Back to Journals » Vascular Health and Risk Management » Volume 16
A Short Series of Laparoscopic Mesenteric Bypasses for Chronic Mesenteric Ischemia
Authors Kazmi SSH , Berge ST, Sahba M , Medhus AW , Sundhagen JO
Received 21 December 2019
Accepted for publication 14 February 2020
Published 20 March 2020 Volume 2020:16 Pages 87—97
DOI https://doi.org/10.2147/VHRM.S243264
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Syed Sajid Hussain Kazmi,1,2 Simen Tveten Berge,1,2 Mehdi Sahba,2,3 Asle Wilhelm Medhus,4 Jon Otto Sundhagen1
1Department of Vascular Surgery, Heart, Lung and Vascular Clinic, Oslo University Hospital Aker, Oslo, Norway; 2Faculty of Medicine, University of Oslo, Oslo, Norway; 3Department of Vascular Surgery, Ostfold Central Hospital, Kalnes, Norway; 4Department of Gastroenterology, Oslo University Hospital, Ullevål, Norway
Correspondence: Syed Sajid Hussain Kazmi
Department of Vascular Surgery, Heart, Lung and Vascular Clinic, Oslo University Hospital Aker, Oslo, Norway
Email [email protected]
Background: Laparoscopic aortomesenteric bypass may be performed to treat the chronic mesenteric ischemia patients who are not suitable for endovascular treatment. This study presents an initial experience with a limited series of laparoscopic mesenteric artery revascularization for the treatment of mesenteric ischemia.
Methods: Chronic mesenteric ischemia (CMI) patients with previous unsuccessful endovascular treatment or with arterial occlusion and extensive calcification precluding safe endovascular treatment were offered laparoscopic mesenteric revascularization. From October 2015 until November 2018, nine patients with CMI underwent laparoscopic revascularization. In addition to demographic data and perioperative results of the treatment, graft patency was assessed with Duplex ultrasound at 1, 3, 6 and 12 months, and annually thereafter. A descriptive analysis of the data was performed.
Results: All bypasses were constructed with an 8 mm ring enforced expanded polytetrafluoroethylene graft in a retrograde fashion (from infrarenal aorta or iliac artery) to either superior mesenteric artery or splenic artery (2 cases). Median operation time was 356 mins (range 247– 492 mins). Five patients had a history of unsuccessful endovascular treatment. Laparoscopic technical success was 78%, and the primary open conversion rate was 22%. All laparoscopic revascularization procedures remained patent after discharge during a median follow-up time of 26 months (range 18– 49 months). The primary graft patency at 30 days was 78%. Primary assisted, and secondary graft patency was 78% and 100%, respectively. Median weight gain was 2 kg (range 2– 18 kg), and all patients achieved relief from postprandial pain and nausea. No mortality was observed during the follow-up period.
Conclusion: Laparoscopic aortomesenteric revascularization procedures for chronic mesenteric ischemia are feasible but require careful patient selection. These procedures should only be performed at referral centers by vascular surgeons with prior experience in laparoscopic vascular surgery.
Keywords: mesenteric ischemia, bypass, laparoscopy, chronic mesenteric ischemia, intestinal ischemia
Introduction
Revascularization procedures have been conducted for the treatment of mesenteric ischemia since 1957.1 Although endovascular procedures for the treatment of chronic mesenteric ischemia (CMI) have superseded the open revascularization procedures, the comparative results on mortality and long-time patency are shown to be better for the open procedures.2–5 Due to reduced perioperative morbidity, guidelines recommend endovascular therapy as the first choice for the treatment of CMI.6,7 However, in case of failed endovascular therapy or in patients with occlusion and extensive calcification precluding safe angioplasty and stenting, a bypass from aorta to either superior mesenteric artery (SMA)/celiac artery (CA) or both is indicated.6 Laparoscopic mesenteric bypass operation techniques have been introduced to achieve the advantages of a minimally invasive surgical technique.8,9 However, no study has yet been performed to investigate the feasibility of the laparoscopic mesenteric bypass operation technique.
