Back to Journals » International Journal of General Medicine » Volume 16
A Systematic Immune and Prognostic Analysis of CD48 Interaction with Tumor Microenvironment in Pan-Cancer
Authors He M, Yu J, Chen S , Mi H
Received 29 July 2023
Accepted for publication 31 October 2023
Published 13 November 2023 Volume 2023:16 Pages 5255—5269
DOI https://doi.org/10.2147/IJGM.S431696
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Mingdong He,1,* Jun Yu,1,* Shaohua Chen,2 Hua Mi1
1Department of Urology, the First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China; 2Department of Urology, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hua Mi; Shaohua Chen, Email [email protected]; [email protected]
Background: The cluster of differentiation 48 (CD48) is a member of the signaling lymphocyte activation molecule family, constitutively expressed on most hematopoietic cells. CD48 was reported to affect immune regulation in certain tumors, thereby influencing tumor development and prognosis, but its impact on the prognosis and immune infiltration in pan-cancer remains unclear.
Material and Methods: We systematically analyzed the raw data from The Cancer Genome Atlas (TCGA), Tumor Immune Estimation Resource (TIMER), and Tumor Immune Dysfunction and Exclusion (TIDE) databases. Initially, we investigated the differences in CD48 expression between pan-cancer and adjacent normal tissues. Then, the correlation analysis of CD48 with tumor mutational burden (TMB), microsatellite instability (MSI), tumor microenvironment (TME), and immune-related genes was evaluated. Moreover, bioinformatics tools: ESTIMATE and gene set enrichment analysis (GSEA) were used for tumor immunology analysis in pan-cancer. We performed validation studies including quantitative real-time PCR (qPCR) and Western blotting.
Results: Differential analysis revealed that CD48 was significantly altered in pan-cancer as compared with normal tissues. Meanwhile, the survival analysis demonstrated that CD48 strongly correlated with overall survival (OS), disease-free interval (DFI), progression-free interval (PFI), and disease-specific survival (DSS), indicating its crucial role in the tumor patients’ prognosis. CD48 expression was also associated with TMB and MSI levels in 17 and 14 types of pan-cancers, respectively. Moreover, CD48 was linked to immune infiltrating cells and stromal components in the TME.
Conclusion: Concludingly, patients with pan-cancer may benefit from evaluating CD48 as a prognostic and immunotherapy response biomarker.
Keywords: CD48, pan-cancer, immunotherapy, tumor microenvironment
Introduction
With an increase in life expectancy, changes in lifestyle, and the global environment, the incidence and mortality of malignant tumors are rising rapidly and have become one of the leading causes of death worldwide.1 Similarly, cancer treatment methods, such as surgery, radiotherapy, and immunotherapy, have evolved gradually over the past few decades;2 immunotherapy is a standard of care in oncology that aims to boost the body’s natural defenses in order to eliminate cancer cells.3 Despite advancements in cancer immunotherapy, the prognosis for many cancers remains dismal. A subset of immunotherapy recipients experienced little or no benefit and may have been exposed to toxicity, despite the high cost of these treatments.4 Consequently, we must identify a biomarker to evaluate the efficacy of immunotherapy.
CD48 is constitutively expressed as an adhesion and stimulation molecule mainly on antigen-presenting cells (APCs).5 Specifically, its distribution and binding properties and ability to act as an activating and inhibitory receptor make it a key player in the immune system’s regulation.6 Recently, research has shown that the combination of CD48 and growth differentiation factor 15 (GDF15) may increase the immunotherapeutic modalities in hepatocellular carcinoma, and their interaction promotes the generation or inhibitory function of immunomodulatory cells.7 Moreover, the occurrence and development of some tumors are also linked to CD48, such as glioma, small cell lung cancer, and acute myeloid leukemia (AML),5,8,9 with evidence indicating that high CD48 expression could reverse immune evasion in AML and activate the function of natural killer (NK) cells in vivo,9 or influence tumor development and prognosis by affecting immune regulation in gliomas.8 Besides, it has been well documented that CD48-positive small cell lung cancer cells may be susceptible to NK cell-mediated cytotoxicity.8 Based on these findings, CD48 may play an important role in regulating immune responses to tumor-induced immune activation or suppression. However, its role in the overall human cancer spectrum remains largely unknown. It is of great interest to understand the relevance of CD48 to pan-cancer for the exploration of immunotherapy targets.
