Back to Journals » Drug Design, Development and Therapy » Volume 17
A Review on the Pharmacological Aspects of Engeletin as Natural Compound
Authors Zhong X, Huang R, Chen X , Lei Y
Received 8 September 2023
Accepted for publication 12 December 2023
Published 23 December 2023 Volume 2023:17 Pages 3833—3843
DOI https://doi.org/10.2147/DDDT.S437703
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Xing Zhong,1,* Rui Huang,2,* Xin Chen,2 Yuhua Lei2
1Cardiovascular Disease Center, Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, Hubei Minzu University, Enshi, People’s Republic of China; 2Cardiovascular Disease Center, Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, Enshi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuhua Lei, Email [email protected]
Background: Engeletin (ENG) is a natural flavonoid compound known for its diverse physiological and pharmacological effects, such as anti-inflammatory, antioxidant, and immunomodulatory properties. It has garnered significant attention as a promising candidate for drug development.
Objective: This article aims to comprehensively review the clinical application, pharmacological action, and potential mechanisms of ENG, while exploring its prospects in clinical pharmacology.
Methods: We conducted a systematic search of PubMed, Science Direct, Google Scholar, Web of Science, Scopus, and MEDLINE for a thorough review of high-quality articles on the source, extraction, and application of ENG, or the primary active ingredient for improving bodily injuries.
Results: ENG exhibits significant potential in treating a variety of diseases across different systems, attributed to its anti-inflammatory, antioxidant, anti-tumor, and metabolic regulatory activities. These effects are linked to direct or indirect interactions with multiple pathways involving key molecules upstream and downstream.
Conclusion: While ENG shows promise, its development requires further exploration. Future studies should focus on elucidating its mechanisms of action, identifying targets through clinical studies, and optimizing compounds for drug development. These research directions are crucial for advancing the development and application of flavonoids. This review underscores the significant research potential of ENG, paving the way for its application in diverse clinical settings.
Keywords: engeletin, pharmacological, agent, liliaceous plants
Introduction
Herbs are one of the oldest medical treatments for a variety of illnesses, and they are still indispensable in modern medicine.1 Natural compounds extracted from plants can cure human ailments as life originated in nature. The public has long been interested in the use of natural compounds to treat human diseases. A new xanthone glycoside, 1, 3, 5, 6-tetrahydroxyxanthone-C-4-β-D-glucopyranoside, has been extracted from mango, which excellent water solubility and skin permeability make it suitable for use as a local anti- aging agent.2 The antioxidant properties of flavonoids extracted from artichokes make them powerful anti-Alzheimer’s disease agents.3 The 25 compounds extracted from Origanum vulgare leaves have been shown to have a good protective effect on thioacetamide-induced liver injury and hepatic encephalopathy through its antioxidant and neuroregulatory properties, and can reduce anxiety and depressive behavior in rats with hepatic encephalopathy.4 ENG (deoxydihydroquercetin-3-β-rhamnoside) is a naturally occurring flavonoid compound found in Liliaceous plants. ENG has the molecular formula C21H22O10 and a molecular weight of 434 g/mol.5 It has beneficial biological activities, including anti-inflammatory,6 antioxidant,7 antibacterial,8 antitumor,9,10 and immunomodulatory.11 Furthermore, ENG is a natural aldose reductase (AR) inhibitor that delays the development of chronic diabetes.12 ENG is effective in the treatment of a variety of circulatory, respiratory, reproductive, endocrine, nervous, and motor diseases. This review aimed to summarize the most recent advances in ENG and to anticipate how they might be applied to the prevention and treatment of diseases in the future.