This small prospective non-comparative cohort aimed to assess the clinical results of an early experience with the laparoscopic revascularization, in the patients with chronic mesenteric ischemia, in addition to critically evaluate the operative technique.
Materials and Methods
Patients with a suspicion of CMI referred to the Department of Vascular Surgery, Oslo University Hospital, Aker, from October 2015 to December 2018, were included in this study. Our department is a tertiary referral hospital for the investigation and treatment of mesenteric ischemia pathology. During the same inclusion period, 72 patients with the diagnosis of CMI were treated with endovascular procedures at the vascular department. All patients in this study were investigated preoperatively with Computed Tomography Angiography (CTA). CMI was defined and diagnosed based on the following criteria:
- Symptom duration > 3 months
- CTA findings of stenosis or occlusion in one or more mesenteric arteries
- Exclusion of differential diagnoses (e.g., cancer disease, peptic ulcer disease, hiatus hernia, irritable and inflammatory bowel disease, gall bladder and pancreas pathologies)
Mesenteric arteries were defined as celiac artery (CA), superior mesenteric artery (SMA), and inferior mesenteric artery (IMA).6 SMA was the main artery selected for revascularization even in cases where both CA and SMA had occlusion or stenosis. The splenic artery was chosen for revascularization when the atherosclerotic occlusion was only confined to CA. All patients had prior investigations (endoscopies, computed tomography, abdominal ultrasound) to exclude other causes of their symptoms of postprandial abdominal pain, weight loss, and changes in food intake patterns. Only patients with a history of prior unsuccessful endovascular treatment or extensive atherosclerotic lesions in the mesenteric arteries precluding safe endovascular treatment were offered a laparoscopic revascularization procedure. An extensive atherosclerotic lesion was defined as a heavily calcified atherosclerotic plaque in Fullen’s zone 1 and 2 on CTA, evaluated to be unavailable for catheterization or stent placement.10 The patients were followed-up postoperatively at 1, 3, 6 and 12 months and annually thereafter. In addition to clinical follow-up, a Duplex ultrasound was used to confirm patency of the graft, and CTA was taken to confirm graft patency if required. Data on changes in the clinical symptoms, complications of the primary surgical treatment, and secondary interventions were collected.
Completion of the laparoscopic procedure without conversion to open surgery was defined as a technical success. Primary patency was defined as patent revascularization at follow-up, confirmed by Duplex ultrasound. Primary assisted patency was defined as patent revascularization, achieved with an intervention for a failing graft (although asymptomatic). Secondary patency was patency achieved after intervention on an occluded graft.
Laparoscopic Technique
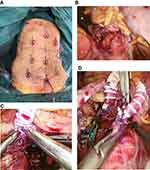
All operations were performed in general anesthesia. All patients received intravenous antibiotic prophylaxis (Cefalotin 2g) after intubation, which was repeated every 3 hrs until a total of four doses. The patient was positioned supined on a split-leg table, and the surgeon stood between the legs. A surgical nurse stood on the left side of the surgeon, and an assistant on each side of the patient. Pneumoperitoneum was achieved with carbon dioxide (CO2) insufflation through a trocar placed under direct visualization of the peritoneum. A pneumoperitoneum pressure of 12 mmHg was maintained during the surgery. If necessary, trocars were placed at anatomical positions suitable for safe peritoneal or omental adhesiolysis (Figure 1A). The small intestine was gently mobilized towards the right side of the abdominal cavity. If necessary, one or two 10 mm fan retractors (Covidien Endo Retract II Ethicon) were used to keep the intestine separated from the surgical field.
Abdominal aorta and iliac arteries were approached by directly opening the overlying peritoneum. The infrarenal aorta was dissected free for a length suitable for application of aortic clamps and the construction of an end-to-side anastomosis (Figure 1B). In one case, the right iliac artery and in another left graft limb, of a previously laparoscopically operated aortobifemoral graft, were also dissected.
