Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 14
A Scoping Review of Gut Microbiome and Bifidobacterium Research in Zimbabwe: Implications for Future Studies
Authors Zhou DT, Mudhluli TE , Hall LJ , Manasa J, Munyati S
Received 18 April 2023
Accepted for publication 13 October 2023
Published 18 December 2023 Volume 2023:14 Pages 483—496
DOI https://doi.org/10.2147/PHMT.S414766
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Danai T Zhou,1 Taona E Mudhluli,1,2 Lindsay J Hall,3– 5 Justen Manasa,1,6 Shungu Munyati6
1Department of Laboratory Diagnostic and Investigative Sciences, University of Zimbabwe, Harare, Zimbabwe; 2Department of Biochemistry, Midlands State University, Gweru, Zimbabwe; 3Gut Microbes & Health, Quadram Institute Bioscience, Norwich, UK; 4Norwich Medical School, University of East Anglia, Norwich, UK; 5Intestinal Microbiome, Technical University of Munich, Freising, Germany; 6Department of Laboratory Sciences, Biomedical Research and Training Institute, Harare, Zimbabwe
Correspondence: Danai T Zhou, Department of Laboratory Diagnostic and Investigative Sciences, University of Zimbabwe, Box A 178, Avondale, Harare, Zimbabwe, Email [email protected]; [email protected]
Background: Gut microbiota play a key role in host health, with certain Bifidobacterium strains critical for immune development. The healthy gut of breastfed infants is dominated by these pioneer microbes, especially the strains that feed on human milk oligosaccharides.
Objective: This is a scoping review of gut microbiome research from Zimbabwe. It focuses on distribution and dynamic changes of bifidobacteria, and milk components that promote growth of microbes in infants, together with the distribution of associated gut microbes in adults.
Design: Online databases were searched for publications from 2000 to 2023.
Results and Analysis: Fourteen publications on microbiota of infants and adults were included in this scoping review. Most were cross-sectional, while three were clinical trials/cohort protocols. Publications focused on pediatrics (78.5%), pregnant women (14.3%), and men (7.2%). Zimbabwe has a high burden of HIV; hence 35.7% of study populations were delineated by HIV status. The laboratory methods used included shotgun metagenomics (62%) or 16S rRNA gene amplicon sequencing. Almost 85% of the studies focused on total microbiome profiles and rarely reported the distribution of different Bifidobacterium species and variants. None of the papers studied human breast milk composition. There were reports of reduced abundance of beneficial genera in pregnant women, children, and adolescents living with HIV. Additionally, gut microbiota was reported to be poorly predictive of child growth and vaccine response, though this was not conclusive.
Conclusion: There are few studies that characterize the gut microbiome by Zimbabwe-based researchers. However, studies on strain level diversity of Bifidobacterium and other key microbes, and their role in health during and beyond infancy, lag behind in Zimbabwe and other low- and middle-income countries. Such cohorts are needed to inform future mechanistic studies and downstream translational work such as next-generation probiotics and prebiotics.
Keywords: Bifidobacterium, gut microbiome, human milk oligosaccharides, LMIC, prebiotics, probiotics, Zimbabwe
Introduction
The Infant Gut Microbiome
Untangling what constitutes a healthy gut microbiome is timely, particularly in low- and middle-income (LMIC) settings where problems of infant nutrition, chronic diarrhea, and failure to thrive are high priority.1,2 Studying the infant gut microbiome is also crucial as many of the events capable of shaping microbial communities (eg birth mode, antibiotic use, and feeding type) take place during this phase of life.2,3 The healthy infant gut microbiota is known to be dominated by the genus Bifidobacterium with certain species particularly dominant during lactation: B. breve, B. bifidum, B. longum subsp. longum, and B. longum subsp. infantis.2,4 Numerous studies have indicated that certain species and strains have immune-modulatory roles, eg B. breve (also a common breast milk-associated species). This species has been reported to have anti-allergic properties and is generally absent from allergic infants.5,6 Additionally, various prospective studies performed in cohorts of allergic infants and formula-fed infants found less bifidobacteria, and this may link to negative health outcomes. Interestingly, certain adult-type Bifidobacterium species, mainly B. adolescentis, B. catenulatum, and B. pseudocatenulatum, appear to be more prevalent in allergic and formula-fed infants, but this requires further inquiry.2,6,7 Within LMICs, previous work has indicated that malnutrition in children causes depletion of bifidobacteria, with enhanced colonization of potential pathogens such as Streptococcus spp., Fusobacterium mortiferum, and Escherichia coli, which is associated with diarrhea and malabsorption of essential nutrients.8 In adults, presence of Bifidobacterium is also associated with “health”, and particular strains have potential roles in the reduction of serum cholesterol, alleviation of lactose intolerance, and treatment of inflammatory bowel diseases, acute diarrhea, colorectal cancer, and other intestinal infections.7,9
Sources of Beneficial Bacteria or “Probiotics”
Probiotics have been isolated from breast milk and fecal samples of infants, eg Bifidobacterium, Lactobacillus, and the yeast Saccharomyces boulardii.10 Other good sources of beneficial Lactobacillus are dairy and dairy-related products, including fermented milk and kefir grains. Non-dairy fermented substrates, for instance Nigerian fermented foods, brines of naturally fermented Aloreña green table olives, meat, and fruits, are also sources of potential probiotic strains, eg Lactobacillus, Staphylococcus carnosus, or Weissella.10 Indeed, Lactobacillus buchneri P2, isolated from pickled juice, has probiotic properties, such as cholesterol reduction, and acid tolerance.9,10 Animals also harbor beneficial bacteria, and several species, eg pigs, rats, and poultry, insects, eg honeybees, and fish are potential probiotic strain sources.10
