Back to Journals » Journal of Inflammation Research » Volume 14
A Synergy Between Endotoxin and (1→3)-Beta-D-Glucan Enhanced Neutrophil Extracellular Traps in Candida Administered Dextran Sulfate Solution Induced Colitis in FcGRIIB-/- Lupus Mice, an Impact of Intestinal Fungi in Lupus
Authors Saithong S, Saisorn W , Visitchanakun P, Sae-khow K, Chiewchengchol D , Leelahavanichkul A
Received 6 February 2021
Accepted for publication 14 May 2021
Published 1 June 2021 Volume 2021:14 Pages 2333—2352
DOI https://doi.org/10.2147/JIR.S305225
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Supichcha Saithong,1– 3 Wilasinee Saisorn,3 Peerapat Visitchanakun,3 Kritsanawan Sae-khow,3 Direkrit Chiewchengchol,2,3 Asada Leelahavanichkul2,3
1Medical Microbiology, Interdisciplinary and International Program, Graduate School, Chulalongkorn University, Bangkok, Thailand; 2Immunology Unit, Department of Microbiology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; 3Translational Research in Inflammation and Immunology Research Unit (TRIRU), Department of Microbiology, Chulalongkorn University, Bangkok, Thailand
Correspondence: Asada Leelahavanichkul; Direkrit Chiewchengchol
Immunology Unit, Department of Microbiology, Chulalongkorn University, Bangkok, 10330, Thailand
Tel +66-2-256-4251
Fax +66-2-252-6920
Email [email protected]; [email protected]
Introduction: The translocation of organismal molecules from gut into blood circulation might worsen the disease severity of lupus through the induction of neutrophil extracellular traps (NETs).
Methods: An impact of lipopolysaccharide (LPS) and (1→ 3)-β-D-glucan (BG), components of gut bacteria and fungi, respectively, on NETs formation, was explored in lupus models, Fc gamma receptor IIB deficiency (FcGRIIB-/-) and Pristane injection, using Candida-administered dextran sulfate solution induced colitis (Candida-DSS) model.
Results: Severity of Candida-DSS in FcGRIIB-/- mice was more prominent than wild-type (WT) and Pristane mice as indicated by (i) colonic NETs using immunofluorescence of Ly6G, myeloperoxidase (MPO) and neutrophil elastase (NE) together with expression of PAD4 and IL-1β, (ii) colonic immunoglobulin (Ig) deposition (immunofluorescence), (iii) gut-leakage by FITC-dextran assay, endotoxemia and serum BG, (iv) systemic inflammation (neutrophilia, serum cytokines, serum dsDNA and anti-dsDNA) and (v) renal injury (proteinuria, glomerular NETs and Ig deposition).
Discussion: The formation of NETs in Candida-DSS mice was more severe than non-Candida-DSS mice and NETs in Candida-DSS were more profound in FcGRIIB-/- mice than Pristane mice. Prominent NETs in Candida-DSS FcGRIIB-/- mice might be due to the profound responses against LPS+BG in FcGRIIB-/- neutrophils compared with WT cells. These data implied an impact of the inhibitory FcGRIIB in NETs formation and an influence of gut fungi in lupus exacerbation. Hence, gut fungi in a DSS-induced gut-leakage lupus model enhanced colonic NETs that facilitated gut translocation of organismal molecules and synergistically exacerbated lupus activity.
Keywords: FcGRIIB deficient mice, Pristane mice, systemic lupus erythematosus, neutrophil extracellular traps, Candida, gut-leakage
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease caused by genetic predispositions and environmental factors. There is a high prevalence of dysfunction polymorphism of Fc gamma receptor IIb (FcGRIIB) gene, the only inhibitory receptor in the Fc gamma receptor (FcgR) family, in Asian populations.1 As such, FcGRIIB-/- mice represent a genetic prone lupus model through the loss of inhibitory FcGRIIB signaling,2,3 while injection of Pristane, a hydrocarbon oil, in wild-type (WT) mice represents a lupus model from environmental activation via the sustained IFN-γ mediated-inflammation.4,5 The gut translocation of endotoxin (a Gram-negative bacterial component) in active lupus, referred to as “gut-leakage”,6,7 and the common enterocolitis from infections or adverse drug reactions in lupus8 are mentioned. Despite the non-prominent gastrointestinal (GI) symptoms in patients with lupus, peritonitis is found in 60–70% of postmortem autopsy in patients with SLE.7 Due to a significant burden of acute diarrhea in developing countries9–11 and a high prevalence of both acute diarrhea and lupus among Asians,1 the co-existence of diarrhea-induced gut leakage and lupus is possible.
Previous reports note that (i) gut-leakage from chronic intestinal inflammation exacerbates lupus disease activity,12–14 (ii) gut translocation of pathogen molecules such as lipopolysaccharide (LPS) and (1→3)-β-D-glucan (BG), the main component of Gram-negative bacteria and fungi, respectively, synergistically induced systemic inflammation4,15 and (iii) the exposure of auto-antigens from neutrophil extracellular traps (NETs; a web-like structure released by activated neutrophils) enhances auto-antibody production in lupus.16 Interestingly, NETs are inducible by different pathogen molecules, including LPS and BG, and the NETs extracellular structure is composed of nuclear components (e.g., chromatin, DNA and histone) and cytosolic anti-microbial molecules from the neutrophils.17,18 Therefore, gut-leakage possibly accelerates immune recognition of the auto-antigens from NETs that is induced by organismal molecules and gut-leakage might become an exacerbating factor of lupus.
In the gut, Gram-negative bacteria are an important source of LPS, an endogenous toxin, in both human and mice. Meanwhile, in mice, there is less abundance of fungi, particularly C. albicans, which is a source of gut BG.19,20 Hence, Candida administration in mice might, at least in part, make the mouse model resemble more that of human conditions.21,22 Indeed, gut translocation of BG enhances systemic inflammation in active lupus6,7 and the synergistic activation of LPS with BG through TLR-4 and Dectin-1, respectively, accelerates inflammation.23,24 Although a crosstalk between TLR-4 and Dectin-1 receptors in lupus macrophages has been previously explored,4,15,25 the effect on neutrophils in lupus remains unclear. Due to the detectable gut-leakage in active lupus,6,7 gut-leakage might exacerbate lupus activity partly through the neutrophils cell death, including NETosis (the cell death after NETs formation), that is induced by gut translocation of LPS and BG.