Aorto/Iliac-SMA Bypass
SMA was approached in the area just below the conjunction of superior and inferior mesenteric veins. Treitz ligament was divided, and duodenum mobilized to free dissect the required length of SMA. Ring enforced expanded polytetrafluoroethylene (ePTFE) 8 mm (Gore-Tex Stretch Vascular Graft) was spatulated at one end and introduced into the abdominal cavity through a 12 mm trocar. Intravenous heparin was given to achieve anticoagulation, and the SMA was clamped distally with either a long vessel loop or with a small laparoscopic bulldog artery clamp. In the case of calcified SMA, a laparoscopic aortic clamp was used for proximal cross-clamping of the SMA, which also helped in keeping the greater omentum and transverse colon, separated from the operation field. Thrombendarterectomy was performed through a longitudinal arteriotomy. A partial stent resection (distal part of the stent) was performed in case of an occluded stent. An end-to-side anastomosis was performed with two hemi-circular 6–0 polypropylene sutures, each about 12–15 cm length and with a beforehand tied Teflon pledgets to their ends (Figure 1C).12 Artery clamps were temporarily removed from SMA and backflow was confirmed through the graft, before it was flushed with heparinized NaCl. A suitable length of the graft was used to allow a generous graft loop to avoid graft kinking, and the rest of the graft length was excised and discarded. The infrarenal aorta was clamped, and after aortotomy and spatulation of the graft end, an end-to-side anastomosis was constructed with the help of 4–0 polypropylene sutures, again with Teflon pledgets at the ends (Figure 1D). Aortic clamps were removed, and mesenteric circulation established through the bypass.
Aorto-Splenic Bypass
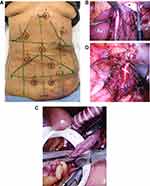
In two cases, the splenic artery was used as the site of the distal anastomosis. Figure 2A–D illustrates the trocar’s positions and different phases of the aorto-splenic bypass. Nathanson liver retractor (Cook Medical) was used to elevate the left liver lobe. The hepatogastric ligament was excised to approach the splenic artery along the cranial edge of the pancreas. The artery was dissected in length suitable for cross-clamping and to construct an end-to-side anastomosis. A laparoscopic iliac clamp (Carl Storz, Germany) was carefully passed anterior to the left renal vein along the right side of the aorta and progressed, cranially behind the pancreas, towards the omental bursa. Care was taken to keep the clamp parallel with the aorta. A spatulated 8 mm ring enforced ePTFE graft was grasped with this clamp and carefully, tunneled from the omental bursa, dorsal to the pancreas, towards the infrarenal aorta.
After systemic heparinization, the splenic artery was clamped with the laparoscopic aortic clamps or small laparoscopic bulldog artery clamps. Longitudinal arteriotomy was performed, and an end-to-side anastomosis was constructed with 6–0 polypropylene sutures. The graft was flushed with heparinized NaCl and cross clamped with a laparoscopic aortic clamp until the anastomosis with the aorta was constructed in an end-to-side fashion (Figure 2D). Nathanson liver retractor was removed, and the proximal portion of the graft was covered by the lesser omentum and the left liver lobe. The retroperitoneum was used to cover all grafts to the SMA.
No intra-operative imaging with either angiography or ultrasound was performed, except in one patient.
Ethics and Trial Registration
All included patients were participating in an ongoing clinical study on chronic mesenteric ischemia.11 The study was approved by the Regional Committees for Medical and Health Research Ethics in the South-Eastern region of Norway (REK sør-øst B 2016/682) and registered in the ClinicalTrials.gov Protocol Registration and Results System (NCT02914912). The patients gave informed written consent for inclusion in the study and the operative procedure. The study was conducted in accordance with the Declaration of Helsinki.
Statistical Analysis
A descriptive analysis of the data was performed. Median and range were calculated.