The Potential Roles of Probiotics and Prebiotics
The major difference between vaginally delivered and exclusively breastfed infants, and those who are not, is their access to natural prebiotics (eg human milk oligosaccharides) and probiotics (eg maternal and breast milk microbes).9,11 The human milk oligosaccharides (HMOs) found in breast milk are not digested by the infant, but rather they favor the growth of certain Bifidobacterium species and strains, eg B. longum subsp. infantis and B. bifidum.12–14 HMOs have other important roles, eg acting as antivirals and antimicrobials, modulating the host immune response, improving gut barrier function, preventing pathogen attachment to mucosal surfaces, developing the immune system, modulating intestinal cell responses, and lowering the abundance of Enterococcus spp. and Escherichia coli.1,8,13
Most HMOs contain a polylactosamine or lacto-N-biose core and lactose at the reducing end. The core and lactose structures are often linked to fucose (70%) and sialic acid (30%).13 HMO metabolism is observed in various members of the Bifidobacterium genus, eg B. longum subsp. infantis and B. bifidum, which is linked to specific HMO degradation enzymatic gene clusters. Such HMO consumers contain all the glycosyl hydrolases required for catabolizing a whole spectrum of HMO linkages.13,14 However, some individual strains of B. breve display poor growth on HMOs but are capable of utilizing more simple structures such as lacto-N-tetrose, and some adult-type strains such as Bifidobacterium adolescentis are incapable of digesting HMOs.8,13,14 Alongside Bifidobacterium, HMO assimilation pathways have been described for a number of gut microbes belonging to other taxa, eg Bacteroides and Akkermansia.15,16
Randomized controlled trials of prebiotic or probiotic interventions have reported significant weight gain in infants fed formula milk containing Bifidobacterium animalis subsp. lactis, and fructogalacto-oligosaccharides.17 B. animalis subsp. lactis is a common probiotic species believed to provide health benefits when consumed, and also improves bone density,17 while fructogalacto-oligosaccharides are prebiotics that have structural similarities to certain HMOs, that promote the growth of normally abundant, beneficial microbes in the human intestine.13,17 Hence, microbiome modulations using HMOs and some Bifidobacterium strains are potential nutritional and therapeutic targets.13
Some strains of Bifidobacterium are included as probiotics in functional dairy products, food supplements, and pharma products.18 This is because of work indicating they may promote health, and the fact that alteration in their number and composition is a frequent feature in intestinal diseases such as inflammatory bowel disease, colorectal cancer, or irritable bowel syndrome, as well as extra-intestinal pathologies, such as those affecting the liver or the respiratory tract (eg, allergy, bronchial asthma, and cystic fibrosis).19 However, the degree of scientific evidence regarding the effectiveness of probiotics is still insufficient, while evidence from African populations is still intermittent.
It has been hypothesized that perturbation of gut Bifidobacterium levels and diversity accounts for differences in immune development and vaccine response.2,20 Understanding the distribution and dynamics of Bifidobacterium species and the roles of different Bifidobacterium strains and HMOs is important for design of context-specific interventions, focusing on immune conditions. For instance, the introduction of Bifidobacterium in clinical studies showed that supplementation with breast milk-metabolizing B. bifidum was associated with long-term establishment and increased Bifidobacterium diversity in the preterm gut.21 In contrast, adding complementary foods to breastfeeding babies has been shown to radically alter the infant intestinal community, leading to a mature, adult-like microbiota.2,22 In light of the above evidence, this scoping review was carried out to systematically map the research performed in the growing area of gut microbiome in Zimbabwe, with focus on Bifidobacterium composition, as well as to identify any knowledge and methodological gaps.
Objectives
The following research question was formulated: What is known from published literature about gut Bifidobacterium and associated gut microbiota, from Zimbabwe? What is the evidence of the gut microbiota evolution in response to diet, infection, and environmental exposures, and what is its contribution to infant growth, susceptibility to viral infections, oral vaccine response, and adult health? What are the implications of a perturbed microbiome for both infant and adult health and disease, respectively? Are there any reports on the fortification of foods with specific Bifidobacterium strains, other potential probiotics, and human milk oligosaccharides?
Method
Protocol Development
Our protocol was drafted using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Extension for Scoping Reviews (PRISMA-ScR)23 and reviewed by two members of the research team (see Figure 1). Ethics approval was not required for this scoping review.
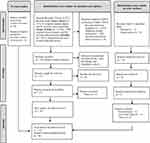 |
Figure 1 Prisma Flow Diagram for Scoping Reviews. Notes: Adapted from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71.23 |
Eligibility Criteria
To be included in this scoping review, papers needed to have been: published between 2000 and 2023, involve human participants from Zimbabwe, measure or incorporate one or two laboratory methods used in gut microbiome studies, and either be an original research or protocol (not review).
Information Sources
Searches were performed in databases and trials or study registries relevant to the research question, ie Science Direct, PubMed, and Google Scholar. The reference lists or bibliographies of included studies were searched, and for unpublished data or grey literature we contacted content experts and authorities in the field. Two co-authors then screened titles and abstracts. We made a final decision on the inclusion criteria for studies one month before completion of this scoping review.