During this study, we aimed to explore the expression of CD48 in pan-cancer, the immune cell infiltration in the TME, and the prognostic impact on the survival of tumor patients. Moreover, we also analyzed the relationship of CD48 with TMB10 and MSI.11 GSEA12 was also used to analyze the gene set’s biological functions and the potential mechanisms behind it. The results helped to comprehend the crucial role of CD48 in pan-cancer, indicating probable links between CD48 with tumor-immune interactions, further illustrating the underlying mechanisms of its functions in the TME.
Materials and Methods
Clinical Specimen Collection and Ethics Statement
We collected pathological specimens of bladder cancer patients from the First Affiliated Hospital of Guangxi Medical University for analysis in 2022. In our study, we included patients diagnosed with muscle-invasive bladder cancer (MIBC) who underwent radical cystectomy as the primary treatment for their bladder cancer. Specifically, we focused on patients with a pathologic stage of T1 and above. The study got approval from the Medical Ethics Committee of The First Affiliated Hospital of Guangxi Medical University (Approval Number: 2022-E386-01). Informed consent was acquired in writing from each patient.
Raw Data Sources and CD48 Expression Analysis
First, RNA sequences, somatic mutations, and survival data were downloaded from the TCGA database through the University of California Santa Cruz (UCSC Xena; https://xena.ucsc.edu/). Next, Practical Extraction and Report Language (Perl) was used to extract CD48 gene expression data from downloaded data sets comprising 12,591 samples of 33 types of tumors, and the expression matrix was used for subsequent analysis. A box plot was designed using the R package “ggpubr” to compare the CD48 expression levels between 33 tumors and corresponding normal tissues. The R language version used in our study was R version 4.0.3. Also, with the help of TIMER (https://cistrome.shinyapps.io/timer/), the expression profiles of CD48 were presented in pan-cancers.
Prognostic Analysis Based on CD48 Expression Levels
The Kaplan-Meier method was used to investigate four prognostic indicators: OS, DFI, DSS, and PFI to explore the connection between CD48 expression and patient survival data. The “survminer” and “survival” packages were used for visualization and statistical analysis. Moreover, a univariate Cox analysis was conducted using the R packages “survival” and “forest plot” to calculate the hazard ratios (HRs) with 95% confidence intervals (CIs) between CD48 expression and prognostic indicators.
Relationship of Clinical Phenotype and CD48 Expression
Tumor stage, age, and gender were used to analyze the relationship between clinical phenotype and CD48 expression. Correlation analysis was performed using the R packages “limma” and “ggpubr”.
qPCR
We extracted total RNA from frozen BLCA tissues using Axygen reagent (CORNING, Nanning, China) and performed quantitative PCR with ABI7500. Primers sequences were shown below (5’-3’): CD48 forward- AGGTTGGGATTCGTGTCTGG, reverse- AGTTGTTTGTAGTTCTCAGGCAG.β-actin forward-GTCATTCCAAATATGAGATGCGT, reverse- GCTATCACCTCCCCTGTGTG. The 2- ΔΔ Ct method was used to calculate the relative expression level, and the results were statistically analyzed using GraphPad Prism.
Western Blotting
Protease inhibitors were added to the RIPA lysate buffer to extract total protein from BLCA tissues, and then the protein concentration was determined. After adding the 5×SDS-PAGE protein loading buffer, the protein sample was placed in a 100 ° C metal bath for 10 minutes. The proteins were separated by 12% SDS-polyacrylamide gels (SDS-PAGE) electrophoresis and then transferred to the polyvinylidene fluoride (PVDF) membrane. The membrane was sealed with 5% skimmed milk for 1 hour, and then washed thrice for 5 minutes in TBST. After that, the membrane was incubated with primary antibody at 4 ° C overnight, then washed in TBST, and incubated with secondary antibody at room temperature for 1 hour. Finally, the target stripe signals were detected using the HRP substrate (Merck Millipore) and analyzed using Image J software and GraphPad Prism.
Relationship of the Immune Microenvironment and CD48 Expression
We used the ESTIMATE method to infer the stromal and immune cell infiltration degree. On this basis, we calculated the stromal and immune scores for each tumor and their correlation with CD48 expression using the R packages “estimate” and “limma”. We used the CIBERSORT algorithm to predict the relative contents of immune cells in the pan-cancer tissues. The immune cells comprised CD4+ T cells, CD8+ T cells, memory B cells, naive B cells, neutrophils, dendritic cells (DCs), monocytes, macrophages, mast cells, eosinophils, NK cells, plasma cells, regulatory T cells (Tregs), gamma delta T cells and follicular helper T (Tfh) cells. Subsequently, we evaluated the correlation coefficient of CD48 expression with immune cell infiltration across diverse cancer types using R-packages “ggplot2”, “ggpubr”, and “ggExtra”.