Results
From October 2015 until May 2018, nine CMI patients with a median age of 60.5 years (range 47–77 years), underwent laparoscopic mesenteric revascularization. Eight patients were females. The median time for symptom duration was 24 months (range 12–48 months). Three patients had constant abdominal pain and could not even take peroral liquid food. Seven patients had previous single or multiple abdominal surgeries. All patients had extensive preoperative investigations to exclude other possible causes of their symptoms. All patients were evaluated for endovascular therapy, and when eligible, “endovascular first practice” was exercised.6 In most of the cases (n=8), the atherosclerotic plaque extended through the entire length of the Fullen’s zone 1 and 2 and incorporated origins of the inferior pancreaticoduodenal artery and middle colic artery. The descriptive data of the nine patients are given in Table 1.
 |
Table 1 Demographic Data, Risk Factors, Comorbidities, and Clinical Findings in the Group of Patients with Chronic Mesenteric Ischemia Treated with Laparoscopic Mesenteric Bypass Procedures |
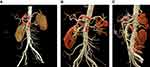
Five laparoscopic retrograde aorto-mesenteric bypass operations to SMA were performed (Figure 3A–C). Two patients were operated with retrograde aorto-splenic bypass. One patient received an iliaco-mesenteric bypass to the SMA and another from the left graft limb of a previously operated aortobifemoral bypass to SMA (Figure 3B). A median hospital stay of the patients in this study was seven days (range 5–35 days). No patient died during the 30 postoperative days. Perioperative details are given in Table 2.
 |
Table 2 Perioperative Data of 9 Chronic Mesenteric Ischemia Patients Treated with Laparoscopic Mesenteric Bypass Procedures |
Two patients reoperated for graft thrombosis also had anticoagulation therapy with a daily subcutaneous, low molecular weight heparin, for three months. The laparoscopic procedure could be successfully completed in 7 patients (78%). In one patient, the laparoscopic procedure was converted to open surgery due to bleeding from the mesenteric venous confluence. In another patient, distal anastomosis on SMA was performed with laparotomy. None of the patients with laparoscopic bypass required postoperative epidural analgesia. The two patients converted to laparotomy required epidural analgesia for three postoperative days.
Primary graft patency at 30 days was 78%. Two graft thromboses were revealed on CTA, taken on the second postoperative day. None of these patients had any clinical symptoms of postoperative intestinal ischemia. One of these underwent a successful laparoscopic thrombectomy. The other had to be operated with open graft thrombectomy. Both patients had a patent bypass during the follow-up and no recurrent symptoms of mesenteric ischemia.
Primary assisted graft patency and secondary graft patency at 30 postoperative days were 78% and 100%, respectively.
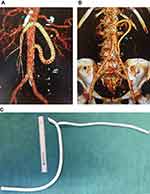
In one patient with a retrograde aorto-splenic bypass, postoperative CTA on the second postoperative day revealed graft stenosis. The patient did not have any symptoms of intestinal ischemia. However, to ensure long-term graft patency, the patient underwent a successful laparoscopic graft length correction, as well as the revision of aortic anastomosis on the 5th postoperative day (Figure 4A–C). Left ureter injury was diagnosed on the third postoperative day in the patient with a prior laparoscopic aortobifemoral bypass. This patient underwent ureter repair and had after almost four years of follow-up, no symptoms from the repaired ureter.
Duplex ultrasound at 1, 3, 6, 12 months, and annually thereafter (n=5), confirmed graft patency in all patients. No patient died during the median postoperative follow-up time period of 26.5 months (range 18–49 months). All patients reported a lasting relief from the symptoms of CMI and had a median increase in body weight of 2 kg (range 2–18 kg).
