were male (Table 1). Overall, the participants had mild disease, with the majority having GOLD grade I COPD1 (n=33; 40%) or GOLD grade II COPD (n=38; 46%).
 |
Table 1 Baseline Patient Demographics and Clinical Characteristics |
 |
Figure 1 Study eligibility flow chart for (A) participants and (B) physicians and facility staff. |
In total, 26 physicians and facility staff were enrolled (Figure 1B), including 21 medical doctors, the majority of whom were pulmonologists and five site coordinators (which included clinical healthcare professionals and administrative employees at the facilities). Of these, two did not complete the study due to changes in roles and patient responsibilities or transferring between hospitals during the study.
Feasibility
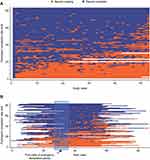
Over the one-year period, the median completion rate for the daily symptoms was 66.6% (interquartile range [IQR] 31.0, 91.8) and for weekly CAT it was 81.0% (45.3, 94.3) (Supplementary Table 4). Overall, 72 of the 84 (86.0%) participants enrolled completed the study, in these the compliance rate for daily symptoms was 75.5% (31.4, 92.6) and for weekly CAT it was 83.0% (49.1, 94.3) (Supplementary Table 4). The survival curves for completion are shown in Figure 2A and B. Over the first six months the rate for both daily and weekly reporting was ≥90% but fell thereafter. The pattern of missing data for the weekly recording is further illustrated at an individual participant level in Figure 3A and B. These suggest that the participants appear to fall into three groups of approximately equal size. One group had very low reporting throughout the study. A second started with a generally high level of reporting that, in some participants, tended to drop in the last few weeks of the study, the third group showed a consistently high level of reporting.
There were some slight differences in participant age and sex subgroups in the compliance of daily questionnaire or weekly CAT entry, but no apparent differences were observed between other participant subgroups including GOLD grade, GOLD group, exacerbation history and allergic medical history (Supplementary Table 5).
A comparison of daily symptom and weekly CAT completion rates is shown in Figure 4A–C. There was only a moderate correlation between them, whether over the first six months or the second six months (rho=0.510 and 0.676, respectively).
Acceptability
The proportion of participants with positive responses to the platform in their exit questionnaire ranged from 22.5% to 83.8% across the seven questions of focus (Figure 5). The statement with the highest proportion of positive responses was “How would you rate the application’s ease of use?”. The question with the highest proportion of participants with negative responses was “Do you think that your family members have benefited from the telemedicine system?”. A summary of responses to the full participant exit questionnaire can be seen in Supplementary Table 6.
 |
Figure 5 Participant acceptability of the YaDoc platform according to their exit questionnairesa. an=80 for each question. |
The proportion of physicians and facility staff with positive responses to questions included in the exit questionnaire ranged from 58.3% to 79.2% (Figure 6). The statement with the highest proportion of positive responses was “I intend to use telemonitoring when necessary to provide healthcare to my patients?”. The highest proportion of physicians and facility staff with negative responses was to “I found telemonitoring easy to use?”. A summary of responses to the full physician/facility staff exit questionnaire can be seen in Supplementary Table 7.
 |
Figure 6 Physician and facility staff acceptability of the YaDoc platform according to their exit questionnairesa. an=24 for each question/statement. |
Impact of the COVID-19 Pandemic
Examining the periods four and eight weeks either side of the declaration of the state of emergency suggests that there was no short-term effect of the state of emergency on reporting, whether weekly or daily (Table 2).
