Back to Journals » Journal of Inflammation Research » Volume 14
A Single-Center Retrospective Observational Study Evaluating the Favorable Predictive Factors for the Disease Control Time of Treatment with Tocilizumab in Patients of Rheumatoid Arthritis
Authors Ishii N, Shimizu T, Ishiura Y , Amuro H, Nishizawa T, Tamaki T , Nomura S
Received 9 June 2021
Accepted for publication 27 July 2021
Published 5 August 2021 Volume 2021:14 Pages 3721—3728
DOI https://doi.org/10.2147/JIR.S323577
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Nobuyasu Ishii, Toshiki Shimizu, Yoshihisa Ishiura, Hideki Amuro, Tohru Nishizawa, Takeshi Tamaki, Shosaku Nomura
First Department of Internal Medicine, Kansai Medical University, Moriguchi-City, Osaka, 570-8507, Japan
Correspondence: Toshiki Shimizu
First Department of Internal Medicine, Kansai Medical University, 10-15 Fumizono-cho, Moriguchi-City, Osaka, 570-8507, Japan
Tel + 81-6-6992-1001
Fax + 81-6-6992-1066
Email [email protected]
Background: Tocilizumab (TCZ) is humanized monoclonal antibody against the interleukin-6 (IL-6) and receptor that has prominent efficacy for the treatment of rheumatoid arthritis (RA). We conducted a retrospective observational study to determine how long TCZ controls RA.
Patients and Methods: We retrospectively reviewed the medical records of RA patients treated with TCZ. The aim of this study was to evaluate the contribution of clinical parameters to disease control time (DCT) in RA patients.
Results: Overall, 144 patients were enrolled in the study. The median age of patients was 66 years (range: 34– 85 years). In univariate analysis, DCT was significantly increased in patients who had never received previous biologic disease-modifying anti-rheumatic drugs treatment (P = 0.0064). We also analyzed the contribution of the base line value of C-reactive protein (CRP) to DCT. We divided the patients with RA into two groups according to a cutoff value of 1.000 mg/dl. The median control times were 77.5 months (95% confidence interval [CI]: 44.8–not reached to median) and 34.5 months (95% CI: 17.0– 79.3) for patients with high and low CRP value, respectively. In univariate analysis, DCT was significantly increased in patients with a high CRP value (P = 0.0283). Multivariate analysis clearly revealed that a high baseline CRP value was an independent favorable predictive factor for longer DCT (hazard ratio, 0.608, 95% CI: 0.378– 0.981, P = 0.0416).
Conclusion: These data clearly demonstrate that the baseline value of CRP was closely associated with long time DCT in patients of RA treated with TCZ.
Keywords: rheumatoid arthritis, tocilizumab, C-reactive protein, platelet large cell ratio, disease control time
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disease characterized by the destruction of the structural architecture of joints.1 The pathogenic process of RA is modulated by a series of proinflammatory cytokines including interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α). Recently, these cytokines have become accepted targets of biologic disease-modifying anti-rheumatic drugs (bDMARDs). Of note, IL-6 has a crucial role in the pathogenic process of RA, and inhibiting its signal transduction cascade is a promising strategy for the treatment for RA. Tocilizumab (TCZ), a humanized monoclonal antibody against the human IL-6 receptor, and strongly blocks IL-6 signal transduction by inhibiting the binding IL-6 to its receptor.2 Numerous clinical reports have demonstrated a significant clinical efficacy of TCZ in the treatment of RA.3–6 However, precise favorable predictive factors for the treatment of RA with TCZ have not been identified. In terms of disease control time (DCT) other than disease remission rate, no obvious predictive factors have been reported. Therefore, we conducted a retrospective observational study to determine how long TCZ controls RA using a statistical technique with a time-to-event analysis. In this study, we revealed that a high value of baseline C-reactive protein (CRP) was an independent favorable predictive factor for the longtime disease control of RA treated with TCZ.
Patients and Methods
Data Collection
The medical records of all patients with RA who received treatment with TCZ from April 2008 to August 2018 at Kansai Medical University Medical Center (Moriguchi-City, Japan) were retrospectively reviewed. The clinical diagnosis of RA was made according to the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) criteria or the 1987 ACR criteria.7,8 Data on sex, age and other covariates in the patients of RA, observed before the administration of TCZ were obtained retrospectively from the patients’ medical records. Informed consent was obtained in the form of opt-out on the website (http://www.kmu.ac.jp/takii/hospital/scq14r0000000yu1.html). Those who rejected consent were excluded from the study.