Discussion
This study is the first to report a series of laparoscopic mesenteric revascularization procedures for the treatment of CMI. To the best of our knowledge, laparoscopic bypass to splenic artery has not been mentioned earlier in the literature. Javerliat et al reported in 2004 a case of a laparoscopic bypass to SMA during a planned laparoscopic aortobifemoral bypass for the treatment of aortoiliac occlusive disease.8 In 2006 Bakoyiannis et al reported a case of the bypass from infrarenal aorta to common hepatic artery in a patient undergoing endovascular stent-graft repair of a thoracoabdominal aortic aneurysm.9 In both these case reports the laparoscopic approach utilized to dissect infrarenal aorta was transabdominal retro-colic. In our study, the sole indication for the treatment was mesenteric ischemia, and the results are followed-up systematically. Besides, we used a transabdominal direct approach to the abdominal aorta and the mesenteric vessels. This direct approach does not require dissection for medial mobilization of the splenic flexure, descending, and sigmoid colon. A longer length of SMA and its branches can be free dissected by this direct approach. The trocar positions also allow the operator to stand, if necessary, on the right side of the patient to perform the aortic anastomosis. Through a retrorenal approach, full aortic length, from the diaphragm to the left iliac artery can be achieved laparoscopically. However, the dissection is more extensive, and the peripheral segment of SMA and CA branches remain inaccessible.
With the patient in the supine position, the small intestine and transverse colon may disturb the operation field. However, we managed this problem by using two fan retractors. All our bypasses were in a retrograde fashion, i.e., from the infrarenal aorta or the iliac artery/graft. The results of retrograde and antegrade mesenteric bypass have comparable patency, as mentioned in the guidelines.6 Although one can transect SMA distal to the occlusion and construct an end-to-end anastomosis, all anastomoses were in an end-to-side fashion to the SMA. In our patients, the atherosclerotic plaque extended through the entire length of Fullen’s zone 1 and 2 and incorporated the origins of the inferior pancreaticoduodenal artery and middle colic artery. The end-to-side anastomosis was better suited for the preservation of these critical branches.
The free dissection of the splenic artery was without any technical difficulty. Elevation of the left liver lobe with Nathanson’s liver retractor provides excellent access to the hepatogastric ligament and the tributaries of the celiac artery. Bakoyiannis et al used a laparoscopic flexible tunneler and placed the graft anterior to the pancreas.9 With the help of CTA, we carefully planned and could, successfully, place the graft in the retroperitoneum along the aorta in two patients. In one of these patients, we did not stretch the flexible Gore-Tex graft properly and ended up with a long graft besides a kinking close to the aortic anastomosis. This long graft was considered to need an early re-laparoscopy correction to optimize long-term patency.
Laparoscopic aortic clamps, although bulky, can be used on the SMA and splenic artery. However, laparoscopic artery bulldog clamps were more appropriate and provided functional working space for anastomosis. Alternatively, long (30 cm) vascular loops through trocars can be used for clamping of the mesenteric arteries. We had two early graft occlusions and one graft stenosis, which resulted in early redo surgery. In all these three cases, instead of reclamping SMA or splenic artery after flushing of the graft with heparinized NaCl, ring enforced ePTFE was clamped, directly, with the laparoscopic aortic clamp. Thrombendarterectomy of the mesenteric artery and the cross-clamping time of the graft during proximal anastomosis might also have contributed to the development of graft thrombosis. Although the greater omentum could be used to cover the graft to avoid contact with the intestine, we successfully covered the graft with the retroperitoneum in all the patients in this study.13 None of our patients has so far developed any graft infection.
Due to graft thrombosis and graft stenosis, we had to modify our technique in later cases. In vitro, a 6 mm ePTFE graft, was anastomosed end-to-side with the main graft (8 mm), close to the site of the distal (mesenteric) anastomosis (Figure 3C). This side canal allowed a safe and effective route for the flushing of the main graft during the operation. If necessary, this side canal can be used for thrombectomy of the main graft. One may also perform completion angiography through this side graft and if required, even stenting of mesenteric arteries. After the bypass completion, Hem-o-loc polymer clips and a large metal clip were applied close to the intergraft anastomosis, and the rest of the 6 mm ePTFE graft was excised and discarded. Furthermore, we avoided cross-clamping of the graft after completion of the distal anastomosis, in the latter cases.
