Back to Journals » Journal of Inflammation Research » Volume 17
A Shortened Diagnostic Interval and Its Associated Clinical Factors and Related Outcomes in Inflammatory Bowel Disease Patients from a Cohort Study in China
Authors Zhou R, Sun X, Guo M, Zhang H, Chen X, Wu M, Liang H, Bai X, Ruan G, Yang H
Received 5 September 2023
Accepted for publication 9 January 2024
Published 18 January 2024 Volume 2024:17 Pages 387—398
DOI https://doi.org/10.2147/JIR.S434673
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Runing Zhou,1 Xiyu Sun,2 Mingyue Guo,1 Huimin Zhang,1 Xuanfu Chen,1 Meixu Wu,1 Haozheng Liang,1 Xiaoyin Bai,1 Gechong Ruan,1 Hong Yang1
1Department of Gastroenterology, Peking Union Medical College Hospital, Chinese Academy Medical Sciences and Peking Union Medical College, Beijing, 100730, People’s Republic of China; 2Department of General Surgery, Peking Union Medical College Hospital, Chinese Academy Medical Sciences and Peking Union Medical College, Beijing, 100730, People’s Republic of China
Correspondence: Hong Yang, Department of Gastroenterology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, No. 1 Shuaifuyuan, Wangfujing Street, Beijing, 100730, People’s Republic of China, Tel +86-10-69155014, Email [email protected]
Aim: The diagnosis of inflammatory bowel disease (IBD) worldwide is complicated and results in diagnostic delay. However, the diagnostic interval of IBD and the factors associated with diagnostic delay in patients in China have not been determined.
Methods: We retrospectively analyzed clinical data of hospitalized IBD patients in Peking Union Medical College Hospital from January 1998 to January 2018. Patients were divided into non-delayed and delayed groups according to their diagnostic interval.
Results: A total of 516 and 848 patients were confirmed to have Crohn’s disease (CD) and ulcerative colitis (UC), respectively. The median diagnostic intervals were 6 and 20 months in patients with UC and CD, respectively (P< 0.05). A decreasing trend in the diagnostic interval for IBD was observed over time, from 9 months to 1 month in UC patients and from 30 months to 3 months in CD patients. The longest diagnostic interval was 29.5 months in CD patients with first symptoms at the age of 51– 60 years and 12.5 months in UC patients at the age of 41– 50 years. In patients with CD, intestinal obstruction (OR=2.71), comorbid diabetes (OR=4.42), and appendectomy history (OR=2.18) were risk factors for diagnostic delay, whereas having fever as the first symptom may reduce its risk (OR=0.39). In patients with UC, the misdiagnosis of chronic enteritis (OR=2.10) was a risk factor for diagnostic delay.
Conclusion: The diagnostic interval for IBD has decreased over the years. Some clinical manifestations, such as initial symptoms and age at symptom onset, may help to shorten this interval. Diseases such as tuberculosis and infectious enteritis should be considered during differentiation.
Keywords: inflammatory bowel disease, diagnostic delay, China
Introduction
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD), is a relapsing chronic inflammatory disorder of the gastrointestinal tract. Its specific etiology is unknown but it possibly involves genetic, immunological, and environmental factors.1 The diagnosis of IBD is a complicated task worldwide, and the number of factors contributing to this disease and possibly causing diagnostic delay (DD) in a considerable number of IBD patients. According to a summary by Gottfried et al from Austria,2 the median diagnostic interval varied from 3 months to 7 years and from 1 month to 1 year in CD and UC patients, respectively. In China, IBD is not a common gastrointestinal disease and has atypical symptoms, physical signs or a lack of sufficient attention. The diagnosis of IBD relies on a combination of clinical, biochemical, stool, endoscopic, and cross-sectional imaging findings; histological investigations; and the exclusion of infectious enteritis. Follow-up for at least six months or one year is essential when the diagnosis is doubtful. A few diseases, such as intestinal tuberculosis (ITB), infectious enteritis, and Behçet’s disease (BD), mimic the manifestations of IBD and are endemic in China, posing a major challenge in differential diagnosis.2,3 Several studies have reported that DD worsens the prognosis of IBD patients. Two Korean studies revealed that DD increased not only the risk of intestinal surgery by 2.54-fold and 6.81-fold in CD and UC patients, respectively, but also the risk of intestinal stenosis or perianal fistula in CD patients.4,5 Moreover, DD may delay the proper treatment of IBD patients, as some medications may have better efficacy in newly diagnosed patients. Therefore, identifying risk factors for DD at an early stage is important.