 |
Table 2 Compliance with YaDoc and Medical Resource Use Four and Eight Weeks Before and After the First State of Emergency in Japan for COVID-19a |
Discussion
This study has shown that telemonitoring with the YaDoc platform for patients with COPD is feasible for use in routine COPD care. Over the one-year study period, median compliance with the daily questionnaire was approximately 67%, with the weekly CAT questionnaire having a higher (81%) rate. This suggests that compliance to a weekly diary is greater than to a daily diary. Over the first six months, median compliance with both the weekly CAT and daily questionnaires was very high at or above 90%, but from six months onwards reporting fell progressively. This is consistent with a previous telemedicine study that demonstrated compliance waning over time from 85% usage in the first week to 40% by Week 12,19 although the completion rate in that study was lower at 12 weeks than we observed at one year. Direct comparisons with other telemedicine studies are not possible because the nature of the diary and duration of the study differ.20–22 Overall, this decrease in compliance over time suggests a need for coaching to ensure good adherence, an approach that has been demonstrated beneficial in improving COPD medication adherence.23
It is noteworthy that three broad groups of participants could be seen in the completion rates. Approximately one-third had low completion rates throughout the study, but the remaining two-thirds had a high completion rate, that only showed a significant fall after about nine months in one-third of participants. There was only a modest correlation between daily and weekly diary reporting, which suggests that participants do not necessarily fall into two distinct groups of “high reporters” and “low reporters”, similarly, there were also no demographic factors that were clearly associated with compliance. These findings make it difficult to speculate about reasons for differences in levels of compliance, however, it is noteworthy that the state of emergency declaration due to COVID-19 demonstrated that external factors that had a significant impact on face-to-face interaction between patients and physicians did not appear to alter data entry.
Ease of use of the platform was rated highly. Previous studies have indicated that perceived ease of use is one of the main determinants of telemedicine compliance, with perceived usefulness also a contributing factor.24–27 It is not possible to make direct comparisons of ease of use to other studies since this was either not assessed or not assessed in the same way. Acceptance of the platform was relatively high and satisfactory according to participant and physician/facility staff exit questionnaires, as seen in previous studies examining telemedicine in COPD.28,29 Overall, approximately half of participants were satisfied with the platform, with over 80% finding it easy to use and nearly 60% suggesting the platform had a positive impact on the management of their health. Interestingly, despite an overall high proportion of participants being satisfied with the platform, half of participants provided a negative response to their family members benefiting from the platform. The high ease of use rating for the platform may reflect perceptions of, and willingness to use, telemedicine among participants and their family members.
Similar to satisfaction results for participants, over two-thirds of physicians and facility staff also found that the platform was useful and simplified patient care, and facilitated patient-physician communication, factors that a previous study has identified as key to physician satisfaction.26 However, whilst the number of participants finding the platform useful in communicating with their physicians was acceptable overall (48% were neutral), 26% of participants provided a negative response to this question. This may be due to a lack of teleconsultations to allow direct communication between patients and physicians, or of participants seeing it as a replacement for face-to-face appointments. If this is the case, it again points to the importance of care and communication when implementing telemedicine, particularly as telemedicine itself poses many challenges to effective communication.30 This finding may also simply reflect patient ambivalence, with many patients responding neutrally, or a preference for face-to-face communication, which may be a barrier to broader implementation.
There are several strengths and limitations of this study, which should be considered when interpreting and generalizing results. This is a real-world observational study assessing the practice of telemedicine for patients with COPD. The findings from this study may directly inform routine clinical practice around the use of telemedicine in patient monitoring in Japan and also aid the design of future studies, by demonstrating the feasibility of telemedicine for monitoring weekly CAT score changes in clinical trials, therefore providing important information on patient-reported outcomes. We included a study population that was typical of Japanese patients with COPD and therefore did not restrict participation to specific subgroups of patients such as those with frequent exacerbations who may not be typical of the broader COPD population in this country. Exacerbation reporting is low in Japan, particularly when compared with Western countries,4–7 however this study has demonstrated the feasibility of telemedicine to monitor symptoms and may therefore lead to improved identification of COPD exacerbations in Japan.
