Back to Journals » Infection and Drug Resistance » Volume 17
A Systematic Review of the Microbial Landscape of Diabetic Foot Ulcers in Uganda
Authors Makeri D , Eilu E , Odoki M , Agwu E
Received 27 October 2023
Accepted for publication 10 January 2024
Published 13 January 2024 Volume 2024:17 Pages 143—151
DOI https://doi.org/10.2147/IDR.S446838
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Danladi Makeri,1 Emmanuel Eilu,1 Martin Odoki,1– 3 Ezera Agwu1,4
1Department of Microbiology and Immunology, Faculty of Biomedical Sciences, Kampala International University-Western Campus, Ishaka, Uganda; 2Department of Microbiology and Immunology, School of Medicine, King Ceasor University, Kampala, Uganda; 3Department of Applied Sciences, School of Sciences, Nkumba University, Entebbe, Uganda; 4Department of Microbiology and Parasitology, College of Medicine and Health Sciences, University of Rwanda, Kigali, Rwanda
Correspondence: Danladi Makeri, Email [email protected]
Background: Diabetes is a growing health concern globally. Poorly managed diabetes may result in diabetic foot ulcers (DFU), which can become a source of chronic infection known as diabetic foot infections. The increasing trend of diabetes in Uganda speaks to the potential for diabetic foot ulcers which may eventually become infected and their attendant impact on the quality of life of diabetic patients. This review assesses the microbial diversity of DFUs in Uganda, aiming to guide treatment and identify research gaps.
Main Body of the Abstract: We searched PubMed, Scopus and Embase for studies conducted in Uganda that reported isolating microorganisms from diabetic foot ulcers. Following the preferred reporting items for systematic reviews and meta-analysis (PRISMA), we included two eligible studies that reported isolating 122 bacteria spread across eleven (11) species using swab samples and conventional culture methods. Significant isolates included World Health Organization priority pathogens including: Enterobacter specie, Staphylococcus aureus, Klebsiella pneumoniae, and Acinetobacter specie. Methicillin resistant Staphylococcus aureus (MRSA) constituted 33.3% of Staphylococci species and 26% of all bacterial isolates while extended-spectrum beta-lactamase producing Escherichia coli and Klebsiella specie constituted 14.29% of total microbial isolates. Most bacteria showed susceptibility to Imipenem, Vancomycin, Ciprofloxacin, and Clindamycin, but resistance to Cotrimoxazole and Ampicillin was noted.
Short Conclusion: We conclude that data on the microbiology of DFUs in Uganda is scarce; however, the bioburden of DFUs in the country is similar to those in other parts of the world, and MRSA poses a challenge to antibiotic therapy. Consequently, the continued use of swab samples and conventional culture and sensitivity methods may limit the isolation, identification, and presentation of other important isolates. We recommend characterization of bacterial isolates to better understand their genetic makeup, and the development of a national guideline for managing diabetic foot infections.
Keywords: diabetic foot ulcers, foot infections, Uganda, wound bacteria, foot complication
Background
Diabetes is a global health issue that affects an estimated 463 million individuals worldwide, with projections indicating a staggering rise to 700 million by 2045.1 This escalating prevalence is not confined to high-income nations but extends to low- and middle-income countries among which Uganda finds itself.2 The nation grapples with a significant burden of diabetes, a condition that significantly impacts the quality of life of affected persons.3,4 However, the challenges posed by diabetes are not limited to the disease itself; its complications, including neuropathy, cardiovascular disease, stroke, retinopathy, and particularly foot complications, compound the already complex landscape of diabetes-related health issues.
Among these complications, diabetic foot ulcers (DFUs) represent a common and perilous manifestation. Left unaddressed, they can lead to severe consequences, including amputation, morbidity, and even mortality.5 It is estimated that 25% of diabetic patients will, at some point in their lives, struggle with foot ulcers (DFUs).6 In Uganda, the occurrence of diabetic foot complications was initially reported in 1996, with a prevalence rate of 4.0%.7 Since that time, the incidence of DFUs in the country has been on the increase.4 A notable study by Bateganya et al,8 even reported that, at one point, diabetic foot ulcers constituted 4.2% of the predominant causes of hospitalizations among people with diabetes in Uganda. The gravity of this issue becomes evident when these foot ulcers become clinically infected. Such infections are often polymicrobial in nature, rendering them challenging to treat effectively.9 Infected diabetic foot ulcers (IDFU) have been reported to account for a substantial number of lower limb amputations in Uganda.4,10,11
Remarkably, Uganda has recorded the highest prevalence rate of diabetic foot ulcers in Africa, standing at 53.9%.12 Yet, despite the substantial prevalence and the heightened risk of clinical infection,13 surprisingly little is known about the microbiology of these ulcers in the Ugandan context. This knowledge gap is further compounded by the absence of critical evaluations regarding the microbial composition and antibiotic susceptibility patterns of diabetic foot ulcers (DFUs) in the country and underscores the need for this study to systematically conduct a comprehensive assessment of the microbiology of DFUs in Uganda.