As an emerging disease in China, IBD has a growing incidence trend, and several studies have reported notable differences in its clinical characteristics compared to West.2 For example, there was a male predominance of CD in China, and less family clustering and lower rates of surgery, extra-intestinal manifestations, and colorectal cancer were observed when compared with Caucasian patients.6–8 All of these findings indicate that it is important to determine the trend in DD and associated factors to compensate for the difference between the east and west. Therefore, we aimed to analyze the characteristics and risk factors for DD in patients with IBD using a retrospective cohort to improve the early diagnosis and prognosis of IBD in the future.
Materials and Methods
Subjects
This was a retrospective hospital-based study. We analyzed the clinical data of hospitalized IBD patients at the Peking Union Medical College Hospital (PUMCH) from January 1998 to January 2018. PUMCH is one of the largest IBD centers in China, and includes 70–80% of IBD patients all over the country. All the data were collected from the electronic database of PUMCH according to the registered International Classification of Diseases 10 codes K50 for CD and K51 for UC. The medical records of all patients were reviewed and collected during constant follow-up or telephone calls. We assigned a definitive diagnosis of IBD based on the European Crohn’s and Colitis Organisation criteria.9 Patients with incomplete medical records and those who refused to undergo follow-up were excluded.
Data Collection and Definition
The following relevant information related to IBD patients who satisfied the inclusion criteria was extracted from clinical data or during follow-up: 1) sociodemographic data (sex, birthday, smoking and drinking status, family history of IBD, allergy history, former appendectomy) and 2) disease details (age at first symptoms, age at diagnosis, first symptoms, misdiagnosed diseases, presence of extra-intestinal manifestations [EIMs], complications, IBD-related surgery, and other comorbidities). The time point of the above-mentioned comorbidities and medications of IBD patients was in their disease period. The diagnostic interval was between the appearance of the first IBD-related symptoms or signs, which included chronic diarrhea, abdominal pain, or bloody or mucus stools, and the establishment of an IBD diagnosis. The final diagnosis was confirmed during the follow-up. Since there is no universal definition of DD in IBD, the diagnostic delay in this study was defined according to the diagnostic interval in which the 76th to 100th percentiles of IBD patients were diagnosed by referring to previous studies.10,11 Patients with IBD with definite diagnostic intervals were divided into delayed and non-delayed groups based on the above definition.
Statistical Analysis
Continuous variables with a normal distribution are expressed as the mean ± standard deviation (SD), while nonnormal variables are reported as medians (interquartile ranges [IQRs]). Categorical and discrete variables are presented as percentages. The means of two continuous normally distributed variables were compared using Student’s t-test, and the Mann–Whitney U-test was applied to compare nonnormally distributed variables. The frequencies of categorical variables were compared using Pearson 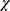 2 or Fisher’s exact test under specific conditions. Logistic regression was used to calculate odds ratios (ORs) with 95% confidence intervals (CIs) to evaluate the risk factors for DD and associated variables were selected after univariate analysis. P<0.05 was considered to indicate statistical significance. All the statistical analyses were performed using SPSS (version 23.0; SPSS Inc., Chicago, IL, USA) and Graphpad Prism (version 10.0; GraphPad Software, Boston, MA, USA).
2 or Fisher’s exact test under specific conditions. Logistic regression was used to calculate odds ratios (ORs) with 95% confidence intervals (CIs) to evaluate the risk factors for DD and associated variables were selected after univariate analysis. P<0.05 was considered to indicate statistical significance. All the statistical analyses were performed using SPSS (version 23.0; SPSS Inc., Chicago, IL, USA) and Graphpad Prism (version 10.0; GraphPad Software, Boston, MA, USA).