One case of conversion due to venous bleeding and another with the left ureter injury occurred in the patients with severe peritoneal adhesions due to earlier abdominal operations. It is estimated that 10 to 37% of patients with elective abdominal surgery will require repeated abdominal surgery.14 The risk of such complications in the patients with previous multiple abdominal surgeries is high, even with open surgery.14,15 Use of preoperative evaluation tools like Hostile Abdomen Index risk stratification may help to select the right patients for laparoscopic procedures.16
One of the significant limitations of our operative technique was the failure to assess the patency of the graft during operation. Only in one case, we used an ultrasound probe (Mira Q Vascular, Medistim) to control the anastomosis and confirm blood flow through the graft. The imaging probe was bulky and not designed for laparoscopic use through a trocar. Retrospectively, we realize that a routinely use of laparoscopic ultrasounds, explicitly manufactured for laparoscopic use, should have been mandatory to confirm graft patency during the operations. Alone, this vital step could have helped us to avoid three major complications in our study, with a limited number of patients included. A completion angiography may be a better alternative, since one may also visualize the periphery of the revascularized artery.
The present early experience with laparoscopic revascularization treatment of chronic mesenteric ischemia suffered from complications related to the technique. This is even though the operating team has a long experience with laparoscopic aortic surgery.17–19 Nevertheless, the previous experience did help during the construction of anastomosis. We experienced that when the operative field for the anastomosis construction was once achieved, the laparoscopic anastomosis could be performed within an acceptable time, as reflected in our results (Table 2). The operative time was long during these procedures. This can be explained by the early experience with the new technique, besides time consumption due to adhesiolysis in most of the patients. Long operation time has also been observed during the initial experience with laparoscopic aortic surgery. However, the operation time for such procedures is not significantly longer in the later experience.17,19 We can, in the future, expect to observe a similar progression in operation time consumption also with laparoscopic mesenteric revascularization procedures. The number of trocars used is to provide a safe peritoneal adhesiolysis and proper positioning for anastomoses construction on the infrarenal aorta, iliac artery, SMA, and splenic artery. Despite many trocars, the patients with laparoscopy seem to have less postoperative abdominal pain, demonstrated using postoperative epidural analgesia.
The benefits of a minimally invasive procedure like laparoscopic mesenteric revascularization can only be achieved by a better patient selection, meticulous free dissection technique, avoidance of graft cross-clamping, and mandatory use of ultrasound during the operation or a completion angiography in future studies. These advanced laparoscopic procedures should only be performed at centers by vascular surgeons with experience in laparoscopic aortic surgery.
Conclusions
Chronic mesenteric ischemia patients can be treated with laparoscopic mesenteric bypass procedures. Careful patient selection and meticulous operative technique are mandatory for achieving acceptable results.
Data Sharing Statement
This small cohort is a subgroup of patients participating in the study of chronic mesenteric ischemia. The study data will be made available online at the completion of the main study in 2022.
Acknowledgments
We are thankful for all the support from our colleagues within the departments of vascular surgery, anesthesiology, and operation theatre.
Author Contributions
All authors, SSHK, STB, MS, AWM, and JOS, made substantial contribution to conception and design, acquisition of data, analysis and interpretation of data; took part in drafting the article and revising it critically. All authors gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors have nothing to disclose.