Methods
Search Strategy
We searched PubMed, Scopus and Embase databases in April, 2023, to identify studies on diabetic foot ulcers conducted in Uganda. The search was not restricted to any study type or year of publication and was executed using the algorithm presented in Table 1. No protocol was registered.
 |
Table 1 Search Query |
Study Selection Criteria
The search yielded fifty-three bibliographies cumulatively from Scopus, PubMed and Embase which we extracted as comma separated value (CSV) files, saved, and merged. Duplicates were removed, and the remaining documents were sought for retrieval and screened for eligibility. Only studies which were conducted in Uganda and reported isolating microorganisms from foot ulcers of patients with diabetes were included. Two authors, DM and EA, carried out independent and overlapping screening of titles and abstracts to establish eligibility. After the screening process, two studies met the inclusion criteria and were included in the review (Figure 1).
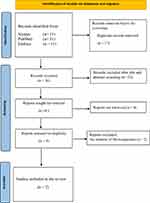 |
Figure 1 Study Selection Flowchart. Notes: PRISMA figure adapted from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. Creative Commons.14 |
Data Extraction
In unison, authors MD, EE, MO and EA extracted and classified the following information: author name, year of publication, study design, number of participants, type of diabetes, sample collected, patient setting, isolation method, type of isolate, number of isolates, list of isolates and antibiotics used.
Statistical Analysis
Data analysis was descriptive. The demographic and clinical data were summarized as percentages. Also, the pooled prevalence of the isolates was estimated. Descriptive analysis was done using Microsoft Excel, (2019) while the pool prevalence of the bacterial isolates was estimated using MedCalc® Statistical Software version 22.006.
Results
Study Selection and Characteristics
The included studies were conducted between 2010 and 2021 and published in 2011 and 2022, respectively. The two studies collectively recruited 122 inpatients, of which more than half (n = 77) were female. Regarding their educational background, 46.7% (n = 57) had primary education, 17.2% had no formal education, and 11.5% (n = 14) possessed post-secondary education. In terms of diabetes classification, one study did not specify the type, whereas the other included participants with either type 1 or 2 diabetes. Methodologically, both studies utilized wound swab samples and culture methods for microbial isolation and identification. The study designs were a prospective cohort and a prospective observational study.
Microbiological Profile of Diabetic Foot Ulcers in Uganda
The studies by Pario et al15 and Sikhondze et al16 are the only studies that explored the microbiology of diabetic foot ulcers in Uganda, and they reported isolating only bacterial species. The studies isolated a total of 122 bacteria including World Health Organization ascribed priority pathogens: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter specie (ESKAPE) were reportedly isolated. Gram-negative bacteria constituted 75% of the total isolates and the rest were gram positives. Also, 14.29% of the isolates were extended beta lactamase producers (ESBL) while 4.92% were methicillin resistant Staphylococcus aureus.