Ethical Considerations
Given that this was a retrospective study, written informed consent was not obtained, and the study was exempted from the Institutional Review Board of Peking Union Medical College Hospital. Only patients whose oral informed consent was obtained from patients during the telephone follow-up were the patients’ data included in the study. Verbal informed consent via the telephone follow-up was acceptable and approved by the committee due to the retrospective and non-invasive study design. And the study complies with the Declaration of Helsinki.
Results
Patient General Characteristics
A total of 627 and 1108 patients were hospitalized for CD and UC, respectively, at PUMCH between January 1998 and January 2018, and 516 CD patients (357 male and 159 female; male-to-female ratio, 2.25:1) and 848 UC patients (456 male and 392 female; male-to-female ratio, 1.16:1) were included in the analysis (Table 1). The follow-up rate was 78.6%. The mean durations of CD and UC were 7.63 years and 10.06 years, respectively. The total follow-up time for patients with CD was 4784 person-years, and that for patients with UC was 11,146 person-years. The male-to-female ratio was greater in patients with CD than in those with UC (P<0.001). The mean age at first symptoms and diagnosis was lower in patients with CD than in those with UC (P<0.001), whereas the median diagnosis interval was shorter in patients with UC (CD, 20 months; UC, 6 months; P<0.001). Diabetes and hypertension were more common in patients with UC and a greater number of patients with CD underwent appendectomy before diagnosis.
 |
Table 1 Baseline Data of Hospitalized IBD Patients at PUMCH |
A greater proportion of CD patients than UC suffered from intestinal obstruction, perforation, and fistula, as well as peritoneal abscess and perianal lesions, while more UC patients developed toxic megacolon than did CD patients (P=0.016). In addition, more patients with UC than patients with CD received 5-aminosalicylic acid therapy, and more patients with CD were treated with immunosuppressants and biologics. Overall, 242 (46.90%) patients with CD underwent IBD-related surgery, and this was a significantly greater percent than that of patients with UC (P=0.000).
Misdiagnosis Analysis
According to the constant follow-up and re-confirmation, although 85 patients out of all hospitalized patients had been diagnosed with CD or UC in our hospital, the ultimate diagnosis was not IBD; among these patients, 11 had intestinal tuberculosis (0.63%), 9 had Behçet’s disease (0.52%), 7 had lymphoma (0.40%), 6 had colonic polyps (0.35%), 4 had vasculitis (0.23%), 4 had colorectal cancer (0.23%), while the rest remained unknown.
Diagnostic Interval of Patients with Different Years of Onset
In CD patients, the longest diagnostic interval was 120 months [48.00, 182.00], which was observed in patients whose first symptoms appeared before 2000 (Figure 1). This interval gradually declined over time. The longest UC interval was also observed in patients with typical symptoms before 2000, and this trend was similar to that of CD patients. The diagnostic interval for CD was significantly longer than that for UC before 2000, in 2001–2010 and in 2011–2015.
 |
Figure 1 Median diagnostic interval of patients in different years after onset. |
The first symptoms and misdiagnoses differed among the patients with different years of disease onset. Abdominal pain was the most common initial symptom in all years, followed by diarrhea and fever (Figure 2A). Patients with CD were most likely to be misdiagnosed with ITB, and the proportion of patients misdiagnosed with infectious enteritis, such as bacillary dysentery, Epstein-Barr virus colitis, and amoebic enteritis, was greater after 2010 (P=0.041) (Figure 2B). Among the UC patients, more started with fever after 2010 (P=0.001) (Figure 2C) and tended to be misdiagnosed with infectious enteritis (Figure 2D); however, the composition of misdiagnosed diseases was not significantly different.
Diagnostic Interval of Patients at Different Ages of Onset
The longest and shortest diagnostic intervals were 29.5 months [9.0, 94.5] and 14.0 months [5.0, 48.5], respectively, in CD patients who had their first symptoms at ages 51–60 and 21–30 years, respectively (Figure 3). For UC, as the age at first symptoms increased, the interval first increased and then decreased and 41–50-year-old patients experienced the longest interval of 12.5 months [3.0, 49.0]. The interval in the CD group was significantly longer than that in the UC group in almost all age groups except for the 41–50-year-old group (Figure 3).
 |
Figure 3 Median diagnostic interval of patients at different ages of onset. |
The first symptoms and misdiagnosis of diseases were different in patients of different ages at the first symptoms. Regarding CD, perianal lesions were more common in patients aged < 16 years than in those aged >40 years (P=0.015) (Figure 2E). However, the composition of the misdiagnosed diseases was not significantly different (Figure 2F). Most patients among the three age groups first suffered from bloody and mucus stools at first among the three age groups (P=0.749) (Figure 2G), and patients aged >40 years tended to be misdiagnosed with chronic enteritis more often than others were (P=0.038) (Figure 2H).