Although infective diarrhea is the most common cause of gut-leakage,26 the administration of a pathogenic organism into the model induces not only gut-leakage but also the immune responses against infection, making it difficult to separate the impact from both factors. Hence, to study only the effect of gut-leakage in acute diarrhea, non-infectious acute diarrheal induction using dextran sulfate solution (DSS), a well-known agent for gut-leakage induction in mice,12,27 was performed. Here, DSS administration, with or without C. albicans, was conducted in two lupus mouse models from genetic predisposition (FcGRIIB-/-) and chemical induction (Pristane).
Materials and Methods
Animals and Animal Model
The Institutional Animal Care and Use Committee of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, gave approval following the protocol of the National Institutes of Health (NIH), USA. FcGRIIB deficient mice on a C57BL/6 background (FcGRIIB-/-) were provided by Dr. Silvia Bolland (NIAID, NIH, Maryland, USA) and wild-type (WT) mice were purchased from Nomura Siam International (Pathumwan, Bangkok, Thailand). Only female mice were used in the experiments.12,26 Because FcGRIIB-/- mice and Pristane (administered in 4-wk-old-mice) model develop anti-dsDNA (a major auto-antibody of lupus) as early as 24 wks old,4–6,15,28 FcGRIIB-/- mice and Pristane mice at 16 wks old were used as a representative model of asymptomatic lupus.
To induce gut-leakage, dextran sulfate solution (DSS) (Sigma-Aldrich, St. Louis, MO, USA) was prepared by dilution into drinking water at the concentration of 3% (v/v) for 1 wk following a previous publication.29 In parallel, C. albicans (American Type Culture Collection, ATCC90028, Fisher Scientific, Waltham, MA, USA) at 1×106 colony forming unit (CFU), using a hemocytometer, diluted in 0.3 mL of phosphate buffer solution (PBS) were orally administered with DSS and at days 1, 3 and 5 of the experiment as previously published.29 Mice were sacrificed at day 7 post-DSS by cardiac puncture under isoflurane anesthesia with blood and organ collection. Urine was collected for 24 h before sacrifice by placing mice into a metabolic cage (Hatteras Instruments, NC, USA) for the determination of urine protein by Bradford protein assay (Sigma-Aldrich). The stool consistency was semi-quantitatively evaluated using the following score; 0, normal; 1, soft; 2, loose and 3, diarrhea, as previously published.30
Serum creatinine and cytokines (IL-6, TNF-α and IL-10) were measured by QuantiChrom Creatinine Assay (BioAssay Systems, Hayward, CA, USA) and ELISA (Invitrogen, Vienna, Austria), respectively. Serum anti-dsDNA was analyzed by a protocol using coated Calf-DNA (Invitrogen, Carlsbad, CA, USA).31 The percentage of peripheral blood neutrophils was determined by Wright staining following a previous publication.32,33 Serum dsDNA, a biomarker of NETs in peripheral blood,17 was determined using Quanti PicoGreen assay. Additionally, expression of several genes in colon was determined using real-time polymerase chain reaction (PCR)34 and a high-capacity reverse transcription assay (Applied Biosystems, Warrington, UK), respectively. Real-time PCR was performed using an Applied Biosystems QuantStudio 6 Flex Real-Time PCR system with SYBR® Green PCR Master Mix (Applied Biosystems) and the results were demonstrated in terms of relative quantitation using the comparative threshold (delta-delta Ct) method (2−ΔΔCt) as normalized by β-actin (an endogenous housekeeping gene). Primer sequences are presented in Table 1. Mouse organs (colon and kidney) were kept in 10% formalin or in Tissue-Tek optimum cutting temperature (O.C.T.) compound (Sakura Finetek, CA, USA) or snap frozen and kept in −80 °C before further use.
 |
Table 1 List of Primers |
Gut Permeability Determination
Fluorescein isothiocyanate-dextran (FITC-dextran, a gut non-absorbable molecule at molecular weight 4.4 kDa; Sigma-Aldrich), was orally administered at the concentration of 25 mg/mL in 0.25 mL PBS at 3 h before sacrifice to determine gut permeability.35 Serum FITC-dextran was measured by fluorospectrometry (microplate reader; Thermo Scientific, Wilmington, DE, USA). Other gut-leakage parameters, serum endotoxin (lipopolysaccharide; LPS) and (1→3)-β-D-glucan (BG) were measured using HEK-Blue LPS detection (InvivoGen, San Diego, CA, USA) and Fungitell (Associates of Cape Cod, Falmouth, MA, USA). The values of LPS < 0.01 EU/mL and BG <7.8 pg/mL were recorded as 0 due to the limitation of the standard curves.
Histology Analysis
The semi-quantitative evaluation of intestinal histology on hematoxylin and eosin (H&E) staining at 200x magnification was determined26 based on mononuclear cell infiltration (in mucosa and sub-mucosa), epithelial hyperplasia (epithelial cell in longitudinal crypts), reduction of goblet cell and epithelial cell vacuolization in comparison with control groups using the following scores; 0: leukocyte < 5% and no epithelial hyperplasia (<10% of control); 1: leukocyte infiltration 5–10% or hyperplasia 10–25%; 2: leukocyte infiltration 10–25% or hyperplasia 25–50% or reduced goblet cells (> 25% of control); 3: leukocyte infiltration 25–50% or hyperplasia >50% or intestinal vacuolization; 4: leukocyte infiltration >50% or ulceration. In parallel, renal tissue with H&E staining was determined only by glomerular injury score due to the very subtle injury in other parts of kidney (tubule and interstitial area) (data not shown). All glomeruli in the slides were examined and the percentage of glomeruli with mesangial expansion (>25% or normal) at 400x magnification represented the glomerular injury score.
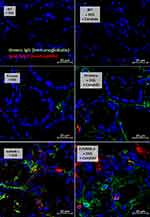
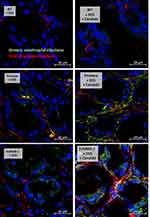
In parallel, NETs formation in colon and in glomeruli was visualized by immunofluorescence prepared in Tissue-Tek O.C.T. compound (Sakura Finetek),34 stained with the primary antibodies against myeloperoxidase (anti-MPO) (ab 25989) and neutrophil peroxidase (anti-NE) (ab68672) with specific secondary antibodies of goat anti-mouse immunoglobulin G (IgG) coupled with Alexa Fluor 647 (ab 150115) and Alexa Fluor 488 (ab150077) with DAPI (4-,6-diamidino-2-phenylindole) (Sigma-Aldrich). Likewise, the goat anti-mouse IgG coupled with Alexa Fluor 488 (ab150113) was used for detection of immunoglobulin deposition in colon and kidney. While IgG deposition on damaged colon indicates the DSS-induced intestinal injury with wound repairing processes,36 the antibody deposition in kidneys with high anti-dsDNA indicates immune complex deposition and lupus disease activation.12 Additionally, the neutrophil infiltration in colon was evaluated by anti-Ly6G (BioLegend 127609). The fluorescence intensity was visualized and analyzed by confocal microscope (ZEISS LSM 800, Carl Zeiss, Germany).