Relationship of TMB, MSI, TIDE, and CD48 Expression
TMB is a quantifiable immune-response biomarker that measures tumor cell DNA mutations.10 MSI occurs when DNA fragments simultaneously gain and lose nucleotides.11 Based on mutation data downloaded from the TCGA database, we calculated the TMB and MSI scores. The correlation between TMB, MSI, and CD48 was analyzed using Spearman correlation analysis. Results were presented as a radar diagram and visualized using the R-package “fmsb”. Furthermore, the TIDE database was used to predict the immunotherapeutic efficacy of CD48 in different tumors.
Relationship of Immunity-Related Genes and CD48 Expression
To analyze the association between immunity-related genes and CD48, we calculated the gene correlation coefficient and p-values using the R-packages “BiocManager” and “limma”. Results were presented as a heatmap and generated using the “reshape2” and “RColorBrewer” packages.
Biological Function of CD48 in Pan-Cancer
GSEA was conducted to investigate potential mechanisms and biological functions of CD48 in pan-cancer. Functional analysis was performed using the R-packages “clusterProfiler” and “enrichplot”.
Statistical Analysis
The R software (version 4.0.3) and attached packages were used for all statistical analyses. The Wilcoxon test was used to compare CD48 expression levels between the 33 tumors and corresponding normal tissues. Kaplan-Meier curves and univariate Cox analyses were used for survival analysis. The relationship between the two variables was investigated using Spearman correlation. A two-tailed p-value of less than 0.05 was considered statistically significant.
Results
Expression Levels of CD48 in Pan-Cancer
We used the pan-cancer datasets provided by the TIMER database to comprehensively analyze the expression of CD48 in pan-cancer and normal tissues, which were typically sampled from areas located more than 2 cm away from the tumor. According to the TIMER database, CD48 expression was significantly higher in the several types of cancers than in normal tissues: head and neck squamous cell carcinoma (HNSC), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), and breast invasive carcinoma (BRCA). Meanwhile, CD48 expression was significantly decreased in colon adenocarcinoma (COAD), bladder urothelial carcinoma (BLCA), lung adenocarcinoma (LUAD), kidney chromophobe (KICH), lung squamous cell carcinoma (LUSC), thyroid carcinoma (THCA), and rectum adenocarcinoma (READ) than in their corresponding normal tissues (Figure 1A). We also dissected the expression of CD48 in pan-cancer using the TCGA database. Similarly, CD48 expression was significantly regulated in some tumors, including COAD, BRCA, BLCA, kidney renal clear cell carcinoma (KIRC), glioblastoma multiforme (GBM), LUAD, KIRP, LUSC, READ, pancreatic adenocarcinoma (PAAD), and THCA (Figure 1B). Taking these results together, CD48 was widely and significantly expressed in different tumors while downregulated in most tumors.
 |
Figure 1 Expression levels of CD48 in pan-cancer and relative normal tissues based on (A) TIMER and (B) TCGA databases. *p < 0.05, **p < 0.01, ***p < 0.001. |
Prognostic Value of CD48 in Pan-Cancer
Univariate Cox and Kaplan-Meier analyses were conducted to investigate the prognostic value of CD48 in pan-cancer. In univariate Cox analysis, OS and DSS demonstrated that high CD48 expression was a favorable factor in cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC) (OS: HR = 0.687, p = 0.003; DSS: HR = 0.687, p = 0.003), HNSC (OS: HR = 0.820, p = 0.005; DSS: HR = 0.773, p = 0.007), LUAD (OS: HR = 0.0.833, p = 0.011; DSS: HR = 0.827, p = 0.035), sarcoma (SARC) (OS: HR = 0.771, p = 0.005; DSS: HR = 0.807, p = 0.039), skin cutaneous melanoma (SKCM) (OS: HR = 0.808, p = 0.001; DSS: HR = 0.784, p < 0.001), and uterine corpus endometrial carcinoma (UCEC) (OS: HR = 0.750, p = 0.026; DSS: HR = 0.703, p = 0.031) (Figure 2A and B). Meanwhile, DFI indicated that high CD48 expression was associated with better prognosis in CESC (HR = 0.622, p = 0.025), COAD (HR = 0.455, p = 0.012), liver hepatocellular carcinoma (LIHC) (HR = 0.756, p = 0.012), and UCEC (HR = 0.712, p = 0.047) (Figure 2C). In addition, by the PFI, higher CD48 expression was a protective factor in BRCA (HR = 0.792, p = 0.004), CESC (HR=0.645, p < 0.001), cholangiocarcinoma (CHOL) (HR = 0.554, p = 0.021), HNSC (HR = 0.838, p = 0.02), LIHC (HR = 0.814, p = 0.036), SKCM (HR = 0.906, p = 0.017), and UCEC (HR = 0.765, p = 0.016) (Figure 2D).