Clinical Factors Associated with DD in CD Patients
There were 98 CD patients with diagnostic delays according to the definition, and the median diagnostic interval was 103.5 months [73.50, 139.0], which was significantly longer than that in the non-delayed group (12.00 months, [5.00, 27.00], P=0.000) (Table 2). A greater proportion of patients with a history of appendectomy and diabetes was found in the delayed group (P=0.002 and P=0.008, respectively). Fever was the only symptom that presented more frequently in the non-delayed group (P=0.007), whereas other symptoms were not significantly different between the two groups. Additionally, patients in the delayed group were more likely to be misdiagnosed with intestinal obstruction than were those in the non-delayed group (P=0.028). No significant difference was found in the proportion of patients who underwent IBD-related surgery or who received biologics between patients who did and did not (P=0.103 and P=0.789, respectively). Multivariate logistic regression analysis, including potential variables, revealed that intestinal obstruction (OR=2.71, 95% CI:1.10–6.66, P=0.03), comorbidity with diabetes (OR=4.42, 95% CI:1.33–14.70, P=0.02), and appendectomy history (OR=2.18, 95% CI:1.13–4.21, P=0.02) were risk factors for DD, while having fever as the first symptom may reduce the risk of DD (OR=0.39, 95% CI:0.15–0.97, P=0.04) (Table 3).
 |
Table 2 Comparison of Diagnostic Delay and Non-Delay in CD Patients |
 |
Table 3 Multivariate Logistic Regression Modelling to Assess DD in CD Patients |
Clinical Factors Associated with DD in UC Patients
A total of 114 UC patients experienced diagnostic delay according to the definition and the median diagnostic interval was 81.5 months [39.75, 122.75], which was significantly longer than that of the non-delayed group (4.00 months, [1.00, 10.00], P=0.000) (Table 4). There were significantly more patients with diabetes in the delayed group than in the delayed group (P=0.035). Bloody and mucus stools were the most common symptoms in both groups, and fever was the only symptom that presented more frequently in the non-delayed group (P=0.024); however, other symptoms were not significantly different. Infectious enteritis was the major misdiagnosed disease in the non-delayed group, whereas chronic enteritis was the most common disease in the delayed group (P=0.012). Although no difference was found in the proportion of patients who underwent IBD-related surgery, there were more patients in the non-delayed group receiving biological treatment than those in the delayed group (P=0.002). A multivariate logistic regression analysis including potential variables revealed that a misdiagnosis of chronic enteritis (OR=2.10, 95% CI:1.10–4.01, P=0.03) was a risk factor for DD (Table 5).
 |
Table 4 Comparison of Diagnostic Delay and Non-Delay UC Patients |
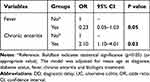 |
Table 5 Multivariate Logistic Regression Modelling to Assess DD in UC Patients |
Mismatch of International Classification of Diseases (ICD) Code and Disease
During the screening of medical data and follow-up, we identified 12 patients without a diagnosis of CD, which was encoded by K50, and 19 patients without a UC diagnosis were mismatched to K51. The overall mismatch rate was 2.30%. The mismatched cases included UC, IBD-Unclassified, intestinal tuberculosis, and Behçet’s disease linked to K50; CD, IBD-Unclassified, Behçet’s disease, and nonspecific colitis mismatched to K51.