This retrospective study was performed in accordance with the Declaration of Helsinki and was approved by the institutional ethics review board for Clinical Research of Kansai Medical University Medical Center (institutional ID: 2019156, UMIN–CTR: UMIN000040027).
Biochemical Analysis of Blood Samples
Complete blood cell count (CBC) and various platelet volume indices were measured using ethylenediaminetetraacetic acid (EDTA)-treated blood. An automated blood cell counter was used for these analyses (Sysmex XE-2100, Kobe, Japan). The CRP concentration was measured using an automatic analyzer (Beckman Coulter AU5400, Miami, FL, USA).
Endpoint Definition
We created a new outcome measure for this study. The DCT was defined as the time from the date patients were initially administrated TCZ to the time the treating physician decided to discontinue the TCZ treatment due to an inadequate efficacy. The event was classified into three categories: (1) TCZ treatment was discontinued due to inadequate efficacy; (2) death of a patient from any reason; (3) TCZ treatment was discontinued due to an unacceptable adverse effect. Censoring was classified into the four categories: (1) TCZ treatment was continued at the time of analysis; (2) TCZ treatment was discontinued at the time of analysis due to disease remission; (3) the last observation of patients lost to follow up; (4) patient refusal to continue TCZ treatment without obvious disease progression.
Statistical Analysis
Statistically significant differences between the groups were compared using the chi-square, Student’s t or Mann–Whitney U-test. Receiver operating characteristics (ROC) curve analysis was used to estimate an optimal cutoff value for the various hematological factors.
Univariate and multivariate analyses of DCT were performed using the Kaplan–Meier product-limit method with the Log rank test and the Cox proportional hazards model, respectively. The 95% confidence interval (CI) for the disease control rate was calculated using Greenwood’s method. To calculate the 95% CI of the median control time (MCT), the Brookmeyer and Crowley method was used.
All statistical analyses were conducted using the JMP (version 9.0.2) software program for Windows (SAS Institute Inc, Cary, NC, USA). All statistical tests were two-sided, and P < 0.05 was considered to be statistically significant.
Results
Patient Characteristics
Overall, 144 patients with RA who were treated with TCZ were enrolled in this study. The characteristics of these 144 patients are summarized in Table 1. All the patients were Asian (Japanese, Korean, or Chinese), their median age was 66 years (range: 34–85 years), and they included 114 women and 30 men. Their median duration of disease of RA was 73.9 months (inter quantile range [IQR]: 23.1, 199.7) and their mean body mass index (BMI) was 23.0 kg/m2. One hundred patients had concomitantly received glucocorticoid, whereas remaining 44 patients had not. Ninety-five patients had received methotrexate (MTX) in addition to TCZ, whereas the remaining 49 patients had received TCZ treatment alone. A history of previous bDMARDs treatment was reported in 84 patients, whereas the remaining 60 patients had been never treated with bDMARDs. The details of the previous bDMARDs treatment included 28 regimens of infliximab, 25 regimens of adalimumab, 12 regimens of golimumab, 7 regimens of certolizumab pegol, 27 regimens of etanercept, 14 regimens of abatacept and 1 regimen of tofacitinib. Median value of base line CRP was 1.329 mg/dl (IQR: 0.3513–3.2138). Eighty-four patients had base line CRP value higher than or equal to 1.000 mg/dl, whereas the base line CRP value of the remaining 60 patients were lower than 1.000 mg/dl.
 |
Table 1 Patient Characteristics |
Clinical Outcomes
We conducted a series of survival analyses on December 31, 2020. At that time, 34 patients had discontinued TCZ treatment due to insufficient efficacy, 1 patient discontinued TCZ treatment due to disease remission, 4 patients had died from any cause, 32 patients discontinued TCZ treatment due to an unacceptable adverse event, 4 patients refused to continue TCZ treatment from any cause, 27 patients were lost to follow-up, and the remaining 42 patients continued TCZ treatment with adequate disease control. Consequently, the censoring rate was 51.4%. In the 34 patients with insufficient efficacy, 9 patients were primary failure (≤6 months) and 25 patients were secondary failure (≥6 months).