The Sources of ENG
Rhizoma Smilacis Chinae (RSC) and Rhizoma Smilacis Glabrae (RSG) contain significant amounts of ENG, an active ingredient in traditional medicinal herbs. However, the amount of ENG in RSC is significantly greater than that in RSG.13 ENG has also been extracted from Artocarpus dadah, Pieris japonica, Dioon spinulosum, and other plants.14–16 ENG is the primary flavonoid compound in wine.17 The chemical structure of ENG and the extraction procedure are plotted in Figure 1, and the sources of ENG in chronological order is summarized Table 1.
 |
Table 1 The Sources of Engeletin |
ENG has been studied as a flavonoid for more than two decades. ENG was first reported in 1998 for immunological hepatocyte damage treatment. Xu et al18 extracted ENG from the roots of Smilax bockii warb in 2005 for the first time and determined the molecular formula of ENG using a white needle-like crystal at normal temperature and a melting point of 169°C–171°C. In 2011, Huang et al19 extracted ENG for the first time from the leaves of Engelhardia roxburghiana. They found that a 50 µM concentration of ENG inhibited the downstream nuclear factor-κB (NF-κB) pathway-mediated inflammatory response and lipopolysaccharide (LPS)-induced interleukin (IL)-1β release. Interestingly, ENG significantly increased IL-1β mRNA expression at 10 µM.19 According to Srisupa in 2012,20 among the flavonoids isolated from the leaves of Engelhardtia chrysolepsis, ENG significantly inhibited the release of prostaglandin E2 from RAW 264.7 cells stimulated by LPS. Moreover, Zhao et al7 confirmed in 2020 that ENG could suppress the phosphorylation of the p65 protein and the NF-κB pathway in an in vitro sepsis model. ENG has good anti-inflammatory activity, but its concentration range and other activities are not well known. On this basis, subsequent researchers investigated its physicochemical properties and found that ENG is an effective achievement with low cytotoxicity. The pharmacokinetics of ENG were identified for the first time in 2017 by Ye et al.21 ENG was rapidly absorbed and widely distributed following a single oral or intravenous administration, with low bioavailability and an oral half-life of 3.686 ± 2.356 h.21 In 2019, Xie et al22 measured the concentrations of ENG in rat plasma after orally administering Poria cocos and discovered that ENG was rapidly absorbed and reached a maximum concentration of 0.30 ± 0.03 µM in the plasma after approximately 0.25 h. In 2020, Chen et al23 identified ENG as one of the main components of the traditional Chinese medicine prescription for treating psoriasis in the absence of purification analysis. Although various methods have been used to extract and purify ENG from plants, no chemical synthesis of ENG has been reported. ENG has a relatively low yield and purity, which limits its clinical study to some extent.
The Biological Activity and Mechanism of ENG
Recent studies have identified ENG as an active agent in several diseases such as the nervous system, the respiratory system, the digestive system, the cardiovascular system, the genitourinary system, the endocrine system and the motor system. Moreover, ENG was found to have anti-tumor activities. The potential role of ENG in different diseases is summarized in Figure 2 and Table 2, and the pathway regulation related to ENG is plotted in Figure 3.
 |
Table 2 The Activity and Mechanisms of ENG in Different Diseases |
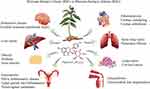 |
Figure 2 The potential role of ENG in different diseases. |
ENG and the Nervous System
In terms of the nervous system, ENG has primarily promoted the regeneration of cerebral ischemia blood vessels, inhibited oxidative stress, reduced inflammation of nerve cells, and preserved nerve function.24 A stroke is a focal injury of the central nervous system caused by vascular events.25 Cerebral ischemia-reperfusion injury (IRI) is the most common cause of neurological impairment. By creating a cerebral IRI model in rats, Liu et al26 revealed that ENG promoted angiogenesis in vivo and in vitro via the vascular endothelial growth factor (VEGF)/vasohibin signaling pathway and stabilized new vessels through the angiopoietin-1/Tie-2 pathway. The study indicated that ENG maintained the blood supply of vital organs and protected cellular activity, providing crucial theoretical support for its potential therapeutic value during IRI. However, this study has several limitations. First, it did not discuss the pharmacokinetic–pharmacodynamic relationship of ENG. Second, because IRI is a complex and multifaceted disease, this study focused only on the effect of ENG on angiogenesis without further examining the specific mechanisms of its protective effect on neurological function injury in IRI.
Based on these findings, another study confirmed that ENG could alleviate oxidative stress and the inflammatory response in BV-2 murine microglial cells induced by amyloid-β (1–42) by adjusting the Kelch like ECH-associated protein 1 (Keap1)/nuclear transcription factor E2-related factor 2 (Nrf2) signaling pathway, hereby arresting the progression of Alzheimer’s disease.24 Collectively, ENG exerted a therapeutic effect on the central nervous system. More basic and clinical studies are warranted to explore its in-depth mechanisms and practical applications.