Antibiotic Regimen for DFU in Uganda
As with most diabetic foot ulcers across the globe, and continent, DFU infections in Uganda are managed in part with antibiotic chemotherapy. The commonly used antibiotics in Uganda includes: Cefoxitin Erythromycin, Co-trimoxazole, Gentamycin, Chloramphenicol, Ciprofloxacin, Clindamycin, Vancomycin, Ceftriaxone, Penicillin, Ampicillin, and Gentamicin. Ampicillin, Ceftriaxone, Augmentin, Cefuroxime, Co-trimoxazole, Ceftazidime, Chloramphenicol, Ciprofloxacin, Imipenem, Piperacillin/Tazobactam, Cefepime, Piperacillin and Amikacin.15,16
The dominant species isolated from diabetic foot ulcers in Uganda include Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa. Remarkably, these isolates exhibited varying susceptibilities and resistance to the commonly used antibiotics. Figure 2 presents different antibiotics and shows the number of dominant bacterial species that are susceptible and resistant to each antibiotic. The figure is useful for understanding which antibiotics are effective against specific bacteria found in DFUs in Uganda, informing treatment choices. We observed that Staphylococcus aureus, the dominant gram-positive bacteria, exhibited significant resistance to Cefoxitin, Erythromycin and Cotrimoxazole (33.3%, 38.8% and 55.5%), respectively. However, all MRSA (n = 6) were 100% susceptible to Vancomycin. Equally, S. aureus showed significant susceptibility to Chloramphenicol (44.4%) and Ciprofloxacin (77.7%). The dominant gram-negative bacteria also exhibited varying level of resistance. Pseudomonas aeruginosa was resistant to Gentamicin (65.2%), Ceftazidime (60.9%), Piperacillin/Tazobactam (78.3%), Cefepime (73.9%) and Amikacin (60.9%). E. coli, exhibited 56.5%, 47.8%, 82.6%, and 100% resistance to Gentamicin, Ceftazidime, Augmentin and Ampicillin, respectively. Klebsiella spp., the dominant gram negative and overall most abundant isolate was resistant Gentamicin (69.2%), Ceftazidime (84.6%), Augmentin (88.5%), Ciprofloxacin (73%), and Ceftriaxone (76.9%); remarkably all dominant gram-negative bacteria were significantly susceptible to Imipenem.
Discussions
The occurrence of diabetic foot complications in Uganda was first reported in 1996, with a prevalence rate of 4.0%.7 Since then, the prevalence of DFU in the country has increased significantly.4 Although the existing studies shade light to the existence of DFU and its subsequent infection, it is essential to note that the exact prevalence of DFUs and DFIs in Uganda is likely higher than reported in the literature as many patients with diabetes in Uganda are not diagnosed or do not receive regular care,17 and DFUs may go undiagnosed.18 Additionally, many people with diabetes in Uganda may not have access to medical facilities or be unable to afford treatment, further exacerbating the problem.
Our review reveals that the bioburden of diabetic foot ulcers in Uganda is predominantly members of the ESKAPE pathogens (Table 2) and this is particularly worrisome because they are notorious for multi-drug resistance.19 We observed a remarkable dominance of Klebsiella spp 21.31% (14.42–29.65) over E. coli 18.85% (12.34–26.93), Pseudomonas spp 18.85% (12.34–26.93), Staphylococcus spp 18.85% (12.34–26.93) and Enterococcus faecalis 4.92% (1.83–10.40), respectively. The result aligns with a recent meta-analysis of the microbial profile of DFU in sub-Saharan Africa which reported E. coli as the dominant gram-negative DFU isolate20 and another, conducted by Makeri et al,21 which reported that Pseudomonas is the dominant gram-negative isolate in Africa. The observed variation in the dominant gram-negative isolate at the continental, sub-continental and national levels speaks to the importance of understanding the local epidemiology of microorganisms infecting diabetic foot ulcers. While the distribution of gram-negative bacteria varies across the continent, Staphylococcus aureus remains the dominant gram-positive isolate as is the case across the globe, emphasizing its role in skin and soft tissue infections.22–24
 |
Table 2 Pooled Prevalence of Bacteria Isolated from DFUs in Uganda |
Another likely explanation for the observed variation in bacterial species isolated across countries and regions of Africa could be the sample specimen used for microbiological assessment which is wound swabs in the case of Uganda. In practice, wound care providers have often adopted wound biopsies (tissue), swabs, curettage, and wound aspirate as principal samples.25 Wound swabs are widely used amidst debates about their reliability and sensitivity in isolating infecting pathogens against contaminants, keeping in mind the abundance of commensal microflora inhabiting healthy skin.26,27 For instance, coagulase-negative Staphylococci (CoNS), Micrococcus, Bacillus spp., and Corynebacterium, which are a part of normal skin flora and have been frequently isolated from DFU swabs are not usually considered pathogenic bacteria, unless the samples are taken from deep tissues.28 While tissue biopsies remain the standard sample as maintained by Ramsay et al,25 Mutluoglu et al29 opine that for any organism isolated using a swab sample, there is an 84.4% chance that the same organism will be isolated when a tissue biopsy sample taken from the same person is analyzed. Other studies have also argued that bacterial species diversity is affected by the sample collected for isolation. For instance, Huang et al27 reported that swab samples are less reliable in isolating gram-negative bacteria such as E. coli and Citrobacter which is quite contrary to our observation of Ugandan studies where swab samples yielded more gram-negative bacteria than gram-positives.