Neutrophil Isolation and the in vitro Experiments
Mice were intraperitoneally injected with 1 mL of 3% Thioglycolate (Sigma-Aldrich) or PBS (control) and the peritoneal neutrophils were harvested after 2 h post-injection by lavages of the peritoneal cavity with 20 mL of cold PBS after sacrifice with cardiac puncture under isoflurane anesthesia. The cells were centrifuged and washed with PBS and resuspended in Roswell Park Memorial Institute (RPMI) media 1640 with 25 mM HEPES, L-Glutamine (Hyclone, Marlborough, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco Life Technologies, Paisley, UK). For human neutrophils, heparinized blood was collected from healthy donors under an approved protocol by the Ethical Institutional Review Board, Faculty of Medicine, Chulalongkorn University, according to the Declaration of Helsinki, with written informed consent. Neutrophils were isolated by density centrifugation with Polymorphprep™ (Axis-Shield, Oslo, Norway) according to the manufacturer’s instructions. The contaminated erythrocytes were removed by hypotonic lysis buffer. Cells were resuspended in RPMI 1640 supplemented with 10% FBS. Cell viability of human and mouse neutrophils was measured by Trypan blue dye (Sigma-Aldrich) exclusion method. Only viable cells which do not take up the dye were counted by a hemocytometer. Cell purity was assessed by Wright Giemsa staining following the manufacturer’s instructions. The cells with segmental lobe nuclei and granules which indicated neutrophil morphology were counted per total 100 cells. Only the samples with cell viability and purity of neutrophils of more than 95% were further processed for the experiments.
Human or mouse neutrophils were placed onto Poly-L-Lysine (Sigma-Aldrich) coated glass coverslips, incubated at 37°C, 5% CO2 for 1 h with BG, using whole glucan particle (WGP) that was purified from Saccharomyces cerevisiae (Biothera, Eagan, MN), at 10 µg/mL with or without LPS (Escherichia coli 026: B6) (Sigma–Aldrich) at 10 µg/mL. After 2 h of the incubation, the coverslips were fixed with 4% formaldehyde, blocked with Tris Buffered Saline (TBS) in 2% bovine serum albumin (BSA) (Sigma-Aldrich) and permeabilized by TBS with 0.05% Tween 20 (Sigma-Aldrich). Then, NETs formation was detected by (i) immunofluorescence (anti-MPO, anti-NE and DAPI) in human neutrophils as previously described and the nucleus morphology, using DAPI staining, in mouse neutrophils before mounting on glass slides using ProLong anti-fade (Invitrogen)37 and (ii) free dsDNA representing NETs in supernatant. After neutrophils were incubated for 2 h, 0.1 M CaCl2 was added, followed by 0.5 unit (U) of micrococcal nuclease (Sigma-Aldrich, Singapore) and incubated at 37°C, 5% CO2 for 10 min. The nuclease reaction was stopped by 0.5 M ethylenediaminetetraacetic acid (EDTA). Supernatant was collected and centrifuged to remove the cell pellets. Subsequently, the Quant-iT™ PicoGreen reagent (Thermo Fisher Scientific, Auckland, New Zealand) was added to measure dsDNA according to the manufacturer’s instructions. In brief, aqueous working solution of the Quant-iT™ PicoGreen® reagent was added into each sample. The mixtures were incubated for 5 min at room temperature, protected from light. The amount of dsDNA in the mixture was measured at 480 nm excitation (520 nm emission) on a fluorescent microplate reader38 to test the possible synergy between LPS with BG.22,23,39,40
Statistical Analysis
Statistical differences among groups were examined using the unpaired Student’s t-test or one-way analysis of variance (ANOVA) with Tukey’s comparison test for the analysis of experiments with two groups or more than two groups, respectively. Time-point data were analyzed by repeated measures ANOVA. Data were presented as the mean ± standard error (SE). Statistical comparisons of data before and after treatment were conducted by paired Student’s t-test. SPSS 11.5 software (SPSS, Chicago, IL, USA) was used for all statistical analyses.
Results
The severity of DSS-induced colitis was enhanced by Candida administration which was more prominent in FcGRIIB-/- mice than Pristane mice, partly due to the accelerated NETs formation from the loss of the inhibitory FcGRIIB signaling.
The Prominent Severity of Colitis in DSS-Administered Candida Gavage FcGRIIB-/- Mice, the Loss of Inhibitory Signaling Induced Hyper-responsiveness Against Organismal Molecules
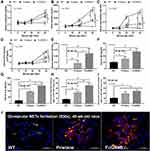
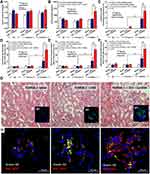
Spontaneous lupus nephritis (anti-dsDNA, proteinuria and serum creatinine) and gut permeability defect (serum FIT-C dextran) in the time-course evaluation (Figure 1A–D) together with the parameters of 40-wk-old mice, including gut translocation of organismal molecules (LPS and BG), systemic inflammation (serum IL-6) and glomerular NETs in FcGRIIB-/- full-blown lupus mice were more prominent than Pristane mice (Figure 1E–J). However, the glomerular neutrophil elastase (NE) showed no difference between FcGRIIB-/- mice and Pristane mice (Figure 1I). Notably, the glomerular NETs, but not the intestinal NETs (data not shown), were detectable only in 40-wk-old lupus mice (FcGRIIB-/- and Pristane) (Figure 1H and I) but not in the 16-wk-old mice of both groups (data not shown). In 40-wk-old full-blown FcGRIIB-/- lupus mice, the spontaneous NETs in glomeruli, but not in colon, indicates a limited impact of colon NETs in the lupus pathogenesis. To explore the impact of gut-leakage in asymptomatic lupus, 16-wk-old mice were used in all experiments.