Discussion
Given that IBD is an emerging disease in China, this is the first study to disclose trends in diagnostic intervals and associated risk factors for diagnostic delay in both CD and UC patients in China. Several findings from our study are worth noting. First, the median diagnostic interval of CD patients was longer than that of UC patients, which was also found in other studies, where the median diagnostic interval of CD was 2–3 times longer than that of UC. Second, a decreasing trend in the diagnostic interval for IBD was observed over time, from 9 months to 1 month in UC patients and from 30 months to 3 months in CD patients between 2001 and 2017. Third, during the process of ultimate diagnosis, ITB and infectious enteritis were the diseases most likely to be misdiagnosed in CD and UC patients, respectively, suggesting the complexity of the differential diagnosis of diseases that mimic clinical manifestations of IBD and have a high prevalence in our country. However, unlike in some Western countries, in this study, the surgery and mortality rates did not significantly differ between the delayed and non-delayed groups of patients with CD and UC.
This study revealed that the diagnostic interval was significantly longer in patients with CD than in patients with UC in different years and in patients of different ages at first symptom. This finding is consistent with those from other Asian countries and regions (Table 6).8,12–17 A few population-based epidemiological studies have indicated that the median diagnostic interval is nearly 2–3 times longer in patients with CD than in those with UC, except for one survey in Hong Kong. The following factors may have contributed to these findings. First, abdominal pain, which is one of the most common symptoms in the majority of gastrointestinal diseases, is mostly observed in CD patients. However, UC patients present with bloody and mucus stools mostly at the beginning, alerting patients to seek help and helping doctors identify UC as soon as possible. Second, the disease behavior of CD, such as intestinal fistula or stricture, can change and can occur simultaneously or at different times, thereby posing a diagnostic burden. Third, some diseases, such as ITB and infectious enteritis, have a higher prevalence in China and present similar symptoms, signs, and pathological findings, increasing the difficulty of differential diagnosis and diagnosis of CD. An early study reported that the rate of misdiagnosis of CD as ITB was 65%, and the rate of misdiagnosis of ITB as CD was 34% before patients underwent surgery at PUMCH,18 indicating the difficulty in distinguishing between these two diseases. A later Korean study revealed a growing tendency in the rate of misdiagnosis of ITB as CD from 1995 to 2015, which was probably due to the decrease in TB incidence in South Korea.19
 |
Table 6 Median Diagnostic Interval for IBD from Several Asian Population-Based Epidemiology Surveys |
We found that the diagnostic interval for both CD and UC decreased over time, similar to the results of a multicenter prospective study of CD patients in China.20 Since 2007, three editions of the Chinese guidelines for IBD diagnosis and treatment have been issued and revised; these publications have significantly improved the accuracy of IBD diagnosis in some tertiary medical centers in China and helped shorten the diagnostic interval. Recently, additional clinical studies on IBD have been carried out in Chinese patients, and the results have improved the diagnostic methods.21 Finally, the convenience of accessing medical services and the establishment of a medical insurance system may shorten the diagnostic interval for patients with IBD.
Patients presenting with initial symptoms at ages 51–60 and 41–50 had the longest diagnostic intervals among CD and UC patients, respectively. Patients with a younger age at first symptom onset had shorter diagnostic intervals, which was also found in some studies in which age >40 years at onset was a risk factor associated with DD.2,22 Considering that the incidence rate of IBD peaks at age 20–40, when bloody and mucus stools, abdominal pain, weight loss, anemia or fever occurred in young people, it is more likely that doctors will associate these manifestations with IBD. On the other hand, elderly individuals with the above presentations were considered to have chronic enteritis or other malignant diseases other than IBD at first sight. However, whether younger or older age is a risk factor remains controversial. Vavricka SR10 noted that patients diagnosed at <40 years of age experienced a longer diagnostic interval due to similar onset symptoms as irritable bowel syndrome. Thus, the relationship between age at first symptom onset and DD requires further investigation.
Three important findings were observed in the comparison between the delayed and non-delayed CD groups. First, more patients in the delayed group underwent appendectomy before CD diagnosis. The removal of the appendix temporarily relieves intestinal inflammation or interrupts the immune system of the digestive tract, which is important in IBD development.23 Moreover, pathologists may overlook the pathological findings of CD because of the lack of specific microscopic features, leading to misdiagnosis and DD. In addition, more patients in the non-delayed group had fever at the beginning, indicating that fever is an important symptom that physicians should be aware of. Third, a few patients with CD receive anti-TB treatment before being diagnosed with CD, and making a differential diagnosis is still a challenging issue, especially when biologics are increasingly used to treat CD in China. Developing better diagnostic tools with higher sensitivity and specificity is challenging and can be an opportunity.