The Kaplan–Meier analysis indicated that high CD48 expression represented better OS in BRCA, CESC, and SKCM. However, brain Lower Grade Glioma (LGG) patients with high CD48 levels presented worse prognoses (Figure 3A). Notably, high CD48 expression had a richer DSS in UCEC, SKCM, and CESC, while LGG and lymphoid neoplasm diffuse large B-cell lymphoma (DLBC) had a poorer DSS (Figure 3B). Moreover, the results identified that high CD48 expression predicted a better DFI in CHOL, COAD, LIHC, and UCEC (Figure 3C). Regarding PFI, patients with high levels of CD48 expression had a better prognosis in CESC, BRCA, and SKCM, while it showed a worse prognosis in LGG (Figure 3D).
In general, high CD48 expression was a protective factor, especially in CESC, HNSC, SKCM, and UCEC. In LGG, CD48 played an undesirable role. Subsequently, CD48 expression was inextricably related to the prognosis of some tumor patients, and it may serve as a biomarker to predict patients’ prognosis.
Relationship of CD48 Expression and Clinical Phenotype
In order to examine the connection between CD48 expression and clinical phenotype, we compared the levels of CD48 expression among patients with different clinical annotations. Remarkably, we found that CD48 expression significantly correlated with tumor stage in KIRC, COAD, SKCM, LUAD, and testicular germ cell tumors (TGCT). Interestingly, CD48 expression increased in the advanced stage of KIRC and SKCM, while it was the other way around in COAD, LUAD, and TGCT (Figure 4A-E). We also analyzed the associations of age and gender with CD48 expression. Notably, older patients had higher CD48 expression in acute myeloid leukemia (LAML), esophageal carcinoma (ESCA), LGG, and LUAD (Figure 4F-M). Additionally, females presented higher expression levels in BLCA, BRCA, LUAD, and LUSC (Figure 4N-Q). The results revealed that CD48 expression was significantly associated with multiple clinical indicators, indicating that CD48 could affect the clinical progression of cancers.
To assess whether CD48 expression in pan-cancer correlates with the stromal and immune components of the TME, we studied its relationship with the stromal and immune scores. CD48 expression was found to be positively correlated with immune score in BRCA, LUAD, UCEC, LGG, SKCM, CESC, and SARC (Figure 5A). Furthermore, CD48 was positively associated with the stromal score in BRCA, LUAD, UCEC, LGG, SKCM, CESC, and SARC (Figure 5B).
Several studies have suggested that immune cells within the TME may play a role in patient survival;13 hence, based on the TCGA database, we examined the relationship between CD48 expression and immune infiltration. The results indicated that CD48 expression was positively related to levels of infiltrating naive B cells (Figure 6A), M1 macrophages (Figure 6B), monocytes (Figure 6C), neutrophils (Figure 6D), plasma cells (Figure 6E), T cells (Figure 6F-H), activated CD4 memory T cells (Figure 6I), resting dendritic cells (Figure 6J), and CD8 T cells (Figure 6K) in most tumors. On the contrary, an inverse relationship was observed between CD48 expression and infiltrating activated mast cells (Figure 6L), activated dendritic cells (Figure 6M), resting NK cells (Figure 6N), M0 macrophages (Figure 6O), M2 macrophages (Figure 6P), resting mast cells (Figure 6Q) and resting CD4 memory T cells (Figure 6R) in most tumors. Interestingly, CD48 was positively interrelated with levels of infiltrating memory B cells in LUAD but negatively linked in PAAD (Figure 6S). Moreover, as the levels of infiltrating activated NK cells were increased, CD48 was also increased in KICH and thymoma (THYM), while it was decreased in KIRC (Figure 6T).
Figures 5 and 6 show that CD48 was strongly associated with immune cell infiltration in TME, prompting that CD48 may have a significant relationship with immune microenvironmental remodeling.