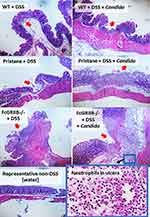
In 16-wk-old mice, the severity of DSS-induced colitis was significantly increased by Candida administration particularly in FcGRIIB-/- mice which was more severe than Pristane mice. The biomarkers of active lupus (serum anti-dsDNA, serum creatinine and urine protein) between WT and lupus mice (FcGRIIB-/- and Pristane) was not different, indicating asymptomatic lupus before the DSS administration (Figure 1A–C, 16-wk-old time-point). At 7 days of DSS, Candida accelerated DSS-induced weight reduction in all groups and the weight loss was more severe in FcGRIIB-/- mice (Figure 2A–D). Despite the comparable diarrheal severity in all DSS-administered mice (WT, FcGRIIB-/- and Pristine) with or without Candida (Figure 2E–H), DSS-Candida FcGRIIB-/- mice demonstrated the most severe colitis (Figure 3A). Of note, Candida worsened DSS-induced colitis in all groups (Figure 3A). Also, the injury in DSS-Candida FcGRIIB-/- mice was more severe than Pristine mice (Figures 3A and 4), despite a similar lupus-prone condition between lupus models (Figure 1A and B, 16-wk-old time-point).
The Prominent Neutrophil Infiltration and NETs in Colon of DSS-Administered Candida-Gavage FcGRIIB-/- Mice, an Impact of Inhibitory FcGRIIB on NETs Formation
Candida accelerated neutrophil infiltration (Ly6G staining) and immunoglobulin deposition, an indicator for the severity of injury, in the colon of DSS lupus mice (more severe in FcGRIIB-/- mice than Pristane mice), but not in WT mice (Figures 3B and C–5). In colon, Candida more prominently enhanced NETs (MPO, NE and PAD4 expression) (Figures 3D–F and 6) and inflammation (gene expression of IL-1β, IL-6 and TNF-α) in both lupus models (more severe in FcGRIIB-/- mice) compared with WT (Figure 3G–I) which implied the high Candida-induced NETs in the immune hyper-responsive condition of lupus. Meanwhile, Candida alone without DSS did not induce intestinal injury in all models (data not shown), indicating the protective property of the healthy gut. Because of the dominant intestinal injury in DSS-Candida FcGRIIB-/- groups, the inflammation might be severe enough to induce systemic inflammatory responses.
The Lupus Disease Exacerbation Through Gut-Leakage Induced Systemic Inflammation, an Enhanced NETs Formation by Endotoxin and (1→3)-β-D-Glucan in Synergy
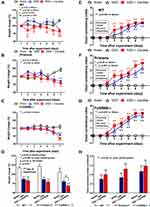
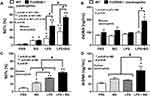
Accordingly, Candida worsened DSS-induced gut-leakage as indicated by FITC-dextran assay and damaged intestinal mucosa that was severe enough for the translocation of LPS and BG, the major organismal components, from gut into blood circulation in all groups (Figure 7A–C). However, the level of endotoxemia, a potent pro-inflammatory activator, was highest in DSS-Candida FcGRIIB-/- mice (Figure 7B). In parallel, the circulating organismal molecules (LPS and BG) prominently increased peripheral blood neutrophils of DSS-Candida mice in comparison with DSS activation alone (similar severity between WT and lupus mice) (Figure 7D). The circulatory NETs, as determined by serum dsDNA, increased only in DSS FcGRIIB-/- mice (with or without Candida), but not in DSS WT mice (Figure 7E). The non-detectable NETs in DSS WT mice with the presentation of NETs inducers (LPS and BG)41,42 in serum supported an impact of Fc gamma receptors (FcGRs) on NETs formation.43 Moreover, the profound NETs in DSS-Candida FcGRIIB-/- mice over Pristane mice resulted in the more prominent inflammatory cytokines and serum anti-dsDNA (Figure 7F–I), the biomarkers of lupus disease activity. Notably, there was neutrophilia in DSS-Candida WT mice, but neither NETs nor serum cytokines (Figure 7E–H), highlighting the impact of inhibitory FcGRIIB on NETs attenuation in WT mice. Therefore, the increased susceptibility to inflammation of FcGRIIB-/- lupus mice toward gut Candida provided several effects, including (i) the worsened intestinal injury (intestinal neutrophils and NETs) (Figure 3B–F), (ii) the enhanced gut translocation of organismal molecules (Figure 7B and C), (iii) the induction of systemic inflammation (Figure 7F and G) and (iv) the aggravation of lupus disease activity (anti-dsDNA) (Figure 7I).
The Induction of Lupus Nephritis in DSS-Administered Candida-Gavage FcGRIIB-/- Mice Due to Prominent Immune Complex Deposition and NETs in Glomeruli
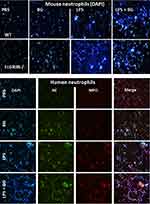
There were no characteristics of lupus nephritis (proteinuria, kidney injury and anti-dsDNA) in control 16-wk-old FcGRIIB-/- mice (Figures 8A and B and 7I). However, the induction by DSS-Candida (but not DSS alone) induced lupus nephritis only in FcGRIIB-/- mice, but neither in WT mice nor Pristane mice, as indicated by proteinuria, glomerular mesangial expansion, immune complex deposition and renal NETs formation (MPO and NE) (Figure 8A–H). These data supported an influence of immune complex deposition and NETs formation in lupus.44 In contrast, DSS-Candida Pristane mice demonstrated only a slight increase in anti-dsDNA (Figure 7I) without lupus nephritis (Figure 8B) compared with DSS-Candida FcGRIIB-/- mice, possibly due to the intact FcGRIIB inhibitory signals in Pristane mice. Since LPS and BG induced NETs,41,42 the effect of combined LPS and BG (LPS+BG) on NETs formation was explored both in human and in mouse neutrophils. As such, LPS+BG more prominently induced NETs, when compared with LPS alone, as indicated by dsDNA in supernatant and the percentage of cells with NETs (human neutrophils) (Figures 9C and D and 10). In mouse neutrophils, NETs in LPS+BG activated FcGRIIB-/- neutrophils was more prominent than the WT cells (Figures 9A and B and 10) supporting an influence of LPS+BG (from gut translocation) and an impact of the loss of inhibitory FcGRIIB in neutrophils on lupus. In LPS+BG activation (but not the activation by each molecule alone), the gene expression of Syk, NFκB and activating-FcGRIV, but not inhibitory-FcGRIIB, were prominently upregulated in FcGRIIB-/- neutrophils compared with WT cells (Figure 11A–G). These data imply a role of FcGRs, Syk and NFκB in neutrophilic NETs formation.
Discussion
A more easily induced NETs in DSS-administered Candida gavage FcGRIIB-/- mice than Pristane mice implied an impact of gut fungi and inhibitory-FcGRIIB signaling on NETs formation.