Search Strategy
The following search terms were used: “gut microbiome research Zimbabwe”. Appropriate controlled vocabulary and free text terms were used to refine searches in the online databases.
Results and Analysis
Data Charting and Data Analysis
Data items relevant to the review questions were extracted. Data charting involved the charting of authors’ or studies’ key information, year of publication, country where the study was conducted, aims/purpose, population and sample size, methodology, study design, and findings that relate to the scoping review questions. Data analysis was undertaken via frequency counts and basic content analysis. The data were presented using tables, boxes, and narrative summaries to address the scoping review’s objectives, and to answer the questions of the review.
Summary of Results
Our searches returned over 2320 manuscripts. We removed duplicates and ineligible studies. Two authors then screened 461 remaining abstracts for eligibility. Most of the screened abstracts were ineligible for being either from elsewhere in Africa, other than Zimbabwe, or being focused on wild animals. Out of 17 eligible publications, three were thesis reports and were excluded, therefore only 14 unique publications were retrieved. Of the 14 studies included, two are clinical trial protocols, one is a prospective cohort protocol, nine are cross-sectional study reports, and two are cohort study reports. A total of 1711 stool specimens were analyzed for gut microbiota composition, by various researchers, in collaboration with experts from South Africa, Switzerland, UK, and USA. None of the microbiota assays were carried out locally, which is a reflection of both skills and technological gaps, in the Zimbabwe setting. As a result of a dearth of research laboratories the total number of relevant papers was low by international standards. Studies on prebiotics (human milk oligosaccharide) and food sources of probiotics are not yet apparent. The few publications that reported on Bifidobacterium suggested reduced abundance and no association of these beneficial genera with child growth and vaccine response, in Zimbabwean populations.24–26 Each of the 14 publications is summarized in Table 1 and in the narrative summaries below.
 |
Table 1 Showing Results of the Systematic Literature Search; Fourteen Original Research Papers Were Retrieved.24–37 |
The 2015 publication by Gough et al presented the protocol for the Sanitation Hygiene Infant Nutrition Efficacy (SHINE) Trial in Zimbabwe, and how it was designed to provide a platform for examining linear growth, anemia, and the evolution of the gut microbiota in response to diet, infection, and environmental exposures.24 The protocol outlines the hypotheses guiding the project and the analytic approaches to be used, such as the longitudinal design, randomized interventions, and advanced DNA sequencing technologies including shotgun DNA (metagenomic) and RNA (meta-transcriptomic) sequencing. The follow-up paper by Humphrey et al described the protocol in greater detail and shared some initial findings.38 Water, sanitation, and hygiene (WASH) interventions included reduced soil ingestion, improved sanitation, water treatment, handwashing, and hygienic preparation of food.24,38,39 The infant and young child feeding (IYCF) interventions included 20 g of a small-quantity lipid-based nutrient supplement per day from 6 to 18 months in addition to counseling that was targeted on key barriers to optimal infant feeding.24 The IYCF interventions increased the mean length-for-age Z-score and the mean hemoglobin concentration by 2.0 g/L (1.3–2.8), reduced stunting and anemia, and improved ponderal growth compared with the non-IYCF interventions. The WASH intervention had no effect on these outcomes. Neither intervention reduced child diarrhea or mortality.24 Subsequent research papers on the infant and maternal microbiota from this cohort are reported below.25–27
In 2021, Gough et al discuss evidence for a persistent public health challenge of preterm birth and low-birth-weight infants in LMICs.25 To identify more effective targets for intervention, the researchers performed shotgun metagenomics sequencing of maternal fecal specimens collected during pregnancy and at 1 month post-partum, from the SHINE Trial. The authors reported that pregnancy gut microbiome taxa accurately predicted birth weight and weight-for-age Z-scores at 1 month. The authors reported remarkably few Bacteroidetes species and that this may reflect dietary or functional microbiome differences. The authors did not discuss specimen storage conditions as a possible factor, but it could be theorized that Bacteroidetes is highly labile and susceptible to long-term storage.39
Taxa that are associated with resistant starch degradation, specifically members of the Ruminococcaceae, Lachnospiraceae, and Eubacteriaceae families, were important predictors of birth weight and length-for-age Z-scores.25 The authors conclude that pregnancy gut microbiome in rural Zimbabwe is characterized by resistant starch degraders, and that these may be an important metabolic target to improve birth weight and neonatal growth (Box 1). There was no mention of Bifidobacterium in the gut microbiome of the studied women,25 which is unusual as B. adolescentis is one of the major starch degraders.40 According to the authors, the prediction accuracy for all outcomes was improved when functional enzyme relative abundances were used, demonstrating the potential value of whole-metagenome shotgun sequencing for investigating the human microbiome and health.25
 |
Box 1 Question About Potential Benefit of Starch Degrading Microbiome |
In the same year (2021), Robertson et al published a manuscript from observations also made in the SHINE Trial.26 The authors first discussed the rotavirus as the leading cause of diarrheal morbidity and mortality in children and that oral rotavirus vaccine (RVV) immunogenicity is considerably lower in low- versus high-income populations. They hypothesized that gut microbiota may have a role because of its established contribution to the maturation of early-life immune function. To verify this, the researchers performed shotgun metagenomics sequencing on stool samples and measured antirotavirus immunoglobulin A in corresponding plasma samples, on a subset of infants. The authors reported presence of diverse Bifidobacterium, dominated by B. longum, followed by B. bifidum and B. pseudocatenulanum. Among the 158 infants with stool samples and anti-rotavirus IgA titers, the authors reported that only 34 (22%) were RVV seroconverters. The authors concluded that further research is warranted to examine the mechanistic role of the gut microbiome in poor oral RVV efficacy in LMICs. Besides the “to-be-expected” presence of B. pseudocatenulanum in the infants, they reported a potential association of Bacteroides thetaiotaomicron with vaccine responsiveness, as it was the only species associated with anti-rotavirus IgA titer.26 The authors debated their findings which failed to find a clear infant microbiome signature that distinguished RVV seroconverters from non-seroconverters, despite using gold-standard metagenome sequencing. This suggests a need for further studies using a larger cohort and long-read metagenomics methods such as MinIon® nanopore sequencing (Box 2).