In our opinion, the existing studies on the microbiology of diabetic foot infections in Uganda down played the importance of utilizing gold standard sample specimen considering that more than 50% of the participants had severe diabetic foot ulcers which were graded between 3 and 5 on the Wagner scale. According to this classification system, a Wagner grade 3 ulcer extends to the bones but not beyond, while grades 4–5 involve the bones and extends beyond into surrounding tissues.30 Considering the severity of the foot ulcers, tissue biopsies would have been the best sample instead of wound swabs. We corroborate our claims with existing literature such as that by Heravi et al,28 who reported that more bacteria were isolated from tissue samples compared to 247 paired swab samples with a 42% concordance; this means that only 42% of the bacteria isolated from the tissue samples were isolated in the paired swab samples. It can be concluded that tissue samples offer more dependable results for identifying bacteria and tracking the bacterial population in diabetic foot infections, although only 0.1–10% of microorganisms are culturable31 and that brings to mind the method of isolation.
The studies included in this review used traditional culture methods for isolation of DFU bacteria, prompting the Spichler et al32 question; as modern clinicians faced with treating complex patients with diabetic foot infections, should we still request traditional and familiar culture and sensitivity methods, or is it time to ask for newer molecular tests? Over time, the traditional culture-based methods have been the most commonly utilized method for microbiological isolation from samples33 yet, they have several limitations, including the requirement for viable organisms, which may be challenging in cases of chronic or treated infections, the time-consuming nature of the procedure, and the potential for misidentification of organisms due to contamination or overgrowth of non-pathogenic flora. According to Liu et al,34 advanced sequencing technologies provide a more thorough analysis of the wound community, providing greater detail regarding the types of microorganisms present and their taxonomic classification. These technologies also identify specific functions such as virulence and antibiotic resistance.
In Uganda today, although bacteria have been isolated from foot ulcers and their antibiogram profile also studied, nothing is known about the molecular makeup of the isolates; moreover, none of the studies has distinguished colonization and infection with the isolated bacteria (say Staphylococcus aureus).
Antibiogram Profile of Diabetic Foot Ulcer Isolates in Uganda
The management of DFU infections in Uganda often involves antibiotic chemotherapy and the commonly used antibiotics include Cefoxitin, Erythromycin, Co-trimoxazole, Gentamycin, Chloramphenicol, Ciprofloxacin, Clindamycin, Vancomycin, Ceftriaxone, Penicillin, Ampicillin, Imipenem and Gentamicin.15,16 The choice of antibiotics is crucial in addressing the varying susceptibilities and resistances exhibited by the dominant bacterial isolates.
We observed that Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa, the dominant species isolated from DFUs in Uganda displayed varying susceptibilities and resistance patterns to commonly used antibiotics.15,16 For instance, E. coli and Klebsiella showed distinct susceptibility and resistance to Imipenem and penicillin, respectively. Additionally, Ciprofloxacin, Chloramphenicol, Ceftazidime, and Ceftriaxone demonstrated high effectiveness against E. coli. In the case of gram-positive bacteria like Staphylococcus aureus, susceptibility was observed to Gentamicin, Ciprofloxacin, Chloramphenicol, Vancomycin, and Clindamycin, while 100% resistance was noted against Cotrimoxazole.
The findings align with reports across the continent and beyond;20,21,35 however, we question clinical considerations (route of administration of antibiotics) when treating DFIs in Uganda, especially, that the country lacks a national guideline for diabetic foot care. While the selection of the initial antibiotic is based on the clinician’s assessment of likely pathogens and their susceptibilities, along with considerations for allergies, adverse effects, and other patient-specific factors,36 studies have shown that for many antibiotics, oral administration can achieve adequate levels in serum and, the infected tissues, provided the patient’s vascular supply is not severely compromised.36–38 This is particularly relevant in mild-to-moderate DFIs, where oral antibiotics like Ciprofloxacin and Clindamycin have been effective.38 However, in cases of severe infection or where there is significant vascular impairment, a common complication in DFUs, the delivery of oral antibiotics to the wound bed may be insufficient,36 prompting parenteral or intravenous administration. Parenteral or intravenous antibiotics ensure higher and more rapid drug concentrations in the blood and, potentially, at the site of infection. This is critical in severe DFIs, where rapid and effective bacterial eradication is necessary.39
In Uganda, we are not aware of the routine for the administration of systemic (oral and parental) antibiotic therapy. Notably, our review underscores the need for a standardized national guideline for diabetic foot infection in Uganda, which would provide essential guidance in the selection and administration of antibiotics.