Prominent Enteropathy in DSS-Administered Candida-Gavage FcGRIIB-/- Mice Over Pristane Mice, an Impact of Gut Fungi and FcGRs in NETs Formation
Since (i) gastrointestinal symptoms in patients with SLE45 are due to silent intestinal vasculitis46 and immunosuppression-induced colitis,47,48 (ii) there is possible co-existence of lupus and acute diarrhea in the developing countries,10,11 (iii) there is a lower abundance of fungi in mouse guts than the human intestines26 and (iv) the double-edged sword of NETs in the pathogen control versus the enhanced autoantigen exposure is reported,49 the impact of gut mucosal injury and fungi on lupus using a model of DSS with Candida gavage (DSS-Candida) was performed. The lupus models were performed in 16-wk-old mice in FcGRIIB-/- mice and Pristane mice (12 wks post-Pristane injection) as the representative models of asymptomatic lupus prone mice caused by a genetic defect and an external stimulation in the mice with normal genetic background, respectively. As such, Pristane (a hydrocarbon from shark oil) induces chronic peritonitis, recruits peritoneal neutrophil accumulation, causes NETs-induced diffuse alveolar hemorrhage (DAH) in some mice50 and also directly triggers the neutrophilic NETs in vitro.51 However, lung hemorrhage, a moribund complication of Pristane, was not detectable in our Pristane mice (data not shown). Although the pathophysiology and the clinical presentations are different between FcGRIIB-/- mice and Pristane mice5 including the hyper-responsive inflammation with dominant lupus nephritis and the potent activation of interferon (IFN) type I with injury in several organs, respectively, both lupus models could be used to compare the lupus severity between the DSS model with versus without Candida administration. Notably, a dominant impact of gut-leakage in FcGRIIB-/- mice over Pristane mice using a non-diarrheal 1% DSS model through macrophage activation12 is demonstrated; however, the influence of symptomatic colitis on neutrophils in these models has never been tested.
With 3% DSS for 7 days, obvious diarrhea with similar clinical manifestations (weight loss and loose stool consistency) presented in all groups (WT, FcGRIIB-/- and Pristane), but DSS induced the more severe pathological injury (ulcers and neutrophil infiltration) in lupus models (more severe in FcGRIIB-/- mice than Pristane mice), possibly through the mild inflammatory status in lupus.12,15 While the intestinal NETs, using MPO and NE with PAD4 (a chromatin de-condensation enzyme), in DSS alone were far less in all groups, DSS-Candida induced more NETs52–55 with severe pathological injury (neutrophil accumulation56,57) in all groups (more prominent in FcGRIIB-/- mice than Pristane mice). These data supported an impact of gut Candida in DSS-induced non-infectious colitis model.26 Likewise, the more severe intestinal NETs in DSS-Candida FcGRIIB-/- mice than Pristane mice supported a possible impact of FcGRs on NETs formation,43 partly through the enhancement of several activating FcGRs due to the loss of inhibitory FcGRIIB.4,15 Meanwhile, the limited intestinal NETs in DSS-Candida Pristine mice implied a balance against the pro-inflammatory signals through an intact inhibitory FcgRIIB due to a normal genetic background. In 40-wk-old FcGRIIB-/- mice (a full-blown lupus), NETs were spontaneously detectable only in kidneys, but not in the intestines, suggesting that NETs did not originate from the intestines in active lupus. In contrast, with DSS-induced gut inflammation, NETs formation started at the intestines and accelerated NETs elsewhere (in blood circulation and in kidneys), implying lupus disease exacerbation through gut-leakage.
Prominent Gut-Leakage Induced Inflammation Aggravated Lupus Nephritis in DSS-Administered Candida-Gavage FcGRIIB-/- Mice
The more severe gut translocation of organismal molecules (LPS and BG) in DSS-Candida FcGRIIB-/- mice prominently activated systemic inflammation6,12,58 (blood neutrophils, circulating NETs and serum cytokines). Since (i) NETs could be induced by LPS, BG and immunoglobulin (Ig) through TLR-4, Dectin-1 and FcGRs,16 (ii) NETs are present in active lupus59–61 and (iii) the collaboration among TLR-4, Dectin-1 and FcGRs is mentioned,6,62 LPS+BG activation might profoundly enhance NETs in FcGRIIB-/- neutrophils. Accordingly, LPS+BG, when compared with LPS alone, more prominently enhanced NETs in FcGRIIB-/- neutrophils than WT cells, which implies the importance of TLR-4, Dectin-1 and FcGRs on NETs formation.43 Furthermore, the more severe inflammation in DSS-Candida FcGRIIB-/- mice also exacerbated lupus disease activity (increased anti-dsDNA and proteinuria), supporting inflammation-aggravating lupus activity. The high level of anti-dsDNA resulted in an accelerated Ig deposition in glomeruli that induced glomerular mesangial expansion, glomerular NETs and proteinuria in FcGRIIB-/- mice, but not in Pristane mice. Indeed, proteinuria is a risk factor of renal progression63 and is also a poor prognostic factor of lupus. Hence, Candida presentation in FcGRIIB-/- lupus prone mice exacerbated lupus activity through the induction of intestinal NETs that were severe enough to cause systemic inflammation and the inflammation aggravated lupus nephritis. Also, gut-leakage induced inflammation in 16-wk-old FcGRIIB-/- mice (asymptomatic lupus) also implied a possibility of lupus exacerbation from acute diarrhea (non-infectious and infectious causes) in full-blown lupus. Although DSS is not administered in 40-wk-old FcGRIIB-/- full-blown lupus mice, DSS-induced gut leakage in the active lupus with a pre-formed Ig deposition in gut might worsen the lupus activity. Moreover, there are several limitations in our lupus mouse models for use as a representative of patients with lupus, including the absence of other important lupus characteristics (polyarthritis, skin manifestations and serositis64) and the diversity of genetic defects in patients. An impact of gut-leakage in other lupus models might be different.