ENG and the Respiratory System
ENG, an important component of anti-inflammation and regulation of apoptosis, autophagy, cell cycle, and endoplasmic reticulum stress,10,27,28 is involved in the pathophysiology of various respiratory diseases, such as the modulation of respiratory epithelial mucin expression,27 pulmonary fibrosis,28 acute lung injury (ALI),29 and lung cancer.10
ENG Inhibited the Secretion of Mucin-5AC (MUC5AC) in Airway Epithelial Cells
A small amount of mucus is present on the surface of the airways, where mucin plays a key role in defending lung epithelial cells. Pathogens that invade the respiratory tract stimulate abnormal mucus secretion and impair respiratory function by inducing an inflammatory response. Hossain27 found that ENG could directly act on airway epithelial cells by reducing the phosphorylation of inhibitor kappa B kinase (IKK) stimulated by phorbol 12-myristate 13-acetate, thereby modulating the phosphorylation and degradation of inhibitor kappa Bα (IkBα) and the phosphorylation of the NF-κB/p65 signaling pathway, which could improve respiratory function by restricting MUC5AC secretion at the mRNA level. These findings suggest that ENG, as a mucosal regulator, is useful in the treatment of inflammatory lung diseases. However, only in vitro data are presented, and this conclusion requires in vivo confirmation.
Effect of ENG on ALI
ALI is a serious threat to human survival, and there is still a need to explore drugs to ameliorate ALI. An animal model of LPS-induced ALI showed that ENG inhibits the production of inflammatory cytokines in the NF-κB pathway, which may be mediated by the upregulation of proliferator-activated receptor γ (PPARγ).29 Although this study suggests that ENG was effective for ALI in animals, in vitro experiments have only confirmed the difference in the expression of tumor necrosis factor (TNF)-α, an inflammatory factor. Moreover, external evidence is scanty, so more research is needed to verify this viewpoint.
Effect of ENG on Pulmonary Fibrosis
The abnormal transformation of pulmonary fibroblasts to myofibroblasts causes pulmonary fibrosis, which is considered a crucial point in the treatment of pulmonary fibrosis. Previous research has shown that ENG can treat anti-pulmonary fibrosis by inhibiting the expression of fibrosis-related markers such as a-SMA, vimentin, collagen I, and collagen III.28,30 Moreover, by modifying the long noncoding RNA (lncRNA) – lnc949, activating the endoplasmic reticulum stress, and blocking the proliferation and migration of fibroblasts, ENG exerts an antifibrosis effect.28 Furthermore, Shen et al30 proposed that ENG was involved in the intermediate regulatory process of pulmonary fibrosis by regulating lnc865 and lnc556. Overall, the current findings demonstrated that ENG plays a beneficial role in pulmonary fibrosis, which would be more convincing if clinical trial data could be used. A recent study showed that ENG had a considerable therapeutic effect on cardiac remodeling,31 so it is expected that its antifibrotic properties will be confirmed in the treatment of other organ fibrosis, such as liver and renal fibrosis.
ENG and Liver Injury
The liver serves as the body’s detoxification factory. Viruses, bacteria, parasites, drugs, alcohol, and autoimmune factors can cause severe liver injury, which is often manifested as inflammatory liver lesions in the early stages. Therefore, inhibiting inflammatory signaling pathways is an effective therapeutic strategy for delaying the development of liver injury. In 1998, Chen et al11 reported that ENG could prevent damaged hepatocytes from infiltrating the liver cells during immune liver injury by causing rhamnose disorder in liver non-parenchymal cells. In 2019, Tian et al32 studied the liver-protective effect of ENG in a mouse liver injury model induced by LPS. They demonstrated that ENG could intercept the NF-κB inflammatory pathway by upregulating PPAR-γ expression, thereby alleviating the pathological changes in liver tissue and reducing the levels of inflammatory cytokines and transaminases in serum. This supported the significant therapeutic value of ENG in liver injury. Chronic liver injury causes liver fibrosis, which can progress to irreversible cirrhosis if treatment is delayed. Because ENG has been confirmed to be an effective antifibrotic agent, its role in the treatment of liver fibrosis needs to be further explored.