Relationship of Immunity-Related Genes, TMB, MSI, TIDE, and CD48 Expression
To clarify the relationship between CD48 expression and immune function, we explored the correlation between immunity-related genes and CD48. In pan-cancer samples, expression of CD48 strongly correlated with most immunity-related genes, as shown in Figure 7A. Notably, CD48 was positively associated with BTLA, TNFRSF14, LAIR1, LAG3, ICOS, CTLA4, CD28, CD276, PDCD1, TMIGD2, HAVCR2, LGALS9, PDCD1LG2, and CD86 in LGG. In summary, CD48 expression played an indispensable role in immune function.
Then, to evaluate the general predictive power of CD48 on immunotherapy response, an analysis of the correlation between CD48 and TMB and MSI was conducted. The results demonstrated that CD48 expression was positively correlated with TMB in UCEC, ovarian serous cystadenocarcinoma (OV), LGG, and COAD but negatively related in adrenocortical carcinoma (ACC), THYM, THCA, TGCT, stomach adenocarcinoma (STAD), prostate adenocarcinoma (PRAD), PAAD, MESO, LUAD, LIHC, HNSC, DLBC, and CHOL (Figure 7B). Similarly, higher expression of CD48 correlated with higher MSI in COAD, while it was correlated with lower MSI in TGCT, STAD, SKCM, SARC, PAAD, OV, LUSC, LUAD, LIHC, LGG, KIRP, HNSC, and ESCA (Figure 7C). TIDE database containing varying cancer cohorts treated with immunotherapy was used to validate above-mentioned results, which indicated that CD48 (AUC > 0.5 in 19 sub-cohorts) showed a better predictive value compared with TIDE (AUC > 0.5 in 18 sub-cohorts), MSI score (AUC > 0.5 in 13 sub-cohorts), TMB (AUC > 0.5 in 8 sub-cohorts), CD8 (AUC > 0.5 in 18 sub-cohorts), interferon-gamma (IFNG) (AUC > 0.5 in 16 sub-cohorts), T.Clonality (AUC > 0.5 in 7 sub-cohorts), B.Clonality (AUC > 0.5 in 7 sub-cohorts), and Merck18 (AUC > 0.5 in 18 sub-cohorts) (Figure 7D). Apart from this, higher CD48 was significantly correlated with longer PFS or OS in melanoma and higher cytotoxic T lymphocyte (CTL) level (Figure 7E). Overall, CD48 could be a powerful predictor of immunotherapy response.
Biological Function of CD48 in Pan-Cancer
Next, we conducted GSEA to investigate the potential biological function of CD48 expression in seven cancers, including BRCA, UCEC, LUAD, LGG, CESC, SKCM, and SARC. As shown in Figure 8, according to the gene ontology (GO) terms (Figure 8A-G), CD48 negatively correlated with mRNA blinding in BRCA, intermediate filament cytoskeleton in CESC, the intermediate filament in LUAD, and negative regulation of endothelial cell differentiation in UCEC. According to our findings, CD48 was positively connected with B cell-mediated immunity in SARC, immune response regulating signaling pathway in SKCM and LGG. Furthermore, as per Kyoto encyclopedia of genes and genomes (KEGG) terms (Figure 8H-M), the results indicated that CD48 had significant enrichment in some immunity-related pathways, such as primary immunodeficiency in CESC, LUAD, and SARC, NK cell-mediated cytotoxicity in SKCM, cytokine receptor interaction in UCEC, and T cell receptor signaling pathway in LGG.
A wide range of functions and signaling pathways were found to be regulated by CD48, especially in immune response regulating signaling pathways and primary immunodeficiency.
Differential Expression of CD48 in BLCA
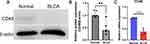
To further confirm the differential expression of CD48 in BLCA and normal neighboring tissues, we conducted qPCR and Western blotting analysis. Our results revealed that CD48 was significantly increased in carcinoma tissues of bladder cancer patients compared to normal tissues (Figure 9A-C). Surprisingly, this finding is in agreement with those analyzed by TCGA and TIMER databases.