Prominent Inflammatory Responses in FcGRIIB-/- Neutrophils Over Wild-Type Cells, an Inhibitory Effect of FcGRIIB
Since (i) spleen tyrosine kinase (Syk) is a common downstream signaling from the activation of TLR-4, Dectin-1 and FcGRs4,15 that activates NFκB, a NETs-associated transcriptional factor,65,66 (ii) LPS and BG could activate NETs through TLR-4 and Dectin-1, respectively,67,68 and (iii) FcGRs are also correlated with NETs formation,43,69 these signals might be associated with the prominent NETs in LPS+BG activated FcGRIIB-/- neutrophils. Here, we hypothesize that the mechanism of NETs formation in neutrophils might be similar to the macrophages due to the presence of all receptors in both cell types.2,3 In FcGRIIB-/- neutrophils, only LPS+BG, but not LPS or BG alone, upregulated Syk, NFκB and activating-FcGRIV. Meanwhile, LPS+BG in WT neutrophils, but not by each molecule alone, upregulated only NFκB and inhibitory-FcGRIIB. In contrast, FcGRI and FcGRIII expression after activation by LPS and/or BG were not different, implying a limited role of these receptors in NETs formation. Notably, mouse FcGRIV is possibly more active than FcGRIII, because FcGRIV recognizes 3 out of 4 isoforms of mouse IgG (IgG1, IgG2a, and IgG2b), while FcGRIII recognizes only mouse IgG1 (mouse IgG3 is non-recognizable by any mouse FcGRs).70,71 Because NETs formation occurs after the activation on TLR-4, Dectin-1 and activating-FcGRs is previously mentioned16,65,69 and NFκB is also associated with NETs formation.65,66 We hypothesize the activation of TLR-4, Dectin-1 and activating-FcGRs (especially FcGRIV), without the inhibitory FcGRIIB, in FcGRIIB-/- neutrophils, though Syk and NFκB is responsible for the prominent NETs (Figure 11G). Additionally, the increased-NETs enhanced systemic inflammation in DSS-Candida FcGRIIB-/- mice.
Then, gut fungi prominently enhanced intestinal NETs in FcGRIIB-/- mice (a genetic prone lupus), compared with Pristane mice (a chemical-induced lupus), supporting the importance of FcGRs and gut fungi in NETs formation. Gut fungi increased BG in gut contents and LPS+BG from gut translocation exacerbated lupus nephritis in FcGRIIB-/- lupus mice through the profound NETs formation in FcGRIIB-/- neutrophils. Because the increase in both BG and LPS in serum is reported in patients with active lupus,6,7 our results demonstrate a possible impact of LPS+BG in lupus exacerbation through the induction of NETs formation.
In conclusion, the evaluation of gut fungi, gut-leakage and NETs formation in lupus are clinically interesting and the exploration of the association between gut and kidney (gut-kidney axis) in lupus might lead to new treatment strategies. Further studies are warranted.
Acknowledgments
Ratchadapisek Sompoch (CU_GR_63_108_3) and the Program Management Unit for Human Resources & Institutional Development Research and Innovation-CU [Global Partnership B16F630071 and Flagship B05F630073], TSRI Fund (CU_FRB640001_01_23_1) and National Research Council of Thailand. The authors would like to thank Dr. Prapaporn Pisitkun for the support of FcGRIIB-/- mice and Supichcha Saithong who supported the scholarship from “The 100th Anniversary Chulalongkorn University Fund for Doctoral Scholarship”.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chu ZT, Tsuchiya N, Kyogoku C, et al. Association of Fcgamma receptor IIb polymorphism with susceptibility to systemic lupus erythematosus in Chinese: a common susceptibility gene in the Asian populations. Tissue Antigens. 2004;63(1):21–27. doi:10.1111/j.1399-0039.2004.00142.x
2. Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13(2):277–285. doi:10.1016/S1074-7613(00)00027-3
3. Clatworthy MR, Willcocks L, Urban B, et al. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malaria. Proc Natl Acad Sci U S A. 2007;104(17):7169–7174. doi:10.1073/pnas.0608889104
4. Issara-Amphorn J, Chancharoenthana W, Visitchanakun P, Leelahavanichkul A. Syk inhibitor attenuates polymicrobial sepsis in FcgRIIb-deficient lupus mouse model, the impact of lupus characteristics in sepsis. J Innate Immun. 2020;12(6):461–479. doi:10.1159/000509111
5. Surawut S, Makjaroen J, Thim-uam A, et al. Increased susceptibility against cryptococcus neoformans of lupus mouse models (pristane-induction and FcGRIIb deficiency) is associated with activated macrophage, regardless of genetic background. J Microbiol. 2019;57(1):45–53. doi:10.1007/s12275-019-8311-8
6. Issara-Amphorn J, Surawut S, Worasilchai N, et al. The synergy of endotoxin and (1→3)-β-D-Glucan, from gut translocation, worsens sepsis severity in a lupus model of Fc gamma receptor IIb-deficient mice. J Innate Immun. 2018;10(3):189–201. doi:10.1159/000486321
7. Shi L, Zhang Z, Yu AM, et al. The SLE transcriptome exhibits evidence of chronic endotoxin exposure and has widespread dysregulation of non-coding and coding RNAs. PLoS One. 2014;9(5):e93846–e93846. doi:10.1371/journal.pone.0093846
8. Tian XP, Zhang X. Gastrointestinal involvement in systemic lupus erythematosus: insight into pathogenesis, diagnosis and treatment. World J Gastroenterol. 2010;16(24):2971–2977. doi:10.3748/wjg.v16.i24.2971
9. Treeprasertsuk S, Thepsuthammarat K, Kitsahawong B, Phaosawasdi K. Acute diarrhea, a significant burden to Thailan„ s universal health care system: a nationwide database. Asian Biomed. 2016;10:s23–s30.
10. Khalil I, El Bcheraoui C, Charara R, et al. Burden of diarrhea in the Eastern Mediterranean Region, 1990–2015: findings from the Global Burden of Disease 2015 study. Int J Public Health. 2018;63(1):109–121.