Limitations include the relatively small sample size (n=84), which was lower than the target sample size of 100 that was calculated as the number required to provide proportion estimates with a margin of error of less than 10% at 95% confidence level. However, this sample size is larger than others of its kind31,32 and large enough to identify three subgroups of participants with some confidence. Additionally, participants were predominantly male and former smokers so may not be fully representative of the Japanese COPD population. Participants and physicians/facility staff were also only selected from six sites where the platform was implemented, although it should be noted that the included sites covered a diverse area and rural/urban locations nationwide. In addition, as physicians and facility staff were included from sites where the platform was implemented, there may have been a selection bias, meaning positive responses to the exit questionnaire were more likely given the physicians and facility staff were already choosing to use the platform in their clinical practice. It is also important to note a risk of bias as some authors were included in the physician and facility staff cohort group. However, their involvement as authors commenced only after study completion. Furthermore, the participants included in this study predominantly had GOLD Grade I/II COPD and were GOLD Group A, with fewer than 10% experiencing an exacerbation requiring a hospitalization or hospitalization visit in the previous year and single-inhaler triple therapy (SITT) users excluded; results may be different for patients with more severe disease and SITT users specifically. Additionally, elderly patients who are not accustomed to using mobile phone applications, or those without smartphones, may have been less willing to participate in this study, causing a selection bias towards participants who have access to and ability to use both a smartphone and the mobile phone application. Thus, this study may have enrolled a population of early adopters of technology rather than a representative sample of all Japanese patients with COPD. However, it is important to note that a proportion of participants enrolled in this study were aged 75 years or older (n=21), therefore telemedicine may be useful in this demographic group. Telemedicine may be further developed by increases in smartphone usage over time. Finally, as the objective of this study was to determine the feasibility and acceptability of telemedicine, the questionnaires used to assess patient health were not validated or evaluated as appropriate for telemedicine; further studies are required to determine this.
Conclusion
These results demonstrate the potential feasibility of a telemedicine platform in routine clinical management of COPD in Japan, and acceptability to both patients and physicians. However, coaching may be required to improve long-term compliance, and further research should be conducted with physicians who are not currently implementing telemedicine in their clinical practice. Results from this study may inform the potential use of telemedicine in clinical practice and the design of future studies aiming to evaluate disease outcomes using a telemedicine approach.
Abbreviations
ACO, asthma-COPD overlap; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; GOLD, Global initiative for Chronic Obstructive Lung Disease; SD, standard deviation.
Data Sharing Statement
The corresponding author had full access to all study data and the final responsibility to submit for publication. Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Ethical Considerations
The study was conducted in accordance with applicable local regulations, and the principles stated in the Declaration of Helsinki. All study documents were reviewed and approved by institutional review boards and/or independent ethics committee(s) at all investigational sites. All patients provided written, informed consent prior to the performance of any study-related procedures.
Consent for Publication
All authors have provided final approval of the published version of the manuscript.
Acknowledgments
Editorial support in the form of preparation of the first draft based on input from all authors, and collation and incorporation of author feedback to develop subsequent drafts, was provided by Alexandra Berry, PhD, at Fishawack Indicia Ltd., UK, part of Fishawack Health, and was funded by GSK. We thank the patients and physicians and facility staff in Japan who participated in this study.
Author Contributions
Takeo Ishii, Paul Jones, Bruce E. Miller, Taizo Matsuki, Yoko Shibata, Takanobu Nishi, Ruth Tal-Singer and Ryan Tomlinson contributed to the conception and design of the study. Masahiro Shinoda, Osamu Hataji, Motohiko Miura, Masaharu Kinoshita, Akira Mizoo and Kazunori Tobino contributed to the acquisition of data. Toru Soutome, Takeo Ishii, Paul Jones, Taizo Matsuki, Yoko Shibata and Takanobu Nishi contributed to the data analysis and interpretation. All authors took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was funded by GSK (GSK Study 212648/HO-19-19947). The funder of the study had a role in study design, data analysis, data interpretation, and writing of the report. Integrity Healthcare Co. Ltd. provided YaDoc, and were responsible for extracting and deidentifying YaDoc data, replacing the patient ID with study participant ID using a match-up table to generate data in accordance with the data set specification.