Discussion
In recent years, more and more studies have focused on genome-wide pan-cancer analysis to reveal genetic mutations and oncogenic driver genes associated with cancer development, which is important for early diagnosis and the identification of sensitive clinical biomarkers.14,15 CD48 belongs to the family of signaling lymphocyte activation molecules, constitutively expressed on most hematopoietic cells.16 Several studies have shown that AML cells evade NK cell surveillance by reducing CD48 or possibly by producing soluble forms.9 It has also been shown that CD48 may be involved in T-cell activation signaling, immune synapse organization, and target lysis of effector lymphocytes in hepatocellular carcinoma.7 However, the basic molecular mechanism of CD48 dysregulation in tumors has not been fully elucidated. Considering the potential prognostic value and biological function of CD48, it is important to explore its basic regulatory mechanisms in pan-cancer and the possibility of using it as a biomarker. Herein, the study analyzed CD48 expression in all 33 cancers using processed data from the TCGA and the TIMER database. There was a significant upregulation of CD48 expression in four types of cancer in the study, while it was downregulated in seven cancer types. In addition, Kaplan-Meier survival analysis indicated that high CD48 expression was correlated with poor prognosis (OS, DSS, DFI, and PFI), especially in CESC, HNSC, and UCEC. Furthermore, CD48 expression was significantly related to survival indicators and clinical annotations, including tumor stages, gender, and age in multiple cancer types. Overall, results suggested that CD48 is a potential prognostic biomarker in tumors. Moreover, our results agreed with a previous study, which reported that overexpressed CD48 might assume the role of interesting potential biomarkers in clear cell renal cell carcinomas (ccRCC), and its blockade may lead to its validation as a new target for immunotherapy.17 CD48 has recently been shown to be a candidate gene for determining the level of immune infiltrating cells in bladder cancer, and it may be represented as a biomarker to guide immunotherapy of bladder cancer.18 Meanwhile, this finding is consistent with the results of our qPCR and Western blotting analysis.
In the current era of precision medicine, the mining of biomarkers is indispensable for the development of immunotherapy, which is playing an increasingly prominent role. TMB and MSI are promising pan-cancer predictive biomarkers.19,20 The TMB biomarker may improve immunotherapy for colorectal and pan-cancers.20 MSI is an independent predictor of clinical immunotherapy success in colorectal cancer.21 The use of programmed death receptor-1/ligand 1 (PD-1/L1) antibodies in many types of cancer can lead to durable remissions and improve the effectiveness of immunotherapy.22 PD-1/PD-L1 blockade therapy is highly effective in tumors with high MSI. Interestingly, previous studies have shown that PD-1 blockade may be more effective on tumors with high MSI when combined with their high TMB, which predicts response to checkpoint inhibitors in many cancer types.23–26 Although the above biomarkers have made great progress in the field of immunotherapy, they still have their limitations. For example, they are expensive for clinical use, technically demanding, and not widely available in clinical pathology laboratories.27 Hence, it is crucial to find more appropriate biomarkers. Our study showed a significant correlation between CD48 expression and TMB levels in 17 cancer types and between CD48 expression and MSI in 14 cancer types. It is worth noting that high CD48 expression showed a consistently significant correlation with the high TMB and MSI in COAD. CD48 expression was linked with expression levels of multiple immunity-related genes in several tumor types. Moreover, our validation results from the TIDE database showed that CD48 has a higher predictive value in immunotherapy response. This indicates that CD48 has better predictive power in immunotherapy response in most tumor types. Based on existing research, our findings may provide a new direction for biomarker mining and development in immunotherapy.
TME is a complicated and dynamic system made up of cells, proteins, and small molecules, the central part of which is mainly tumor cells and infiltrating immune cells.28 Immunotherapy is based on infiltrating immune cells as an emerging approach to oncology treatment, including APCs, helper T cells (Th), NK cells, and cytotoxic T lymphocytes (CTLs).29 Notably, the infiltrating immune cells of TME play an instrumental role in the immunotherapy of tumors.30 Alternatively, some immune cells could skillfully evade immune surveillance, including tumor-associated macrophages (TAMs), Tregs, and myeloid-derived suppressor cells (MDSCs).31 Furthermore, recent findings suggested that B cells can also play other vital roles in cancer therapy, acting as APCs and secreting antibodies.32 Meanwhile, finding the appropriate immune checkpoint for TME remains a necessity.