The causes of death of four patients were two acute myocardial infarctions, one infection of clostridium difficile, and one primary lung cancer. The Kaplan–Meier curve for DCT is shown in Figure 1A. The MCT was 59.4 months (95% CI: 36.4–79.3). The 1-, 2-, and 5-year disease control rates were 79.3% (95% CI: 72.5–86.0), 67.8% (95% CI: 59.9–75.7), and 49.9% (95% CI: 40.6–59.1), respectively. In univariate analysis, DCT was significantly increased in patients who had never received previous bDMARDs treatment (P = 0.0064) (Table 2, Figure 1B). However, age (P = 0.1017), sex (P = 0.7950), disease duration (P = 0.2225), concomitant use of glucocorticoid (P = 0.5865) and concomitant use of MTX (P = 0.3983) were not statistically significant (Table 2). We also analyzed the contribution of the base line value of CRP to DCT. Because a previous report showed that a cut off value of CRP predicted disease remission, we divided the patients with RA into two groups according to a cutoff value of 1.000 mg/dl for CRP.9 The MCT was 77.5 months (95% CI: 44.8–NR) and 34.5 months (95% CI: 17.0–79.3) for patients with high and low CRP value, respectively (Table 2, Figure 1C). In univariate analysis, the DCT was significantly increased in the patients with a high CRP value (P = 0.0283).
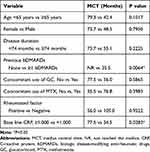 |
Table 2 Univariate Analysis of Disease Control Time |
Comparisons According to the Baseline CRP Value
Next, we compared the various characteristics between the two groups: RA patients with a high and those low CRP values. The characteristics of the two groups are summarized in Table 1. There were no significant differences in age, sex, BMI, disease duration, concomitant use of glucocorticoid and concomitant use of MTX between the two groups. Although a history of bDMARDs treatment was a significant predictive factor for a shorter DCT, there were no significant differences between the two groups (Table 1). We also analyzed various hematological indices in two groups (Table 3). The erythrocyte sedimentation rate (ESR), white blood cell (WBC) count, and platelet count (PC) were significantly increased in RA patients with a high baseline CRP value compared with those with a low baseline CRP value (P < 0.0001, P = 0.0011 and P < 0.0001 respectively). In contrast, hematocrit (Ht), hemoglobin (Hb), red blood cell distribution width (RDW), mean platelet volume (MPV), platelet distribution width (PDW) and platelet-large cell ratio (P-LCR) were significantly decreased in RA patients with a high baseline CRP value compared with those with a low baseline CRP value (P = 0.0139, P = 0.0217, P = 0.0353, P = 0.0009, P = 0.0017 and P = 0.0010 respectively). All other all variates were not significantly different between the two groups. These covariates that correlated with baseline CRP value were analyzed using logistic regression analysis followed by ROC curve analysis, and the optimal cut off values were determined. Using these cut off value, we conducted a series of univariate analysis for DCT (Table 4). In conclusion, only a low proportion of P-LCR and a low value of Hb were significantly correlated with a longer DCT (MCT: 105.0 Mo vs 39.4 Mo, P = 0.0239, MCT: 105.0 Mo vs 55.1 Mo, P = 0.0454, respectively) (Table 4, Figure 1D).
 |
Table 3 Hematological Parameters |
 |
Table 4 Univariate Analysis of Confounding Factors of Base Line CRP for Disease Control Time |
Multivariate Analysis for DCT
We also conducted a multivariate analysis for DCT (Table 5). Multivariate analysis clearly revealed that a high baseline CRP value was an independent favorable predictive factor for longer DCT (hazard ratio [HR], 0.608, 95% CI: 0.378—0.981, P = 0.0416). In addition, no history of bDMARDs was also an independent favorable predictive factor for longer disease control (HR, 0.470, 95% CI: 0.275—0.780, P = 0.0031). In contrast, age younger than 65 years old (P = 0.0673), being male (P = 0.8743), concomitant use of glucocorticoid (P = 0.4073), and concomitant use of MTX (P = 0.7099) were not significant factors. These results clearly showed that concomitant use of glucocorticoid or MTX in addition to TCZ treatment had no additional benefit for disease control of RA compared with TCZ monotherapy.