 |
Box 2 Long-Range Sequencing Methods |
In 2023, the SHINE Trial Team (first author Robertson RC) published a manuscript, which started by describing traditional determinants of stunting and child undernutrition, then proposed some yet unestablished determinants.27 The authors then described their study of early-life fecal microbiome composition and function, in which stool samples were collected longitudinally from infants. After shotgun metagenomics sequencing, the authors found that B. longum was the predominant species at all time-points up to 12 months of age. Four other Bifidobacterium species (B. breve, B. bifidum, B. pseudocatenulatum, and B. kashiwanohense) were consistently among the most abundant species at the earlier time points before the children attained 12 and 18 months of age, before being outnumbered by different gut bacteria. The study also showed that children who were HIV-exposed but uninfected (HEU) exhibited reduced abundance of Bifidobacterium species compared to the HIV-unexposed. The authors proposed that this may partially explain deficits in growth in the HEU childhood and interventions may complement efforts to combat child undernutrition.27 Of interest is the presence of B. pseudocatenulatum in the infants, associated with bottle (formula)-fed infants, which needs exploring (Box 3). Formula feeding is rare in Zimbabwe due to Ministry of Health and Child Care campaigns and the Zimbabwe Public Health (Breast-milk Substitutes and Infant Nutrition) Regulations, 1998, restricting the advertising and sales promotion of breast milk substitutes to the public.
 |
Box 3 Bifidobacterium pseudocatenulatum and Formula Milk in Infants from Zimbabwe |
According to a 2022 Child Health, Agriculture and Integrated Nutrition (CHAIN) protocol, the authors redesigned infant and young child feeding (IYCF) interventions using locally available foods to improve intake, uptake, and utilization of nutrients.28 The objectives of the trial are to improve infant growth, and interrogate underlying pathogenic pathways during the complementary feeding period with the aim of reducing global burden of stunting. Results of this study are not yet published.
A 2020 paper by Flygel et al29 compared the composition of gut microbiota of children and adolescents living with perinatal transmitted HIV, taking antiretroviral therapy, in the Bronchopulmonary function in REsponse to Azithromycin Treatment for chronic lung disease in HIV-infected children (BREATHE) Study, with that of children and adolescents without HIV. Rectal swabs were collected from 177 participants living with HIV and 103 controls living without HIV. Gut microbial composition was explored using 16S rRNA gene (V4 hypervariable region) amplicon sequencing. The authors reported enriched levels of Corynebacterium, Finegoldia, and Anaerococcus in participants living with HIV, and enrichment of Enterobacteriaceae in participants with low CD4+ counts (<400 cells/mm3). Prolonged ART-treatment (≥10 years) was significantly associated with a richer gut microbiota by alpha diversity.29 They concluded that children living with HIV have altered gut microbiota and that prolonged ART may restore the richness of the microbiota closer to that of children and adolescents without HIV. This result may be crucial for high HIV-burdened settings like Zimbabwe, though more information on reasons for differences and presence of potential probiotic microbes and potential pathogens in the birth canal, breast milk, gut, and skin of mothers of HIV-exposed infants needs to be explored (Box 4).
 |
Box 4 Microbiota Differences in HIV-Exposed Children |
In 2020, Osakunor et al focused on preschool-aged infants living in a rural community endemic with bilharzia.30 Bilharzia, also known as schistosomiasis, is caused by helminth parasitic worms and has been shown to have systemic effects within the host. The authors first discussed established factors associated with the composition of the gut microbiome, then deliberated about some yet-to-be-determined environmental factors such as protozoa and helminth parasites. In their study, the researchers tested the hypothesis that infection with the human helminth parasite, Schistosoma haematobium, is associated with changes in gut microbial and antimicrobial resistance gene abundance or diversity. They used shotgun metagenomics sequencing to characterize the gut microbiome and the resistome of Zimbabwean preschool infants aged 1 to 5 years, in which the S. haematobium infection prevalence was 15.9%. The most abundant bacteria phyla in decreasing order were Bacteroidota (previously Bacteroidetes), Bacillota (previously Firmicutes), and Pseudomonadota (previously Proteobacteria).30 This study was one of the first to describe a metagenomics dataset of Zimbabwean preschool-aged infants and indicates an association between urogenital schistosome infection and abundance of Pseudomonadota in the gut microbiome. They concluded that the gut microbiome but not the resistome is associated with urogenital schistosomiasis in preschool-aged children. The study also provided insight into microbial composition and function of preschool infants, though more information about bacterial strain level diversity is required.