ENG and the Cardiovascular System
Currently, research on ENG in cardiovascular events is lacking. Owing to its anti-inflammatory and antifibrotic effects,6,28 ENG has been shown to have great potential in the treatment of atherosclerosis (AS)33 and cardiac remodeling.31 AS is the leading cause of death worldwide,34 and it is caused by a combination of factors such as lipid metabolism disorders, endothelial dysfunction injuries, inflammation, and oxidative stress.35–37 Based on Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis and animal experiments, Wei et al33 confirmed that ENG inhibits the occurrence and progression of AS via the following mechanisms: 1. downregulating the expression of p65 and its downstream targets TNF-α, vascular cell adhesion molecule-1, and IL-1β in the NF-κB pathway to reduce the arterial inflammatory response; 2. directly interacting with the low-density lipoprotein receptor (LDLR), thereby targeting and regulating essential cholesterol metabolic pathway genes such as APOB, APOE, and LDLR, resulting in a reduction in lipid levels; and 3. inhibiting the expression of diabetes-related target genes via the insulin signaling pathway and acting as an antioxidant. The study identified potential targets and pathways for AS, providing ideas for future AS research as well as elucidating various biological roles of ENG. The latest research results show that the antioxidant properties of ENG improve isoproterenol-induced myocardial fibrosis, electrical remodeling, and ion channel remodeling as well as ameliorate cardiac remodeling and ventricular fibrillation susceptibility.31 Despite recent studies demonstrating that ENG has positive effects on cerebral IRI26 and other diseases, the potential role and mechanisms of ENG in cardiac IRI have not been discussed yet and need to be confirmed by further research.
ENG and the Genitourinary System
Researchers have also studied the effects of ENG on reproductive system disorders. It has been proven to be effective in treating endometritis,38 genital tract inflammation,39 pelvic inflammatory disease,40 and other conditions because of its anti-inflammatory activity. Its unique antibacterial activity is effective in treating vaginal candidiasis.11 It can also prevent tumor cells from proliferating and metastasizing, thereby inhibiting reproductive system tumors.9
ENG and Inflammation of the Reproductive System
Currently, antibiotics are the primary treatment modality for inflammation of the female reproductive system. Antibiotics can effectively inhibit pathogens, but long-term use will inevitably lead to bacterial resistance due to chronic inflammation in the genital tract. Therefore, new therapeutic measures must be explored. In one study, ENG extracted from Poria cocos was found to be a natural AR inhibitor with strong anti-inflammatory and antioxidant activities.12 At present, many studies have suggested that ENG plays an important role in inflammatory diseases of the reproductive system.38,40 Wang et al40 found that ENG could exert an anti-pelvic inflammatory disease (PID) effect by specifically blocking AR-dependent phospholipase C (PLC)/ protein kinase C (PKC)/NF-κB and mitogen-activated protein kinase (MAPK) inflammatory pathways, inhibiting nuclear translocation of NF-κB p65, and enhancing the phagocytotic ability of RAW 264.7 macrophages induced by LPS. Further research using the PID rat model showed that ENG significantly reduced the uterine inflammatory response and improved the epithelial tissue exfoliation of the endometrial edema, inflammatory cell infiltration, and endometrial fibrosis pathology. In addition, ENG plays a role in endometritis and inflammatory diseases of the upper reproductive system.38,39 Therefore, given the present findings, we hypothesize that ENG could be a valuable and effective alternative drug for gynecological inflammatory diseases.
ENG and Vaginal Candidiasis
Candida albicans produces secreted aspartyl proteinases (SAP) to exert its virulence,41 and vulvovaginal candidiasis mediated by Candida albicans is the second most common and frequently recurring vaginal inflammation in women.42 Molecular simulations and docking simulations performed by Poorna et al8 revealed that ENG has the highest free energy of binding with the target amino acid residues and can occupy the bioactive amino acid residues in the target cell, demonstrating strong antifungal activity by inhibiting SAP. Therefore, we can expect that the use of ENG in inflammatory diseases of the reproductive system will greatly improve the quality of life of women. ENG can also inhibit the proliferation and metastasis of cervical cancer cells.9 As a result, researchers believe that ENG may have adjuvant therapeutic value in gynecological diseases, but further research is needed to confirm this.