 |
Table 5 Multivariate Analysis of Disease Control Time |
Discussion
CRP was firstly reported by Tillett and Francis at 1930.10 It was induced in vivo in response to a streptococcal capsular polysaccharide.11 Most CRP is produced by hepatocytes, and it forms a pentamer in circulating blood. Currently, it is widely accepted as an important acute-phase protein and is used as a universal surrogate marker of inflammatory condition in various clinical situations. Castel et al clearly showed that IL-6 was a major regulator of CRP synthesis in adult human hepatocytes.12 Thus, CRP is thought to be a candidate predictive factor for the clinical efficacy of TCZ treatment, because the target of TCZ is the IL-6 receptor. Pers et al reported that the baseline CRP value ≥1.0 mg/dl was a favorable predictive factor for better disease remission in patients with RA treated with TCZ.9 On the basis of their results, we assessed the contribution of baseline value of CRP in patients with RA treated with TCZ. We clearly showed that a baseline CRP value ≥1.0 mg/dl was an independent favorable predictive factor for longtime disease control. These findings strongly suggested that TCZ treatment is suitable for patients in whom inflammatory condition have accelerated. Unfortunately, we could not obtain adequate data to evaluate the disease remission (for example, Disease Activity Score-28 for Rheumatoid Arthritis with ESR (DAS28-ESR) or Clinical Disease Activity Index (CDAI) because of the retrospective nature of this study.13 Therefore, we could not assess whether the predictive factors for DCT and for the disease remission rate were identical. Therefore, a prospective study is warranted.
Previous experimental data clearly showed that at least distinct two mechanisms are involved in the pathogenic process of RA. IL-6 blockade did not affect the pathogenesis of the spontaneous arthritis in TNF-α transgenic mice although it completely inhibited the pathogenic process of collagen-induced arthritis in DBA/1J mice.14 Moreover, Fujimoto et al showed that anti-mouse IL-6 receptor monoclonal antibody inhibited Th17 mobilization and pathogenic process of collagen-induced arthritis in DBA/1J mice.15 These findings suggested that patients suitable for TCZ or TNF-α inhibitors might be quite different.
In the past decade, the time-to-event analysis technique has been applied in the clinical research field of RA. Numerical studies employed “drug retention” for time-to-event analysis.16–18 However, there are some issues regarding the definition of “event” and “censored for drug retention”. Regarding the definition of drug retention, treatment discontinuation due to disease remission was also treated as an event. Therefore, time-to-event analysis has become complicated and unintuitive, and additional subset analyses related to the reason for treatment discontinuation must be performed. Therefore, we defined new outcome measures, DCT and disease control rate, in this study. Using our new outcome measures, discontinuation due to disease remission was excluded from events; therefore, a simple time-to-event analysis was possible.
We also assessed the correlation between various hematological parameters and baseline CRP value. However, only two factors, Hb and P-LCR, were significant variates in the Log rank test for DCT. Previous reports showed that Hb and P-LCR were closely correlated with the serum concentration of IL-6.19,20 However, IL-6 induces the production of CRP. Therefore, we concluded that Hb and P-LCR were confounding factors for baseline CRP and they were excluded from our multivariate analysis for DCT. Nevertheless, they are ubiquitous blood biomarkers that predict the longtime disease control of TCZ treatment because Hb and P-LCR data are obtained from a common complete blood count test.
We also revealed that no treatment history with bDMARDs was an independent favorable predictive factor for longtime disease control for TCZ treatment. However, all patients included in this study had no history of treatment with anti-IL-6 receptor antibody. There is an enigma that the previous history of treatment failure with bDMARDs other than anti-IL-6 receptor antibody contributed to the clinical efficacy of anti-IL-6 receptor antibody treatment whereas all patients had never received anti-IL-6 receptor antibody. One possible explanation to this issue is that previous failure of any bDMARDs treatment might be a selection bias in favor of refractory RA.21 The patients of refractory RA demonstrated an insufficiency to multiple bDMARDs targeting various cytokines. In such a situation, a selection of patients who had no treatment history with bDMARDs could be a “cherry picking”, resulting in reduce the likelihood of refractory RA and leading to better efficacy of TCZ treatment.
Despite the retrospective nature and small size of the present study, we revealed that a high baseline CRP value might indicate a dominant inflammatory pathogenic process of RA patients for whom TCZ treatment might achieve longer disease control. Further investigation should lead to a better understanding of therapeutic selection of appropriate patients in the treatment of TCZ.
Acknowledgments
We thank J. Ludovic Croxford, PhD, from Edanz Group for editing a draft of this manuscript.