Kay et al carried out a study to characterize the gut microbiome of children also exposed to the helminth S. haematobium, then pre- and post-treatment with the drug praziquantel.31 Stool DNA was extracted at baseline and 12 weeks following anti-helminthic treatment with praziquantel (PZQ), as appropriate. The 16S rRNA gene amplicon (V3-V4 hypervariable region) sequencing included baseline and post-treatment samples, with profiles clustered into operational taxonomic units (OTUs). Pre-treatment, the most abundant phyla were Bacteroidota, followed by Bacillota and Pseudomonadota, respectively. The relative abundance of taxa among bacterial classes and communities showed limited variation by age group or sex. Some OTUs including Veillonella, Streptococcus, Bacteroides, and Helicobacter were more abundant in children ≤1 year old compared to older children. Furthermore, the gut microbiome differed in schistosome-infected vs uninfected children, with 27 OTUs occurring in infected but not uninfected children. PZQ treatment did not appear to alter microbiome structure in infected or uninfected children from that observed at baseline.31 The data suggest changes in the gut microbiota with age, which agrees with reports from other settings.
Pfavayi et al studied the association between gut microbiome and fungal allergic sensitization in young rural Zimbabwean children.32 They carried out the research to interrogate increased prevalence of allergic diseases over the last few decades. The gut microbiome of stool samples was characterized using shotgun metagenomics sequencing, and sensitization to common fungi was assayed using skin prick tests. The mycobiome formed <1% of the sequenced gut microbiome, and 228 fungal genera were identified. According to the authors, the most abundant fungal genera detected were Protomyces, Taphrina, and Aspergillus, and individuals were frequently sensitized to Saccharomyces cerevisiae. The paper concluded that further studies with larger populations are required to understand the role of the microbiome in allergic diseases.32
Katsidzira et al explored gut microbiome signatures associated with colorectal cancer (CRC) in 10 male and 10 female adults from rural and urban Zimbabwe.33 In their hypothesis, they propose that the rise in CRC incidence in Zimbabwe is related to shifts in the colonic microbiota and metabolome. Therefore, the researchers conducted an exploratory study comparing diet and fecal markers associated with CRC risk in the 20 apparently healthy adults, all aged >50 years. The full-length 16S rRNA gene was sequenced. The researchers reported minor differences in fecal microbiota composition between urban and rural participants. In particular, there were significant differences in the relative abundances of a few genera, ie Blautia obeum, Streptococcus bovis, and Subdoligranulum were more abundant among urban residents. Oscillospira guilliermondii, Sporobacter termitidis, and Clostridia were more abundant among rural participants. The study detected no difference in the abundance of Fusobacteria in feces, which has been associated with tumor formation in the colon. In addition, there was no difference in the abundance of Bilophila wadsworthia, a bacterium associated with experimental colitis. The authors concluded that the higher abundance of Subdoligranulum in the urban participants highlights the potentially beneficial impact of retained fibre intake in the urban study population.33
In a 2020 publication, Gough et al described results of strain-level analysis of gut-resident pro-inflammatory viridans group Streptococci (VGS) for infants in the Anti-Retrovirus Research for Watoto (ARROW) Trial.34 Six stool samples from children who continued and fourteen from children who stopped cotrimoxazole were positive for VGS, in an earlier study. The authors further explored strain-level patterns within the VGS identified as being suppressed by long-term cotrimoxazole prophylaxis, Streptococcus salivarius being the most prevalent. They used a strain-level metagenomic profiling tool – The Pangenome-based Phylogenomic Analysis (PanPhlAn) – to further characterize S. salivarius sub-species variants. They found that S. salivarius strains in HIV-positive children on ART who continued cotrimoxazole use are no different from those of children who stopped, but that the S. salivarius strains are different from those of children from high-income countries.34
According to Bourke et al, in a publication from 2019, stunting in Zimbabwean infants is characterized by low-grade chronic inflammation.35 The study therefore focused on cotrimoxazole, an antibiotic that reduces systemic inflammation in children living with HIV. The research used shotgun metagenomics sequencing of total fecal DNA from children randomized to continue versus stop cotrimoxazole, in the ARROW Trial. Randomized groups did not differ in species-level diversity. Bacterial community composition was also similar between groups. However, 7 bacterial species (Alistipes onderdonkii, Eggerthella lenta, Clostridium bartlettii, Haemophilus parainfluenzae, Streptococcus mutans, Streptococcus parasanguinis, and Streptococcus vestibularis) and 11 protein families mapping to Streptococcus parasanguinis, Streptococcus salivarius, and Haemophilus parainfluenzae were consistently less abundant in the continue versus stop group. The relative abundance of Enterobacteriaceae, which includes gastrointestinal pathogens (eg Salmonella, E. coli, and Shigella) that are frequently resistant to cotrimoxazole, was not affected by cotrimoxazole and was increased in those continuing versus stopping cotrimoxazole at week 96.35