11. Pinzón-Rondón ÁM, Zárate-Ardila C, Hoyos-Martínez A, Ruiz-Sternberg ÁM, Vélez-van-meerbeke A. Country characteristics and acute diarrhea in children from developing nations: a multilevel study. BMC Public Health. 2015;15(1):811. doi:10.1186/s12889-015-2120-8
12. Thim-Uam A, Surawut S, Issara-Amphorn J, et al. Leaky-gut enhanced lupus progression in the Fc gamma receptor-IIb deficient and pristane-induced mouse models of lupus. Sci Rep. 2020;10(1):777. doi:10.1038/s41598-019-57275-0
13. Podolska M, Biermann M, Maueröder C, Hahn J, Herrmann M. Inflammatory etiopathogenesis of systemic lupus erythematosus: an update. J Inflamm Res. 2015;8:161–171. doi:10.2147/JIR.S70325
14. Deng GM, Tsokos GC. Cholera toxin B accelerates disease progression in lupus-prone mice by promoting lipid raft aggregation. J Immunol. 2008;181(6):4019–4026. doi:10.4049/jimmunol.181.6.4019
15. Issara-Amphorn J, Somboonna N, Pisitkun P, Hirankarn N, Leelahavanichkul A. Syk inhibitor attenuates inflammation in lupus mice from FcgRIIb deficiency but not in pristane induction: the influence of lupus pathogenesis on the therapeutic effect. Lupus. 2020;29(10):1248–1262. doi:10.1177/0961203320941106
16. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2017;18(2):134–147. doi:10.1038/nri.2017.105
17. Masuda S, Nakazawa D, Shida H, et al. NETosis markers: quest for specific, objective, and quantitative markers. Clin Chim Acta. 2016;459:89–93. doi:10.1016/j.cca.2016.05.029
18. Yang H, Biermann MH, Brauner JM, Liu Y, Zhao Y, Herrmann M. New insights into neutrophil extracellular traps: mechanisms of formation and role in inflammation. Front Immunol. 2016;7:302. doi:10.3389/fimmu.2016.00302
19. Koh AY. Murine models of Candida gastrointestinal colonization and dissemination. Eukaryot Cell. 2013;12(11):1416–1422. doi:10.1128/EC.00196-13
20. Borges FM, de Paula TO, Sarmiento MRA, et al. Fungal diversity of human gut microbiota among eutrophic, overweight, and obese individuals based on aerobic culture-dependent approach. Curr Microbiol. 2018;75(6):726–735. doi:10.1007/s00284-018-1438-8
21. Iliev ID, Leonardi I. Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat Rev Immunol. 2017;17(10):635–646. doi:10.1038/nri.2017.55
22. Panpetch W, Somboonna N, Bulan DE, et al. Gastrointestinal colonization of candida albicans increases serum (1→3)-β-D-Glucan, without candidemia, and worsens cecal ligation and puncture sepsis in murine model. Shock. 2018;49(1):62–70. doi:10.1097/SHK.0000000000000896
23. Dennehy KM, Ferwerda G, Faro-Trindade I, et al. Syk kinase is required for collaborative cytokine production induced through dectin-1 and toll-like receptors. Eur J Immunol. 2008;38(2):500–506. doi:10.1002/eji.200737741
24. Goodridge HS, Reyes CN, Becker CA, et al. Activation of the innate immune receptor dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472(7344):471–475. doi:10.1038/nature10071
25. Koenderman L. Inside-out control of Fc-receptors. Front Immunol. 2019;10:544.
26. Panpetch W, Hiengrach P, Nilgate S, et al. Additional Candida albicans administration enhances the severity of dextran sulfate solution induced colitis mouse model through leaky gut-enhanced systemic inflammation and gut-dysbiosis but attenuated by Lactobacillus rhamnosus L34. Gut Microbes. 2020;11(3):465–480. doi:10.1080/19490976.2019.1662712
27. Panpetch W, Chancharoenthana W, Bootdee K, et al. Lactobacillus rhamnosus L34 attenuates gut translocation-induced bacterial sepsis in murine models of leaky gut. Infect Immun. 2017;86:
28. Surawut S, Ondee T, Taratummarat S, et al. The role of macrophages in the susceptibility of Fc gamma receptor IIb deficient mice to Cryptococcus neoformans. Sci Rep. 2017;7(1):40006. doi:10.1038/srep40006
29. Hiengrach P, Panpetch W, Worasilchai N, et al. Administration of candida albicans to dextran sulfate solution treated mice causes intestinal dysbiosis, emergence and dissemination of intestinal Pseudomonas Aeruginosa and Lethal Sepsis. Shock. 2020;53(2):189–198. doi:10.1097/SHK.0000000000001339
30. Kim JJ, Shajib MS, Manocha MM, Khan WI. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp. 2012;60:e3678.
31. Mihara M, Tan I, Chuzhin Y, et al. CTLA4Ig inhibits T cell-dependent B-cell maturation in murine systemic lupus erythematosus. J Clin Invest. 2000;106(1):91–101. doi:10.1172/JCI9244
32. Dang CP, Leelahavanichkul A. Over-expression of miR-223 induces M2 macrophage through glycolysis alteration and attenuates LPS-induced sepsis mouse model, the cell-based therapy in sepsis. PLoS One. 2020;15(7):e0236038. doi:10.1371/journal.pone.0236038
33. Leelahavanichkul A, Somparn P, Bootprapan T, et al. High-dose ascorbate with low-dose amphotericin B attenuates severity of disease in a model of the reappearance of candidemia during sepsis in the mouse. Am J Physiol Regul Integr Comp Physiol. 2015;309(3):R223–R234. doi:10.1152/ajpregu.00238.2014
34. Dinallo V, Marafini I, Di Fusco D, et al. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J Crohns Colitis. 2019;13(6):772–784. doi:10.1093/ecco-jcc/jjy215
35. Leelahavanichkul A, Worasilchai N, Wannalerdsakun S, et al. Gastrointestinal leakage detected by serum (1→3)-β-D-Glucan in mouse models and a Pilot Study in patients with sepsis. Shock. 2016;46(5):506–518. doi:10.1097/SHK.0000000000000645
36. Nishio N, Ito S, Suzuki H, Isobe K-I. Antibodies to wounded tissue enhance cutaneous wound healing. Immunology. 2009;128(3):369–380. doi:10.1111/j.1365-2567.2009.03119.x
37. Sae-Khow K, Tachaboon S, Wright HL, et al. Defective neutrophil function in patients with sepsis is mostly restored by ex vivo Ascorbate Incubation. J Inflamm Res. 2020;13:263–274. doi:10.2147/JIR.S252433
38. Wright HL, Makki FA, Moots RJ, Edwards SW. Low-density granulocytes: functionally distinct, immature neutrophils in rheumatoid arthritis with altered properties and defective TNF signalling. J Leukoc Biol. 2017;101(2):599–611. doi:10.1189/jlb.5A0116-022R
39. Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10(10):2058–2066. doi:10.1111/j.1462-5822.2008.01188.x