For UC, more patients initially had fever in the non-delayed group, similar to that in the CD group. Although IBD can cause inflammation of the intestine, it can be accompanied by systemic manifestations, such as fever, weight loss, weakness, and anemia, and doctors should pay attention to this condition. In addition, UC and chronic enteritis are challenging conditions, and endoscopy or imaging studies may help to confirm this phenomenon.
The advantage of our survey was the establishment of a retrospective cohort with systematic follow-up, in which we not only estimated the trend of the diagnostic interval of UC for the first time, but also established a foundation for tracing patients’ outcomes in the future. Nevertheless, this study has several limitations. Owing to the retrospective design and single-center recruitment, recall bias occurred in the description of the first symptoms. Since PUMCH is a well-known tertiary center, hospitalized patients are usually seriously ill and have already undergone a few referrals; overestimation of the diagnostic interval and rate of adverse events has rarely been avoided. In addition, the mismatch between the codes and diagnoses may result in the absence of patients with IBD without a code of K50 or K51. Additionally, we did not separate the diagnostic interval into two parts: patient-dependent and physician-dependent. Finally, some socioeconomic factors, such as educational level, occupation, and income, which may influence the diagnostic interval, were not included in this study and require further investigation.
Conlcusions
In summary, the diagnostic interval for IBD decreased, and CD patients had a longer interval than UC patients did. DD in IBD patients is due to various factors, including initial symptoms and age at symptom onset. Differential diagnosis of IBD remains a difficult issue, especially in China, where tuberculosis and infectious enteritis are endemic. Comprehensive scoring systems that have been validated in Chinese IBD cohorts are essential for differential diagnoses. The promotion of national guidelines and academic activities may help narrow the gap in diagnostic quality across the entire country.
Abbreviations
IBD, Inflammatory bowel disease; CD, Crohn’s disease; UC, Ulcerative colitis; OR, Odds ratios; ITB, Intestinal tuberculosis; BD, Behçet’s disease; DD, Diagnostic delay; PUMCH, Peking Union Medical College Hospital; EIMs, Extra-intestinal manifestations; SD, Standard deviation; IQR, Interquartile range; CIs, Confidence intervals.
Data Sharing Statement
The datasets used during and/or analyzed during the current study are available from the corresponding author (Dr. Hong Yang, email: [email protected]) on reasonable request.
Ethics Approval and Consent to Participate
Given that this was a retrospective study, written informed consent was not obtained, and the study was exempted from the Institutional Review Board of Peking Union Medical College Hospital (phone number: +86-10-69156874/69165709). Only patients whose oral informed consent was obtained from patients during the telephone follow-up were the patients’ data included in the study. Verbal informed consent via the telephone follow-up was acceptable and approved by the committee due to the retrospective and non-invasive study design. And the study complies with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from National High-Level Hospital Clinical Research Funding [2022-PUMCH-C-018, 2022-PUMCH-B-022, 2022-PUMCH-A-074, 2022-PUMCH-A-179], The Capital Health Research and Development of Special Foundation [2022-2-4014], the National Key Clinical Specialty Construction Project [ZK108000], the National Natural Science Foundation of China [81970495] and the CAMS Innovation Fund for Medical Sciences [2022-I2M-C&T-B-011].
Disclosure
The authors declare that they have no competing interests.
References
1. Graham DB, Xavier RJ. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature. 2020;578:527–539. doi:10.1038/s41586-020-2025-2
2. Novacek G, Grochenig HP, Haas T, et al. Diagnostic delay in patients with inflammatory bowel disease in Austria. Wien Klin Wochenschr. 2019;131:104–112. doi:10.1007/s00508-019-1451-3
3. IBD CSo. Consensus of diagnosis and management of inflammatory bowel disease (2018 Beijing). Chin J Inflamm Bowel Dis. 2018;2:173–190.