While the lack of a national guideline in Uganda is itself a challenge to the treatment and management of diabetic foot infections in the country, the observed multidrug resistance (MDR) among DFU isolates is worrisome in a country with the 165th highest age-standardized mortality rate per 100,000 population associated with AMR across 204 countries.40 In light of the growing concern over antimicrobial resistance and the limitations of current antibiotic therapies in Uganda, it is imperative to explore alternative treatment options for diabetic foot infections. One promising avenue is the use of medical-grade honey (MGH), which has demonstrated broad-spectrum antimicrobial activity, including effectiveness against biofilms.41,42 Unlike traditional antibiotics, MGH does not pose a risk of developing resistance; moreover, MGH combats infection and also promotes wound healing. According to Nolan et al,42 the MDR status of bacterial strains has no impact on the susceptibility of the organism to MGH thus simplifies the treatment process by eliminating the need for identifying specific microorganisms or their sensitivity profiles. Given these advantages, MGH should be considered for over-the-counter (OTC) accessibility, offering a viable, effective solution in the management of DFUs in settings with limited resources and high resistance to conventional antibiotics.
Recommendation and Future Direction
To address the microbial complexities of diabetic foot ulcers (DFUs) in Uganda, we propose the establishment of a robust surveillance system for accurate DFU reporting, as well as the standardization of diagnostic techniques, including appropriate sample collection and molecular testing for enhanced microbial identification. Consequently, future researchers should adopt advanced molecular approaches such as whole-genome sequencing for genetic insights, investigate biofilm presence and other virulence factors, explore host-microbe interactions, and conduct longitudinal studies for infection progression understanding.
Conclusions
This systematic review illuminates the microbial landscape of diabetic foot ulcers (DFUs) in Uganda, highlighting the urgent need for comprehensive research and intervention. While data on the microbiology of DFUs in Uganda are limited, the bioburden aligns with global trends, with noteworthy prevalence of multidrug-resistant bacteria, particularly Methicillin-resistant Staphylococcus aureus (MRSA). The use of traditional culture-based methods and wound swabs may have limitations in capturing the full spectrum of infecting pathogens, prompting the recommendation for advanced molecular techniques. This review underscores the urgent need for a comprehensive national guideline in Uganda that includes alternative therapeutic options such as MGH, alongside traditional antibiotic therapies, to effectively combat DFU infections along with the establishment of a robust surveillance system for reporting diabetic foot ulcers.
Abbreviations
DFU, diabetic foot ulcer; DFI, diabetic foot infections; MGH, medical grade honey; MRSA, methicillin resistant Staphylococcus aureus; OTC, over the counter.
Data Sharing Statement
Extracted and synthesized studies are cited in this manuscript.
Ethics Approval and Consent to Participate
Ethics approval not required for this study.
Acknowledgments
We acknowledge Kampala International University for an enabling environment and promotion of research activities.
Funding
There was no financial aid for this study.
Disclosure
All the authors declare that they have no competing interests.
References
1. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabet Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
2. Lam AA, Lepe A, Wild SH, Jackson C. Diabetes comorbidities in low-and middle-income countries: an umbrella review. J Glob Health. 2021;11. doi:10.7189/jogh.11.04040
3. Asiimwe D, Mauti GO, Kiconco R. Prevalence and risk factors associated with type 2 diabetes in elderly patients aged 45–80 years at Kanungu District. J Diabetes Res. 2020;2020:1–5. doi:10.1155/2020/5152146
4. Nyanzi R, Wamala R, Atuhaire LK. Diabetes and quality of life: a Ugandan perspective. J Diabetes Res. 2014;2014:1–9. doi:10.1155/2014/402012
5. Rubio JA, Jiménez S, Lázaro-Martínez JL. Mortality in patients with diabetic foot ulcers: causes, risk factors, and their association with evolution and severity of ulcer. J Clin Med. 2020;9(9):3009. doi:10.3390/jcm9093009
6. Yazdanpanah L, Shahbazian H, Nazari I, et al. Incidence and risk factors of diabetic foot ulcer: a population-based diabetic foot cohort (ADFC study)-two-year follow-up study. Int J Endocrinol. 2018;2018:1–9. doi:10.1155/2018/7631659
7. Nambuya AP, Otim MA, Whitehead H, Mulvany D, Kennedy R, Hadden DR. The presentation of newly-diagnosed diabetic patients in Uganda. QJM - Mon J Assoc Physicians. 1996;89(9):705–712. doi:10.1093/qjmed/89.9.705
8. Bateganya MH, Luie JR, Nambuya AP, Otim MA. Morbidity and mortality among diabetic patients admitted to Mulago Hospital, Uganda. Malawi Med J. 2003;15(3):91–94.