Duri et al published the protocol of The University of Zimbabwe College of Health Sciences (UZ-CHS) Birth Cohort study in 2020.36 This is a prospective observational cohort study comparing infants born to 1200 mothers living with and without HIV, attending primary health care clinics in Harare’s high-density suburbs. Half of the recruited pregnant women were living with HIV. This is a prospective observational cohort study comparing infants born to women living with HIV and those without. The pregnant women were recruited at ≥20 weeks of gestation between February 2016 and June 2019. Participants are being followed up as mother–infant pairs at birth, within 10 days of birth and at 6, 10, 14, 24, 48, 72, and 96 weeks post-partum. Questionnaires are administered and bio-samples for laboratory tests are acquired at each visit. Biomaterials are for specific tests including gut microbiome profiling, as well as tests for current infections, clinical biochemistry, full blood counts, plus immune status. One paper has been published on maternal microbiome profiles.37
The paper from the sub-study of mothers from the University of Zimbabwe (UZ) Birth Cohort,36 mentioned above, was published by Chandiwana et al in 2023.37 It assessed the composition of the intestinal microbiota in pregnant women residing in Zimbabwe.37 Pregnant women living with HIV (PWLWH) had a lower species diversity (α-diversity) than those without. The differences in microbiome composition between samples (ie β-diversity) also differed between them. High abundance of Spirochaetaceae, Veillonellaceae, and Treponema in the third trimester of pregnancy was associated with low birth weight in the infants. This study focused on beneficial bacteria in the pregnant women and reported that living with HIV was associated with a lower abundance of Bacteroides and Bifidobacterium (Box 5), and a higher abundance of Actinomyces and Succinivibrio.37
 |
Box 5 Identity of Potential Microbes |
Results and Analysis
We searched the literature for publications about the gut microbiota and Bifidobacterium. We found very few publications on this and expanded our search to include papers describing components of a healthy or an unhealthy gut microbiome of neonates, infants, and adults from Zimbabwe. Some of the published papers explored evolution of the gut microbiota in response to diet, infection (eg bilharzia and HIV), and environmental exposures (such as antibiotics and antiretroviral drugs). Some explored the contribution of the gut microbiota and attempted to unravel the implications of a perturbed microbiome to mostly infant health and disease. There were no reports on the fortification of foods with specific Bifidobacterium strains and human milk oligosaccharides in the Zimbabwe context.
Most publications leveraged randomized clinical trials, eg the SHINE Trial and ARROW Trial, and international collaborations. The microbiome studies were carried out in collaboration with researchers from Europe (UK and Switzerland), South Africa, and USA. The laboratory methods applied to the microbiome work included Illumina shotgun metagenome sequencing and 16S rRNA gene sequencing (mainly sequencing of the V3 hypervariable region).
Discussions and Conclusions
There are commendable efforts to characterize the gut microbiome by individual researchers and groups, leveraging on-going mother–infant cohorts in Zimbabwe. Most of the assays were carried out in laboratories outside of Zimbabwe, mainly due to technological and skills gaps. Efforts to include local research institutions in microbiome research are likely to receive local and global support because issues of diversity, equity, and inclusion have risen to the top of the list of critical concerns in science and medicine. All of the published microbiome studies focused on short-read sequencing. There may be useful insights to be gained from long-read sequencing, eg by using Nanopore sequencing technologies. In the Zimbabwe context MinIon® Nanopore sequencers would be most attractive and relevant due to their low cost and small size.41,42
Based on the few numbers of studies and participants, there are many important microbiome-focused questions that still need to be answered. For example, studies of strain level diversity of Bifidobacterium communities and their role in immune development and vaccine response ought to be carried out, in Zimbabwe and other LMIC. In order to benefit and leverage advances made in other settings, Zimbabwean researchers must carry out local cohort studies that compare findings to other LMIC and high-income country groups. Such cohorts are valuable when designing mechanistic studies and downstream translational work such as next-generation probiotics, and prebiotics. The dynamics of Bifidobacterium during early life, and across the lifespan, needs to be explored in experimental studies and clinical trials. Culturing of microbes and sequencing of isolates are important for identifying local strains for therapy development. This is relevant because strategies to counteract malnutrition, enhance vaccine response, and improve health are of significant public health interest.1,8,36,43 Other pertinent questions that remain unanswered are placed in boxes throughout this scoping review.
Acknowledgments
The authors acknowledge the support of fund administrators Alberta Davis (AREF, UK), Leonie Corry (TUM, Germany), and Mildred Pepukai (BRTI, Zimbabwe).
Disclosure
D.T.Z. is supported by the Africa Research Excellence Fund (AREF) DDI.02073952402, and NIH Fogarty International Center Grant # 1D43TW011326, awarded to the Biomedical Research Training Institute (BRTI), Zimbabwe’s D43 ATCHIVR Program. T.E.M. is supported by NIH Fogarty International Center Grant # D43TW010313, awarded to the University of Buffalo, SUNY, USA and the University of Zimbabwe’s HIV Research Training Program. L.J.H. is supported by Wellcome Trust Investigator Awards 100974/C/13/Z and 220876/Z/20/Z; and a BBSRC Institute Strategic Programme, Gut Microbes and Health BB/R012490/1, and its constituent projects BBS/E/F/000PR10353 and BBS/E/F/000PR10356. The authors report no other conflicts of interest in this work.