4. Lee DW, Koo JS, Choe JW, et al. Diagnostic delay in inflammatory bowel disease increases the risk of intestinal surgery. World J Gastroenterol. 2017;23:6474–6481. doi:10.3748/wjg.v23.i35.6474
5. Moon CM, Jung SA, Kim SE, et al. Clinical factors and disease course related to diagnostic delay in Korean crohn’s disease patients: results from the CONNECT study. PLoS One. 2015;10:e0144390.
6. Lu CL. Clinical presentations of inflammatory bowel disease: east meets West. J Chin Med Assoc. 2017;80:51–52. doi:10.1016/j.jcma.2016.09.005
7. Ng SC. Epidemiology of inflammatory bowel disease: focus on Asia. Best Pract Res Clin Gastroenterol. 2014;28:363–372. doi:10.1016/j.bpg.2014.04.003
8. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158–165 e2. doi:10.1053/j.gastro.2013.04.007
9. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144–164. doi:10.1093/ecco-jcc/jjy113
10. Vavricka SR, Spigaglia SM, Rogler G, et al. Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:496–505. doi:10.1002/ibd.21719
11. Nahon S, Lahmek P, Lesgourgues B, et al. Diagnostic delay in a French cohort of Crohn’s disease patients. J Crohns Colitis. 2014;8:964–969. doi:10.1016/j.crohns.2014.01.023
12. Zeng Z, Zhu Z, Yang Y, et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study. J Gastroenterol Hepatol. 2013;28:1148–1153. doi:10.1111/jgh.12164
13. Yang H, Li Y, Wu W, et al. The incidence of inflammatory bowel disease in Northern China: a prospective population-based study. PLoS One. 2014;9:e101296. doi:10.1371/journal.pone.0101296
14. Zhao J, Ng SC, Lei Y, et al. First prospective, population-based inflammatory bowel disease incidence study in mainland of China: the emergence of ”western” disease. Inflamm Bowel Dis. 2013;19:1839–1845. doi:10.1097/MIB.0b013e31828a6551
15. Park SH, Kim YJ, Rhee KH, et al. A 30-year trend analysis in the epidemiology of inflammatory bowel disease in the Songpa-Kangdong District of Seoul, Korea in 1986–2015. J Crohns Colitis. 2019;13:1410–1417. doi:10.1093/ecco-jcc/jjz081
16. Ng SC, Leung WK, Shi HY, et al. Epidemiology of inflammatory bowel disease from 1981 to 2014: results from a territory-wide population-based registry in Hong Kong. Inflamm Bowel Dis. 2016;22:1954–1960. doi:10.1097/MIB.0000000000000846
17. Shin DH, Sinn DH, Kim YH, et al. Increasing incidence of inflammatory bowel disease among young men in Korea between 2003 and 2008. Dig Dis Sci. 2011;56:1154–1159. doi:10.1007/s10620-010-1403-2
18. Tonghua Liu GP, Mai C, Chen M. Crohn’s disease: III. Differentiate diagnosis between Cronh’s disease and intestinal tuberculosis. Chinese J Int Med. 1981;20:211–215.
19. Seo H, Lee S, So H, et al. Temporal trends in the misdiagnosis rates between Crohn’s disease and intestinal tuberculosis. World J Gastroenterol. 2017;23:6306–6314. doi:10.3748/wjg.v23.i34.6306
20. Li Y, Chen B, Gao X, et al. Current diagnosis and management of Crohn’s disease in China: results from a multicenter prospective disease registry. BMC Gastroenterol. 2019;19(1):145. doi:10.1186/s12876-019-1057-2
21. Li Y, Qian JM. The challenge of inflammatory bowel disease diagnosis in Asia. Inflamm Intest Dis. 2017;1:159–164. doi:10.1159/000448384
22. Cantoro L, Di Sabatino A, Papi C, et al. The time course of diagnostic delay in inflammatory bowel disease over the last sixty years: an Italian multicentre study. J Crohns Colitis. 2017;11:975–980. doi:10.1093/ecco-jcc/jjx041
23. Radford-Smith GL, Edwards JE, Purdie DM, et al. Protective role of appendicectomy on onset and severity of ulcerative colitis and Crohn’s disease. Gut. 2002;51:808–813. doi:10.1136/gut.51.6.808
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