Disclosure
No authors received payment for the development of the manuscript. Masahiro Shinoda, Osamu Hataji, Motohiko Miura, Masaharu Kinoshita, Akira Mizoo and Kazunori Tobino report having received grants from the GSK group of companies for the conduct of this study. Osamu Hataji also reports personal fees from AstraZeneca, Novartis Pharma, and Boehringer Ingelheim for research funding consulting fee, outside the submitted work. Yoko Shibata reports having received personal fees from the GSK group of companies during the conduct of the study, and lecture fees from AstraZeneca, Novartis and Boehringer Ingelheim. Takanobu Nishi is an employee of GSK. Takeo Ishii, Ryan Tomlinson, Taizo Matsuki and Paul W Jones are employees of GSK and hold stocks/shares. Bruce E. Miller, Toru Soutome and Ruth Tal-Singer are former employees at the time of the study and shareholders of GSK. Ruth Tal-Singer is a Board member of ENA Respiratory and holds stock options from ENA Respiratory, she reports honorarium for conference talks from AAAAI and ATS, travel reimbursement from AAAAI, and personal fees from Immunomet, VOCALIS Health, ENA Respiratory, and Teva until January 2021. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (2022 report); 2022. Available from: https://goldcopd.org/.
2. Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res. 2017;18(1):67. doi:10.1186/s12931-017-0548-3
3. Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. 2010;19(116):113–118. doi:10.1183/09059180.00002610
4. Ishii T, Nishimura M, Akimoto A, James MH, Jones P. Understanding low COPD exacerbation rates in Japan: a review and comparison with other countries. Int J Chron Obstruct Pulmon Dis. 2018;13:3459–3471. doi:10.2147/COPD.S165187
5. Tamaki K, Sakihara E, Miyata H, et al. Utility of Self-administered questionnaires for identifying individuals at risk of COPD in Japan: the OCEAN (Okinawa COPD casE finding AssessmeNt) study. Int J Chron Obstruct Pulmon Dis. 2021;16:1771–1782. doi:10.2147/COPD.S302259
6. Fukuchi Y, Fernandez L, Kuo HP, et al. Efficacy of tiotropium in COPD patients from Asia: a subgroup analysis from the UPLIFT trial. Respirology. 2011;16(5):825–835. doi:10.1111/j.1440-1843.2011.01982.x
7. Betsuyaku T, Kato M, Fujimoto K, et al. A randomized trial of symptom-based management in Japanese patients with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:2409–2423. doi:10.2147/COPD.S152723
8. Donner CF, ZuWallack R, Nici L. The role of telemedicine in extending and enhancing medical management of the patient with chronic obstructive pulmonary disease. Medicina. 2021;57(7):726. doi:10.3390/medicina57070726
9. McCabe C, McCann M, Brady AM. Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;5:Cd011425. doi:10.1002/14651858.CD011425.pub2
10. Ministry of Health LaW, Japan. Guidance for defined the procedure and requirement for telemedicine (Japanese). Available from: https://www.mhlw.go.jp/file/05-Shingikai-10801000-Iseikyoku-Soumuka/0000201789.pdf2018.
11. Miyawaki A, Tabuchi T, Ong MK, Tsugawa Y. Age and social disparities in the use of telemedicine during the COVID-19 pandemic in Japan: cross-sectional study. J Med Internet Res. 2021;23(7):e27982. doi:10.2196/27982
12. Kato M, Tomii K, Hashimoto K, et al. The IMPACT study - single inhaler triple therapy (FF/UMEC/VI) versus FF/VI and UMEC/VI in patients with COPD: efficacy and safety in a Japanese population. Int J Chron Obstruct Pulmon Dis. 2019;14:2849–2861. doi:10.2147/COPD.S226601
13. Integrity Healthcare Co. Ltd. The YaDoc telemonitoring platform (Japanese); 2022. Available from: https://www.yadoc.jp/about/feature.
14. Personal Information Protection Commission Japan. Amended act of the protection of personal information; 2016.
15. Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi:10.1183/09031936.04.00014304
16. Tamaoki A. Diagnosis and Treatment for ACO. Intern Med. 2018;107(9):1852–1857.
17. Yamamura K, Hara J, Kobayashi T, et al. The prevalence and clinical features of asthma-COPD overlap (ACO) definitively diagnosed according to the Japanese Respiratory Society Guidelines for the Management of ACO. J Medical Invest. 2019;66(1.2):157–164. doi:10.2152/jmi.66.157
18. Government of Japan. [COVID-19] the declaration of the lifting of the state of emergency in response to the novel coronavirus disease; 2020. Available from: https://japan.kantei.go.jp/ongoingtopics/_00027.html.