Correspondingly, this study demonstrated a significant correlation between CD48 expression and several types of immune cells infiltrating the body, such as B cells, CD4+ and CD8+ T cells, DCs, macrophages, and neutrophils. In the meantime, CD48 also had strong relevance to stromal and immune scores, particularly in BRCA, LUAD, UCEC, LGG, SKCM, CESC, and SARC. The outcomes of the GSEA analysis established that CD48 was widely engaged in immune response-regulating signaling pathways. Based on previous findings and our results, it is concluded that CD48 may affect the TME by influencing immune components and stromal components, thereby affecting the performance of immunotherapy. Notably, research suggested that transforming growth factor-β (TGF-β) secreted in the TME modulated immunotherapy sensitivity in acute leukemia through downregulation of CD48.33 CD48 mediated NK cell dysfunction in hepatocellular carcinoma.34 As a result of these innovations, CD48’s crucial role in immune infiltration has been better understood. Nevertheless, further research and exploration are needed to uncover the specific regulatory mechanisms of CD48 in the TME.
It is worth noting that despite our analysis and integration of information from different databases, this research has certain limitations. First, even though analysis of the biological information of CD48 in the field of pan-cancer provided us with novel insights, experimental studies are expected to verify our findings in vitro and in vivo. Second, the specific regulatory mechanisms or immune signaling pathways of CD48 in the TME are still not elucidated, and further summary studies are necessary. Furthermore, CD48 expression in tumors has been associated with immune and clinical survival, but its impact on clinical survival through the immune system remains unclear.
Conclusions
In summary, CD48 was exceptionally expressed in pan-cancer compared with normal tissues. Additionally, high CD48 was significantly associated with TMB, MSI, and TME, demonstrating its potential as a biomarker for predicting immunotherapy response. These surveys will provide new and improved options for innovative targets for tumor immunotherapy.
Data Sharing Statement
The raw data can be gained from the University of California Santa Cruz (UCSC Xena, https://xenabrowser.net/). Further inquiries can be directed to the corresponding author.
Ethics Approval and Consent to Participate
The study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Medical Ethics Committee of The First Affiliated Hospital of Guangxi Medical University (Approval Number: 2022-E386-01). Informed consent was acquired in writing from each patient.
Patient Consent for Publication
Informed consent was acquired from each patient.
Acknowledgments
We would like to express our gratitude to the TIMER, TCGA, and TIDE databases for their valuable contributions to this study. The availability of these databases has played a crucial role in our research, providing us with comprehensive datasets and analytical tools.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (81860142).
Disclosure
All authors declare no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Wang JJ, Lei KF, Han F. Tumor microenvironment: recent advances in various cancer treatments. Eur Rev Med Pharmacol Sci. 2018;22(12):3855–3864. doi:10.26355/eurrev_201806_15270
3. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–821. doi:10.1038/s41423-020-0488-6
4. Maleki Vareki S, Garrigos C, Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Crit Rev Oncol Hematol. 2017;116:116–124. doi:10.1016/j.critrevonc.2017.06.001
5. Zou C, Zhu C, Guan G, et al. CD48 is a key molecule of immunomodulation affecting prognosis in glioma. Onco Targets Ther. 2019;12:4181–4193. doi:10.2147/OTT.S198762
6. Pahima H, Puzzovio PG, Levi-Schaffer F. 2B4 and CD48: a powerful couple of the immune system. Clin Immunol. 2019;204:64–68. doi:10.1016/j.clim.2018.10.014
7. Wang Z, He L, Li W, et al. GDF15 induces immunosuppression via CD48 on regulatory T cells in hepatocellular carcinoma. J Immunother Cancer. 2021;9(9):78.