9. Sharifah Aisyah SH, Siti Asma’ H, Nurahan M. The significant association between polymicrobial diabetic foot infection and its severity and outcomes. Malaysian J Med Sci. 2019. doi:10.21315/mjms2019.26.1.10
10. Basimbe F, Muganga D, Mutyaba F. Outcomes of major lower limb amputation among patients at a Referral Hospital. J Clin Res. 2022;6:2020–2023. doi:10.37421/2795-6172.2022.6.167
11. Okello TR, Magada SM, Atim P, et al. Major limb loss (MLL): an overview of etiology, outcomes, experiences and challenges faced by amputees and service providers in the post-conflict period in Northern Uganda. J Glob Heal Rep. 2019;3. doi:10.29392//001c.11990
12. Rigato M, Pizzol D, Tiago A, Putoto G, Avogaro A, Fadini GP. Characteristics, prevalence, and outcomes of diabetic foot ulcers in Africa. A systemic review and meta-analysis. Diabet Res Clin Pract. 2018;142:63–73. doi:10.1016/j.diabres.2018.05.016
13. Edmonds M, Manu C, Vas P. The current burden of diabetic foot disease. J Clin Orthop Trauma. 2021;17:88–93. doi:10.1016/j.jcot.2021.01.017
14. Page MJ et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71
15. Pario A, Nambuya A, Nakwagala FN. Clinical presentation and bacteriology of diabetic foot ulcers in Mulago Hospital. Cardiovasc J Afr. 2011;22(3):S19.
16. Sikhondze MM, Twesigye D, Odongo CN, et al. Diabetic foot ulcers: surgical characteristics, treatment modalities and short-term treatment outcomes at a Tertiary Hospital in South-Western Uganda. Open Access Surg. 2022;15:75–87. doi:10.2147/oas.s384235
17. Birabwa C, Bwambale MF, Waiswa P, Mayega RW. Quality and barriers of outpatient diabetes care in rural health facilities in Uganda - A mixed methods study. BMC Health Serv Res. 2019;19(1). doi:10.1186/s12913-019-4535-x
18. Kubiak RW, Sveum EM, Faustin Z, et al. Prevalence and risk factors for hypertension and diabetes among those screened in a refugee settlement in Uganda. Confl Health. 2021;15(1). doi:10.1186/s13031-021-00388-z
19. World Health Organization. WHO publishes list of bacteria for which new antibiotics are urgently needed. Saudi Medical Journal; 2017.