40. Engstad CS, Engstad RE, Olsen JO, Osterud B. The effect of soluble beta-1,3-glucan and lipopolysaccharide on cytokine production and coagulation activation in whole blood. Int Immunopharmacol. 2002;2(11):1585–1597. doi:10.1016/S1567-5769(02)00134-0
41. Clark H, Abbondante S, Minns M, Greenberg E, Sun Y, Pearlman E. Protein deiminase 4 and CR3 regulate aspergillus fumigatus and β-glucan-induced neutrophil extracellular trap formation, but hyphal killing is dependent only on CR3. Front Immunol. 2018;9. doi:10.3389/fimmu.2018.01182
42. Li RHL, Ng G, Tablin F. Lipopolysaccharide-induced neutrophil extracellular trap formation in canine neutrophils is dependent on histone H3 citrullination by peptidylarginine deiminase. Vet Immunol Immunopathol. 2017;193–194:29–37. doi:10.1016/j.vetimm.2017.10.002
43. Alemán OR, Mora N, Cortes-Vieyra R, Uribe-Querol E, Rosales C. Differential use of human neutrophil fcγ receptors for inducing neutrophil extracellular trap formation. J Immunol Res. 2016;2016:2908034. doi:10.1155/2016/2908034
44. Liu Z, Davidson A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med. 2012;18(6):871–882. doi:10.1038/nm.2752
45. Gravito-Soares E, Gravito-Soares M, Cipriano MA, Amaro P. Fatal colitis associated with active systemic lupus erythematosus complicated by cytomegalovirus superinfection. BMJ Case Rep. 2017;2017:
46. Katsanos KH, Voulgari PV, Tsianos EV. Inflammatory bowel disease and lupus: a systematic review of the literature. J Crohns Colitis. 2012;6(7):735–742. doi:10.1016/j.crohns.2012.03.005
47. Dalbeni A, Capoferro E, Bernardoni L, et al. Pancolitis with ischemic injury as a complication of immunosuppressive treatment in a patient with autoimmune hepatitis: a case report. Case Rep Gastrointest Med. 2012;2012:698404. doi:10.1155/2012/698404
48. Vodusek Z, Feuerstadt P, Brandt LJ. Review article: the pharmacological causes of colon ischaemia. Aliment Pharmacol Ther. 2019;49(1):51–63. doi:10.1111/apt.15052
49. Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189(6):2689–2695. doi:10.4049/jimmunol.1201719
50. Jarrot P-A, Tellier E, Plantureux L, et al. Neutrophil extracellular traps are associated with the pathogenesis of diffuse alveolar hemorrhage in murine lupus. J Autoimmun. 2019;100:120–130. doi:10.1016/j.jaut.2019.03.009
51. Herman S, Kny A, Schorn C, et al. Cell death and cytokine production induced by autoimmunogenic hydrocarbon oils. Autoimmunity. 2012;45(8):602–611. doi:10.3109/08916934.2012.719948
52. Branzk N, Lubojemska A, Hardison SE, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15(11):1017–1025. doi:10.1038/ni.2987
53. Guiducci E, Lemberg C, Küng N, Schraner E, Theocharides APA, LeibundGut-Landmann S. Candida albicans-induced NETosis is independent of peptidylarginine deiminase 4. Front Immunol. 2018;9(1573). doi:10.3389/fimmu.2018.01573
54. Röhm M, Grimm MJ, D’Auria AC, Almyroudis NG, Segal BH, Urban CF. NADPH oxidase promotes neutrophil extracellular trap formation in pulmonary aspergillosis. Infect Immun. 2014;82(5):1766–1777. doi:10.1128/IAI.00096-14
55. Urban CF, Nett JE. Neutrophil extracellular traps in fungal infection. Semin Cell Dev Biol. 2019;89:47–57. doi:10.1016/j.semcdb.2018.03.020
56. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi:10.1038/nri3399
57. Swidergall M, Khalaji M, Solis NV, et al. Candidalysin is required for neutrophil recruitment and virulence during systemic candida albicans infection. J Infect Dis. 2019;220(9):1477–1488. doi:10.1093/infdis/jiz322
58. Ondee T, Surawut S, Taratummarat S, et al. Fc gamma receptor IIB deficient mice: a lupus model with increased endotoxin tolerance-related sepsis susceptibility. Shock. 2017;47(6):743–752. doi:10.1097/SHK.0000000000000796
59. Granger V, Peyneau M, Chollet-Martin S, de Chaisemartin L. Neutrophil extracellular traps in autoimmunity and allergy: immune complexes at work. Front Immunol. 2019;10(2824). doi:10.3389/fimmu.2019.02824
60. Moore S, Juo -H-H, Nielsen CT, Tyden H, Bengtsson AA, Lood C. Neutrophil extracellular traps identify patients at risk of increased disease activity and cardiovascular comorbidity in systemic lupus erythematosus. J Rheumatol. 2019;
61. Yu Y, Su K. Neutrophil extracellular traps and systemic lupus erythematosus. J Clin Cell Immunol. 2013;4(02):139. doi:10.4172/2155-9899.1000139
62. Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4(1):31–36. doi:10.1038/nprot.2008.214
63. Medina-Rosas J, Touma Z. Proteinuria: assessment and utility in lupus nephritis. J Rheumatol Muscular Syst. 2016;1:001.
64. Nikpour M, Bridge JA, Richter S. A systematic review of prevalence, disease characteristics and management of systemic lupus erythematosus in Australia: identifying areas of unmet need. Intern Med J. 2014;44(12a):1170–1179. doi:10.1111/imj.12568
65. Lapponi MJ, Carestia A, Landoni VI, et al. Regulation of neutrophil extracellular trap formation by anti-inflammatory drugs. J Pharmacol Exp Ther. 2013;345(3):430–437. doi:10.1124/jpet.112.202879
66. Tonello S, Rizzi M, Migliario M, Rocchetti V, Renò F. Low concentrations of neutrophil extracellular traps induce proliferation in human keratinocytes via NF-kB activation. J Dermatol Sci. 2017;88(1):110–116. doi:10.1016/j.jdermsci.2017.05.010
67. Khan MA, Farahvash A, Douda DN, et al. JNK activation turns on LPS- and gram-negative bacteria-induced NADPH oxidase-dependent suicidal NETosis. Sci Rep. 2017;7(1):3409. doi:10.1038/s41598-017-03257-z
68. Liu S, Su X, Pan P, et al. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci Rep. 2016;6(1):37252. doi:10.1038/srep37252
69. Behnen M, Leschczyk C, Möller S, et al. Immobilized immune complexes induce neutrophil extracellular trap release by human neutrophil granulocytes via FcγRIIIB and Mac-1. J Immunol. 2014;193(4):1954–1965. doi:10.4049/jimmunol.1400478
70. Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24(1):19–28. doi:10.1016/j.immuni.2005.11.010
71. Stewart R, Hammond SA, Oberst M, Wilkinson RW. The role of Fc gamma receptors in the activity of immunomodulatory antibodies for cancer. J Immunothe Cancer. 2014;2(1):29. doi:10.1186/s40425-014-0029-x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.