References
1. Iddrisu I, Monteagudo-Mera A, Poveda C, et al. Malnutrition and gut microbiota in children. Nutrients. 2021;13(8):2727. doi:10.3390/nu13082727
2. Lin C, Lin Y, Zhang H, et al. Intestinal ‘infant-type’ bifidobacteria mediate immune system development in the first 1000 days of life. Nutrients. 2022;14(7):1498. doi:10.3390/nu14071498
3. Huda MN, Ahmad SM, Alam MJ, et al. Bifidobacterium abundance in early infancy and vaccine response at 2 years of age. Pediatrics. 2019;143:e20181489. doi:10.1542/peds.2018-1489
4. Saturio S, Nogacka AM, Alvarado-Jasso GM, et al. Role of bifidobacteria on infant health. Microorganisms. 2021;9(12):2415. doi:10.3390/microorganisms9122415
5. Wong CB, Iwabuchi N, Xiao J-Z. Exploring the science behind Bifidobacterium breve M-16V in infant health. Nutrient. 2019;11(8):1724. doi:10.3390/nu11081724
6. Cukrowska B, Bierła JB, Zakrzewska M, et al. The relationship between the infant gut microbiota and allergy. the role of bifidobacterium breve and prebiotic oligosaccharides in the activation of anti-allergic mechanisms in early life. Nutrients. 2020;12(4):946. doi:10.3390/nu12040946
7. He F, Ouwehand AC, Isolauri E, et al. Comparison of mucosal adhesion and species identification of bifidobacteria isolated from healthy and allergic infants. FEMS Immunol Med Microbiol. 2001;30(1):43–47. doi:10.1111/j.1574-695X.2001.tb01548.x
8. Gotoh A, Katoh T, Sakanaka M, et al. Sharing of human milk oligosaccharides degradants within bifidobacterial communities in faecal cultures supplemented with Bifidobacterium bifidum. Sci Rep. 2018;8(1):13958. doi:10.1038/s41598-018-32080-3
9. O’Neill I, Schofield Z, Hall LJ, Marchesi JR. Exploring the role of the microbiota member Bifidobacterium in modulating immune-linked diseases. Emerging Top Life Sci 2017;1:333–349. doi:10.1042/ETLS20170058
10. Kozak K, Charbonneau D, Sanozky-Dawes R, et al. Characterization of bacterial isolates from the microbiota of mothers’ breast milk and their infants. Gut Microbes. 2015;6(6):341–351. doi:10.1080/19490976.2015.1103425
11. Yakoob R, Pradeep BV. Bifidobacterium sp as probiotic agent - roles and applications. J Pure Appl Microbiol. 2019;13(3):1407–1417. doi:10.22207/JPAM.13.3.11
12. Phillips S, Watt R, Atkinson T, et al. on behalf of the PEARL study team, The Pregnancy and EARly Life study (PEARL) - a longitudinal study to understand how gut microbes contribute to maintaining health during pregnancy and early life, BMC. Pediatr. 2021;21:357.
13. Walsh C, Lane JA, van Sinderen D, et al. Human milk oligosaccharides: shaping the infant gut microbiota and supporting health. J Funct Foods. 2020;72:104074. doi:10.1016/j.jff.2020.104074
14. Lawson MAE, O’Neill IJ, Kujawska M, et al. Breast milk-derived human milk oligosaccharides promote Bifidobacterium interactions within a single ecosystem. ISME J. 2020;14(2):635–648. doi:10.1038/s41396-019-0553-2
15. Kostopoulos I, Elzinga J, Ottman N, et al. Akkermansia muciniphila uses human milk oligosaccharides to thrive in the early life conditions in vitro. Sci Rep. 2020;10:14330. doi:10.1038/s41598-020-71113-8
16. Kijner S, Cher A, Yassour M. the infant gut commensal Bacteroides dorei presents a generalized transcriptional response to various human milk Oligosaccharides. Front Cell Infect Microbiol. 2022;12:854122. doi:10.3389/fcimb.2022.854122
17. Abrahamse-Berkeveld M, Alles M, Franke-Beckmann E, et al. Infant formula containing galacto-and fructo-oligosaccharides and Bifidobacterium breve M-16V supports adequate growth and tolerance in healthy infants in a randomised, controlled, double-blind, prospective, multicentre study. J Nutr Sci. 2016;5:e42. doi:10.1017/jns.2016.35
18. Hidalgo-Cantabrana C, Delgado S, Ruiz L, et al. Bifidobacteria and their health-promoting effects. Microbiol Spectr. 2017;5(3). doi:10.1128/microbiolspec.BAD-0010-2016.