8. Park EJ, Jun HW, Na IH, et al. CD48-expressing non-small-cell lung cancer cells are susceptible to natural killer cell-mediated cytotoxicity. Arch Pharm Res. 2022;45(1):1–10. doi:10.1007/s12272-021-01365-z
9. Wang Z, Xiao Y, Guan W, et al. Acute myeloid leukemia immune escape by epigenetic CD48 silencing. Clin Sci (Lond). 2020;134(2):261–271. doi:10.1042/CS20191170
10. Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30(1):44–56. doi:10.1093/annonc/mdy495
11. Cortes-Ciriano I, Lee S, Park WY, Kim TM, Park PJ. A molecular portrait of microsatellite instability across multiple cancers. Nat Commun. 2017;8(1):15180. doi:10.1038/ncomms15180
12. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi:10.1073/pnas.0506580102
13. Bonanno L, Pavan A, Dieci MV, et al. The role of immune microenvironment in small-cell lung cancer: distribution of PD-L1 expression and prognostic role of FOXP3-positive tumour infiltrating lymphocytes. Eur J Cancer. 2018;101:191–200. doi:10.1016/j.ejca.2018.06.023
14. Miao Y, Wang J, Li Q, et al. Prognostic value and immunological role of PDCD1 gene in pan-cancer. Int Immunopharmacol. 2020;89(Pt B):107080. doi:10.1016/j.intimp.2020.107080
15. Chen C, Cai Y, Liu Y, et al. Pan-Cancer Analysis of Microbiome Quantitative Trait Loci. Cancer Res. 2022;82(19):3449–3456. doi:10.1158/0008-5472.CAN-22-1854
16. McArdel SL, Terhorst C, Sharpe AH. Roles of CD48 in regulating immunity and tolerance. Clin Immunol. 2016;164:10–20. doi:10.1016/j.clim.2016.01.008
17. Ziblat A, Iraolagoitia XLR, Nunez SY, et al. Circulating and Tumor-Infiltrating NK Cells From Clear Cell Renal Cell Carcinoma Patients Exhibit a Predominantly Inhibitory Phenotype Characterized by Overexpression of CD85j, CD45, CD48 and PD-1. Front Immunol. 2021;12:681615. doi:10.3389/fimmu.2021.681615
18. Pan S, Zhan Y, Chen X, Wu B, Liu B. Bladder Cancer Exhibiting High Immune Infiltration Shows the Lowest Response Rate to Immune Checkpoint Inhibitors. Front Oncol. 2019;9. doi:10.3389/fonc.2019.01101
19. Fumet JD, Truntzer C, Yarchoan M, Ghiringhelli F. Tumour mutational burden as a biomarker for immunotherapy: current data and emerging concepts. Eur J Cancer. 2020;131:40–50. doi:10.1016/j.ejca.2020.02.038
20. Lee DW, Han SW, Bae JM, et al. Tumor Mutation Burden and Prognosis in Patients with Colorectal Cancer Treated with Adjuvant Fluoropyrimidine and Oxaliplatin. Clin Cancer Res. 2019;25(20):6141–6147. doi:10.1158/1078-0432.CCR-19-1105
21. Chen S, Su X, Mo Z. KCNN4 is a Potential Biomarker for Predicting Cancer Prognosis and an Essential Molecule that Remodels Various Components in the Tumor Microenvironment: a Pan-Cancer Study. Front Mol Biosci. 2022;9:812815. doi:10.3389/fmolb.2022.812815
22. Goodman AM, Sokol ES, Frampton GM, Lippman SM, Kurzrock R. Microsatellite-Stable Tumors with High Mutational Burden Benefit from Immunotherapy. Cancer Immunol Res. 2019;7(10):1570–1573. doi:10.1158/2326-6066.CIR-19-0149
23. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–2520. doi:10.1056/NEJMoa1500596
24. Goodman AM, Kato S, Bazhenova L, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther. 2017;16(11):2598–2608. doi:10.1158/1535-7163.MCT-17-0386
25. Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med. 2017;377(25):2500–2501. doi:10.1056/NEJMc1713444
26. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi:10.1126/science.aaa1348
27. Duffy MJ, Crown J. Biomarkers for Predicting Response to Immunotherapy with Immune Checkpoint Inhibitors in Cancer Patients. Clin Chem. 2019;65(10):1228–1238. doi:10.1373/clinchem.2019.303644
28. Su JY, Li WH, Li YM. New opportunities for immunomodulation of the tumour microenvironment using chemical tools. Chem Soc Rev. 2022;51(18):7944–7970. doi:10.1039/D2CS00486K
29. Yenyuwadee S, Aliazis K, Wang Q, et al. Immune cellular components and signaling pathways in the tumor microenvironment. Semin Cancer Biol. 2022;86(Pt 2):187–201. doi:10.1016/j.semcancer.2022.08.004
30. Grisaru-Tal S, Rothenberg ME, Munitz A. Eosinophil-lymphocyte interactions in the tumor microenvironment and cancer immunotherapy. Nat Immunol. 2022;23(9):1309–1316. doi:10.1038/s41590-022-01291-2
31. Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117(5):1137–1146. doi:10.1172/JCI31405
32. Demaria O, Cornen S, Daeron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574(7776):45–56. doi:10.1038/s41586-019-1593-5
33. Huang CH, Liao YJ, Chiou TJ, Huang HT, Lin YH, Twu YC. TGF-beta regulated leukemia cell susceptibility against NK targeting through the down-regulation of the CD48 expression. Immunobiology. 2019;224(5):649–658. doi:10.1016/j.imbio.2019.07.002
34. Wu Y, Kuang DM, Pan WD, et al. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology. 2013;57(3):1107–1116. doi:10.1002/hep.26192
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.