References
1. Shaw RS, Maynard EP
2. Schermerhorn ML, Giles KA, Hamadan AD, Wyers MC, Pomposelli FB. Mesenteric revascularization: management and outcomes in the United States, 1988-2006. J Vasc Surg. 2009;50:341–348. doi:10.1016/j.jvs.2009.03.004
3. Oderich GS, Bower TC, Sullivan TM, Bjarnason H, Cha S, Gloviczki P. Open versus endovascular revascularization for chronic mesenteric ischemia: risk-stratified outcomes. J Vasc Surg. 2009;49(1472–1479.e3). doi:10.1016/j.jvs.2009.02.006
4. Rawat N, Gibbons CP; Joint Vascular Research Group. Surgical or endovascular treatment for chronic mesenteric ischemia: a multicenter study. Ann Vasc Surg. 2010;24:935–945. doi:10.1016/j.avsg.2010.05.007
5. Lejay A, Georg Y, Tartaglia E, et al. Chronic mesenteric ischemia: 20-year experience of open surgical treatment. Eur J Vasc Endovasc Surg. 2015;49:587–592. doi:10.1016/j.ejvs.2015.01.004
6. Bjorck M, Koelemay M, Acosta S, et al. Management of the disease of mesenteric arteries and veins. Clinical Practice Guidelines of the European Society of Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53:460–510. doi:10.1016/j.ejvs.2017.01.010
7. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHH 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi:10.1161/CIRCULATIONAHA.106.174526
8. Javerliat I, Coggia M, Bourriez A, Centa D, Cerceau P, Goeau-brissonniere OA. Total laparoscopic aortomesenteric bypass. Vascular. 2004;12(2):126–129. doi:10.2310/6670.2004.20780
9. Bakoyiannis C, Cagiannos C, Wasilljew S, Pinter L, Kolvenbach R. Totaly laparoscopic aortohepatic bypass for aortic debranching during endovascular thoracoabdominal aneurysm repair. Eur J Vasc Endovasc Surg. 2007;34:173–175. doi:10.1016/j.ejvs.2006.12.032
10. Fullen WD, Hunt J, Altemeier WA. The clinical spectrum of penetrating injury to the superior mesenteric arterial circulation. J Trauma. 1972;12:656–664. doi:10.1097/00005373-197208000-00003
11. Berge ST, Safi N, Medhus AW, Ånonsen K, Sundhagen JO, Hisdal J. Gastroscopy assisted laser Doppler flowmetry and visible light spectroscopy in patients with chronic mesenteric ischemia. SJCLI. 2019;79(7):541–549.
12. Coggia M, Javerliat I, Di Centa I, et al. Total laparoscopic bypass for aortoiliac occlusive lesions: 93-case experience. J Vasc Surg. 2004;40:899–906. doi:10.1016/j.jvs.2004.08.013
13. Kazmers A. Operative management of chronic mesenteric ischemia. Ann Vasc Surg. 1998;12(3):299–308. doi:10.1007/s100169900158
14. Chema S, Stommel MW, Schipper LJ, et al. Risk factors for future repeat abdominal surgery. Langenbecks Arch Surg. 2016;401:829–837. doi:10.1007/s00423-016-1414-3
15. Seetahal S, Obirieze A, Cornwell, EEet al. Open abdominal surgery: a risk factor for future laparoscopic surgery? Am J Surg. 2014;209:623–626.
16. Goldfarb MA, Protyniak B, Schultheis M. Hostile abdomen index risk stratification and laparoscopic complications. JSLS. 2014;18:14–19. doi:10.4293/108680813X13693422518993
17. Kazmi SS, Jørgensen JJ, Sundhagen JO, et al. A comparative cohort study of totally laparoscopic and open aortobifemoral bypass for the treatment of advanced atherosclerosis. Vasc Health Risk Manag. 2015;11:541–547. doi:10.2147/VHRM
18. Krog AH, Sahba M, Pettersen EM, Wisløff T, Sundhagen JO, Kazmi SSH. Cost-utility analysis comparing laparoscopic versus open aortobifemoral bypass surgery. Vasc Health Risk Manag. 2017;13:217–224. doi:10.2147/VHRM.S138516
19. Sahba M, Krog AH, Pettersen EM, et al. Cost comparison analysis of laparoscopic versus open aortobifemoral bypass surgery: a randomized controlled trial. Open Access J Clin Trials. 2019;11:11–18. doi:10.2147/OAJCT.S192552
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.