19. Tojo R, Suárez A, Clemente MG, et al. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20(41):15163–15176. doi:10.3748/wjg.v20.i41.15163
20. Jordan A, Carding SR, Hall LJ. The early-life gut microbiome and vaccine efficacy. Lancet Microbe. 2022;3:e787–94. doi:10.1016/S2666-5247(22)00185-9
21. Alcon-Giner C, Dalby MJ, Caim S, et al. Microbiota supplementation with Bifidobacterium and Lactobacillus modifies the preterm infant gut microbiota and metabolome: an observational study. Cell Rep Med. 2020;1(5):100077. doi:10.1016/j.xcrm.2020.100077
22. Laursen MF. Gut microbiota development: influence of diet from infancy to toddlerhood. Ann Nutr Metab. 2021;1–14. doi:10.1159/000517912
23. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:
24. Gough EK, Prendergast AJ, Mutasa KE, et al. The Sanitation Hygiene Infant Nutrition Efficacy (SHINE) trial team, assessing the intestinal microbiota in the SHINE Trial. Clin Infect Dis. 2015;61(suppl 7):S685–702. doi:10.1093/cid/civ850
25. Gough EK, Edens TJ, Geum HM, et al. For SHINE trial team, maternal fecal microbiome predicts gestational age, birth weight and neonatal growth in rural Zimbabwe. EBiomedicine J. 2021;68:103421. doi:10.1016/j.ebiom.2021.103421
26. Robertson RC, Church JA, Edens TJ, et al. SHINE Trial Team, The fecal microbiome and rotavirus vaccine immunogenicity in rural Zimbabwean infants. Vaccine. 2021;39:5391–5400. doi:10.1016/j.vaccine.2021.07.076
27. Robertson RC, Edens TJ, Carr L, et al. The gut microbiome and early-life growth in a population with high prevalence of stunting. Nat Commun. 2023;14:654. doi:10.1038/s41467-023-36135-6
28. Smith LE, Chagwena DT, Bourke C, et al. Child Health, Agriculture and Integrated Nutrition (CHAIN): protocol for a randomised controlled trial of improved infant and young child feeding in rural Zimbabwe. BMJ Open Nutr Metab. 2021;12(12). doi:10.1136/bmjopen-2021-056435
29. Flygel TT, Sovershaeva E, Claassen-Weitz S, et al. BREATHE study team, composition of gut microbiota of children and adolescents with perinatal human immunodeficiency virus infection taking antiretroviral therapy in Zimbabwe. The J of Infect Dis. 2020;221:3.
30. Osakunor DNM, Munk P, Mduluza T, et al. The gut microbiome but not the resistome is associated with urogenital schistosomiasis in preschool-aged children. Commun Biol J. 2020;3:155. doi:10.1038/s42003-020-0859-7
31. Kay GL, Millard A, Sergeant MJ, et al. Differences in the faecal microbiome in schistosoma haematobium infected children vs. uninfected children. PLoS Negl Trop Dis. 2015;9(6):e0003861. doi:10.1371/journal.pntd.0003861
32. Pfavayi LT, Sibanda EN, Baker S, et al. Fungal allergic sensitisation in young rural Zimbabwean children: gut mycobiome and seroreactivity characteristics. Curr Res Microb Sci. 2021;2(2021):100082. doi:10.1016/j.crmicr.2021.100082
33. Katsidzira L, Ocvirk S, Wilson A, et al. Differences in fecal gut microbiota, short-chain fatty acids and bile acids link colorectal cancer risk to dietary changes associated with urbanization among Zimbabweans. Nutr Cancer. 2019;71:8. doi:10.1080/01635581.2019.1602659
34. Bourke CD, Gough EK, Pimundu G, et al. Cotrimoxazole reduces systemic inflammation in HIV infection by altering the gut microbiome and immune activation. Sci Transl Med. 2019;11(486):eaav0537. doi:10.1126/scitranslmed.aav0537
35. Gough EK, Bourke CD, Berejena C, et al. Strain-level analysis of gut-resident pro-inflammatory viridans group Streptococci suppressed by long-term cotrimoxazole prophylaxis among HIV-positive children in Zimbabwe. Gut Microbes. 2020;11(4):1104–1115. doi:10.1080/19490976.2020.1717299
36. Duri K, Gumbo FZ, Munjoma PT, et al.; the UZ-CHS Birth Cohort Team. The University of Zimbabwe College of Health Sciences (UZ-CHS) BIRTH COHORT study: rationale, design and methods. BMC Infect Dis. 20;2020:725. doi:10.1186/s12879-020-05432-6
37. Chandiwana P, Munjoma PT, Mazhandu AJ, et al. Antenatal gut microbiome profiles and effect on pregnancy outcome in HIV infected and HIV uninfected women in a resource limited setting. BMC Microbiol. 2023;23:4. doi:10.1186/s12866-022-02747-z
38. Humphrey JH, Mbuya MNN, Ntozini R, et al. for the Sanitation Hygiene Infant Nutrition Efficacy (SHINE) Trial Team, Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: a cluster-randomised trial. The Lancet Global Health J. 2019. doi:10.1016/S2214-109X(18)30374-7
39. Holzhausen EA, Nikodemova M, Deblois CL, et al. Assessing the impact of storage time on the stability of stool microbiota richness, diversity, and composition. Gut Pathog. 2021;13(75). doi:10.1186/s13099-021-00470-0
40. Jung D-H, Chung W-H, Seo D-H, et al. Complete genome sequence of Bifidobacterium adolescentis P2P3, a human gut bacterium possessing strong resistant starch-degrading activity, 3. Biotech. 2020;10(2):31. doi:10.1007/s13205-019-2019-7
41. Leggett RM, Alcon-Giner C, Heavens D, et al. Rapid MinION profiling of preterm microbiota and antimicrobial-resistant pathogens. Nat Microbiol. 2020;5(3):430–442. doi:10.1038/s41564-019-0626-z
42. Komiya S, Matsuo Y, Nakagawa S, et al. MinION, a portable long-read sequencer, enables rapid vaginal microbiota analysis in a clinical setting. BMC Med Genomics. 2022;15:68. doi:10.1186/s12920-022-01218-8
43. Pekmez CT, Dragsted LO, Brahe LK. Gut microbiota alterations and dietary modulation in childhood malnutrition—The role of short chain fatty acids. Clin Nutr. 2018;1–16. doi:10.1016/j.clnu.2018.02.014.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