20. Wada FW, Mekonnen MF, Sawiso ED, et al. Bacterial profile and antimicrobial resistance patterns of infected diabetic foot ulcers in sub-Saharan Africa: a systematic review and meta-analysis. Sci Rep. 2023;13(1):14655. doi:10.1038/s41598-023-41882-z
21. Makeri D, Odoki M, Eilu E, Agwu E. Update on prevalence and antimicrobial resistance of Staphylococcus aureus and Pseudomonas aeruginosa isolated from diabetic foot ulcers in Africa: a systematic review and meta‑analysis. Bull Natl Res Cent. 2023;47(1). doi:10.1186/s42269-023-01119-5
22. Arfaoui A, Sallem RB, Fernández-Fernández R, et al. Methicillin-resistant Staphylococcus aureus from diabetic foot infections in a Tunisian Hospital with the First Detection of MSSA CC398-t571. Antibiotics. 2022;11(12):1755. doi:10.3390/antibiotics11121755
23. Anafo RB, Atiase Y, Dayie NTKD, et al. Methicillin-resistant staphylococcus aureus (MRSA) infection of diabetic foot ulcers at a tertiary care hospital in Accra, Ghana. Pathogens. 2021;10(8):937. doi:10.3390/pathogens10080937
24. Pius T, Irege R, Makeri D, Tamale A. among patients with skin and soft tissue infections: a cross-sectional study at a Tertiary Hospital in Bushenyi, Western, Uganda. Open Access Libr J. 2023;10(5):1–12. doi:10.4236/oalib.1110186
25. Ramsay S, Cowan L, Davidson JM, Nanney L, Schultz G. Wound samples: moving towards a standardised method of collection and analysis. Int Wound J. 2016;13(5):880–891. doi:10.1111/iwj.12399
26. Abdulbasith K, Bhaskar M, Munisamy M, Nagarajan R. Study of fine-needle aspiration microbiology versus wound swab for bacterial isolation in diabetic foot infections. Indian J Med Res. 2020;152(3):312–315. doi:10.4103/ijmr.IJMR_1151_18
27. Huang Y, Cao Y, Zou M, et al. A comparison of tissue versus swab culturing of infected diabetic foot wounds. Int J Endocrinol. 2016;2016:1–6. doi:10.1155/2016/8198714
28. Heravi FS, Zakrzewski M, Vickery K, Armstrong DG, Hu H. Bacterial diversity of diabetic foot ulcers: current status and future prospectives. J Clin Med. 2019. doi:10.3390/jcm8111935
29. Oliver TI, Mutluoglu M. Diabetic foot ulcer. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537328/.
30. Shah P, Inturi R, Anne D, et al. Wagner’s classification as a tool for treating diabetic foot ulcers: our observations at a Suburban Teaching Hospital. Cureus. 2022. doi:10.7759/cureus.21501
31. Pipite A, Lockhart PJ, McLenachan PA, et al. Isolation, antibacterial screening, and identification of bioactive cave dwelling bacteria in Fiji. Front Microbiol. 2022;13. doi:10.3389/fmicb.2022.1012867
32. Spichler A, Hurwitz BL, Armstrong DG, Lipsky BA. Microbiology of diabetic foot infections: from Louis Pasteur to “crime scene investigation. BMC Med. 2015;13(1). doi:10.1186/s12916-014-0232-0
33. Laupland KB, Valiquette L. The changing culture of the microbiology laboratory. Can J Infect Dis Med Microbiol. 2013;24(3):125–128. doi:10.1155/2013/101630
34. Liu C, Ponsero AJ, Armstrong DG, Lipsky BA, Hurwitz BL. The dynamic wound microbiome. BMC Med. 2020;18(1). doi:10.1186/s12916-020-01820-6
35. Macdonald KE, Boeckh S, Stacey HJ, Jones JD. The microbiology of diabetic foot infections: a meta-analysis. BMC Infect Dis. 2021;21(1). doi:10.1186/s12879-021-06516-7
36. Lipsky BA, Aragón-Sánchez J, Diggle M, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016;32(S1):45–74. doi:10.1002/dmrr.2699
37. Kwon KT, Armstrong DG. Microbiology and antimicrobial therapy for diabetic foot infections. Infect Chemother. 2018;50(1):11. doi:10.3947/ic.2018.50.1.11
38. Lipsky BA. Evidence-based antibiotic therapy of diabetic foot infections. FEMS Immunol Med Microbiol. 1999;26(3–4):267–276. doi:10.1016/S0928-8244(99)00143-1
39. Haug F, Waibel FWA, Lisy M, Winkler E, Uçkay I, Schöni M. The impact of the length of total and intravenous systemic antibiotic therapy for the remission of diabetic foot infections. Int J Infect Dis. 2022;120:179–186. doi:10.1016/j.ijid.2022.03.049
40. Kariuki S, Kering K, Wairimu C, Onsare R, Mbae C. Antimicrobial resistance rates and surveillance in Sub-Saharan Africa: where are we now? Infect Drug Resist. 2022;Volume 15:3589–3609. doi:10.2147/IDR.S342753
41. Pleeging CCF, Coenye T, Mossialos D, et al. Synergistic antimicrobial activity of supplemented medical-grade honey against pseudomonas aeruginosa biofilm formation and eradication. Antibiotics. 2020;9(12):866. doi:10.3390/antibiotics9120866
42. Nolan VC, Harrison J, Wright JEE, Cox JAG. Clinical significance of manuka and medical-grade honey for antibiotic-resistant infections: a systematic review. Antibiotics. 2020;9(11):766. doi:10.3390/antibiotics9110766
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