Disclosure
The authors have declared no conflicts of interest.
References
1. Pope JE, Choy EH. C-reactive protein and implications in rheumatoid arthritis and associated comorbidities. Semin Arthritis Rheum. 2021;51(1):219–229. doi:10.1016/j.semarthrit.2020.11.005
2. Nishimoto N, Kishimoto T, Yoshizaki K. Anti-interleukin 6 receptor antibody treatment in rheumatic disease. Ann Rheum Dis. 2000;59(Suppl1):i21–i27. doi:10.1136/ard.59.suppl_1.i21
3. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67(11):1516–1523. doi:10.1136/ard.2008.092932
4. Smolen JS, Aletaha D. Interleukin-6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: the role of acute-phase reactants. Arthritis Rheum. 2011;63(1):43–52. doi:10.1002/art.27740
5. Burmester GR, Rubbert-Roth A, Cantagrel A, et al. A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). Ann Rheum Dis. 2014;73(1):69–74.
6. Choy EH, Bernasconi C, Aassi M, Molina JF, Epis OM. Treatment of rheumatoid arthritis with anti-tumor necrosis factor or tocilizumab therapy as first biologic agent in a global comparative observational study. Arthritis Care Res. 2017;69(10):1484–1494. doi:10.1002/acr.23303
7. Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi:10.1002/art.1780310302
8. Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology. 2012;51(suppl_6):vi5–vi9. doi:10.1093/rheumatology/kes279
9. Pers YM, Fortunet C, Constant E, et al. Predictors of response and remission in a large cohort of rheumatoid arthritis patients treated with tocilizumab in clinical practice. Rheumatology. 2014;53(1):76–84. doi:10.1093/rheumatology/ket301
10. Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med. 1930;52(4):561–571. doi:10.1084/jem.52.4.561
11. Mold C, Nakayama S, Holzer TJ, Gewurz H, Du Clos TW. C-reactive protein is protective against Streptococcus pneumoniae infection in mice. J Exp Med. 1981;154(5):1703–1708. doi:10.1084/jem.154.5.1703
12. Castell JV, Gómez-Lechón MJ, David M, et al. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989;242(2):237–239. doi:10.1016/0014-5793(89)80476-4
13. Aletaha D, Funovits J, Keystone EC, Smolen JS. Disease activity early in the course of treatment predicts response to therapy after one year in rheumatoid arthritis patients. Arthritis Rheum. 2007;56(10):3226–3235. doi:10.1002/art.22943
14. Alonzi T, Fattori E, Lazzaro D, et al. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187(4):461–468. doi:10.1084/jem.187.4.461
15. Fujimoto M, Serada S, Mihara M, et al. Interleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory Th17 responses. Arthritis Rheum. 2008;58(12):3710–3719. doi:10.1002/art.24126
16. Ebina K, Hashimoto M, Yamamoto W, et al. Drug tolerability and reasons for discontinuation of seven biologics in 4466 treatment courses of rheumatoid arthritis-the ANSWER cohort study. Arthritis Res Ther. 2019;21(1):91. doi:10.1186/s13075-019-1880-4
17. Kawabe A, Nakano K, Kubo S, Asakawa T, Tanaka Y. Differential long-term retention of biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis by age group from the FIRST registry. Arthritis Res Ther. 2020;22(1):136. doi:10.1186/s13075-020-02233-9
18. Alpay-Kanitez N, Pehlivan O, Omma A, et al. Favorable retention rates and safety of conventional anti-rheumatic drugs in older patients with rheumatoid arthritis. Medicine. 2020;99(16):e19696. doi:10.1097/MD.0000000000019696
19. Nikolaisen C, Figenschau Y, Nossent JC. Anemia in early rheumatoid arthritis is associated with interleukin 6-mediated bone marrow suppression, but has no effect on disease course or mortality. J Rheumatol. 2008;35(3):380–386.
20. Zhang D, Zhou X, Yan S, et al. Correlation between cytokines and coagulation-related parameters in patients with coronavirus disease 2019 admitted to ICU. Clin Chim Acta. 2020;510:47–53. doi:10.1016/j.cca.2020.07.002
21. Buch MH. Defining refractory rheumatoid arthritis. Ann Rheum Dis. 2018;77(7):966–969. doi:10.1136/annrheumdis-2017-212862
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