19. North M, Bourne S, Green B, et al. A randomised controlled feasibility trial of E-health application supported care vs usual care after exacerbation of COPD: the RESCUE trial. Npj Digital Med. 2020;3(1):145. doi:10.1038/s41746-020-00347-7
20. Cordova FC, Ciccolella D, Grabianowski C, et al. A telemedicine-based intervention reduces the frequency and severity of COPD exacerbation symptoms: a randomized, controlled trial. Telemed J E Health. 2016;22(2):114–122. doi:10.1089/tmj.2015.0035
21. Smith HS, Criner AJ, Fehrle D, Grabianowski CL, Jacobs MR, Criner GJ. Use of a smartphone/tablet-based bidirectional telemedicine disease management program facilitates early detection and treatment of COPD exacerbation symptoms. Telemed J E Health. 2016;22(5):395–399. doi:10.1089/tmj.2015.0135
22. Rassouli F, Baty F, Stolz D, et al. Longitudinal change of COPD assessment test (CAT) in a telehealthcare cohort is associated with exacerbation risk. Int J Chron Obstruct Pulmon Dis. 2017;12:3103–3109. doi:10.2147/copd.s141646
23. Willard-Grace R, Chirinos C, Wolf J, et al. Lay health coaching to increase appropriate inhaler use in COPD: a randomized controlled trial. Ann Fam Med. 2020;18(1):5–14. doi:10.1370/afm.2461
24. Almathami HKY, Win KT, Vlahu-Gjorgievska E. Barriers and facilitators that influence telemedicine-based, real-time, online consultation at patients’ homes: systematic literature review. J Med Internet Res. 2020;22(2):e16407. doi:10.2196/16407
25. Shahrabani S, Mizrachi Y. Factors affecting compliance with use of online healthcare services among adults in Israel. Isr J Health Policy Res. 2016;5:15. doi:10.1186/s13584-016-0073-8
26. Cook EJ, Randhawa G, Sharp C, et al. Exploring the factors that influence the decision to adopt and engage with an integrated assistive telehealth and telecare service in Cambridgeshire, UK: a nested qualitative study of patient ‘u/sers’ and ‘non-users’. BMC Health Serv Res. 2016;16:137. doi:10.1186/s12913-016-1379-5
27. Rho MJ, Choi IY, Lee J. Predictive factors of telemedicine service acceptance and behavioral intention of physicians. Int J Med Inform. 2014;83(8):559–571. doi:10.1016/j.ijmedinf.2014.05.005
28. Soriano JB, García-Río F, Vázquez-Espinosa E, et al. A multicentre, randomized controlled trial of telehealth for the management of COPD. Respir Med. 2018;144:74–81. doi:10.1016/j.rmed.2018.10.008
29. Mínguez Clemente P, Pascual-Carrasco M, Mata Hernández C, et al. Follow-up with telemedicine in early discharge for COPD exacerbations: randomized clinical trial (TELEMEDCOPD-trial). COPD. 2021;18(1):62–69. doi:10.1080/15412555.2020.1857717
30. Coleman C. Health literacy and clear communication best practices for telemedicine. Health Lit Res Pract. 2020;4(4):e224–e229. doi:10.3928/24748307-20200924-01
31. Tabak M, Brusse-Keizer M, van der Valk P, Hermens H, Vollenbroek-Hutten M. A telehealth program for self-management of COPD exacerbations and promotion of an active lifestyle: a pilot randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2014;9:935. doi:10.2147/COPD.S60179
32. Calvo GS, Gómez-Suárez C, Soriano J, et al. A home telehealth program for patients with severe COPD: the PROMETE study. Respir Med. 2014;108(3):453–462. doi:10.1016/j.rmed.2013.12.003
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.