The Effect of ENG in the Endocrine System
ENG and Gouty Arthritis (GA)
GA is a chronic inflammatory disease disorder characterized by synovial joint swelling; its acute onset is associated with the deposition of monosodium urate crystals in the joints,43,44 whereas chronic development and impaired renal function are caused by long-term serum hyperuricemia. Researchers found that RSG with ENG as the primary active ingredient had promising effects in the treatment of acute and chronic GA. For example, Liang et al45 demonstrated using gout animal models of acute GA and chronic hyperuricemia that ENG could dose-dependently reduce toe swelling, serum inflammatory factors, uric acid, and BUN levels; inhibit uric acid secretion; promote uric acid excretion; and decelerate the development of chronic gout. This study suggests that ENG, as the main active ingredient in RSG, plays a role in GA by reducing xanthine oxidase activity in the liver; improving inflammatory cell infiltration, renal tubular dilatation, vacuole formation, and synovial hyperplasia; and reducing the infiltration of inflammatory cells into the synovial membrane. The therapeutic effect and mechanism of ENG on gout still need to be verified experimentally in this study because it was not purified and analyzed separately.
ENG and Obesity
Obesity is a risk factor for diabetes, coronary AS, hyperuricemia, and other diseases.46,47 The activation of brown adipocytes reduces lipid accumulation and prevents obesity.48 Kong et al49 discovered that ENG can stimulate brown adipose tissue by activating mitochondria. In this mechanism, the β3-AR/ AMP-activated protein kinase (AMPK) signaling pathway is activated, leading to an increase in the expression of related metabolic genes such as lipolysis and oxidation-related metabolic genes, activating browning transcription factors, stimulating the transformation of white adipocytes into beige or brown-like adipocytes, promoting fat metabolism and thermogenesis, and decreasing fat storage. The effect of ENG on adipocyte browning is described for the first time in this study, which provides a new direction for developing drugs to improve human health.
ENG and Diabetes
Diabetes mellitus and its complications pose a serious threat to human health. Controlling blood glucose levels is currently the most important treatment for diabetes mellitus. According to Nguyen,50 ENG, which is extracted from plants such as Smilax glabra and lotus, cannot directly stimulate insulin secretion but can lower blood glucose levels in animals and cells. However, these effects have not yet been unanimously confirmed by exact mechanisms. In the body, a large amount of glucose is converted to sorbitol by AR during long-term hyperglycemia. Sorbitol accumulates in small blood vessels, nerves, retinas, kidneys, and other organs, leading to a hyperosmotic effect, which is the primary reason for chronic complications in diabetes mellitus.51 AR is the first rate-limiting enzyme in glucose-to-sorbitol conversion. Based on this theory, researchers have developed a method to control sorbitol levels to treat diabetic complications. Wirasathien12 confirmed that ENG extracted from cauliflower had the highest AR inhibitory activity and that it inhibited AR activity via non-competitive inhibition of the formation of inactive enzyme–substrate–inhibitor complexes, thereby terminating sorbitol accumulation and slowing the development of diabetic complications.
The Role of ENG in the Motor System
The pathogenesis of osteoarthritis (OA) and intervertebral disc degeneration (IDD) is similar. The pathological feature of OA is cartilage degeneration, and its pathological mechanism is as follows: reactive oxygen species (ROS) inhibit the expression of extracellular matrix (ECM) in the joint and promote the synthesis of ECM-degrading enzymes, alter mitochondrial membrane potential, and induce apoptosis, thereby leading to chondrocyte decline and cartilage integrity destruction.52 IDD is caused by the loss of EMC cells and the apoptosis of nucleus pulposus cells in the nucleus pulposus of intervertebral discs, and inflammatory cytokines accelerate this process. According to the in vitro and in vivo findings of Wang et al.53 ENG inhibits the production of ROS, the degradation of ECM, the apoptosis of chondrocytes, and the destruction of mitochondrial membrane potential and suppresses the phosphorylation of NF-κB and MAPK signaling pathways associated with OA progression to alleviate the joint inflammatory response. Li et al54 demonstrated that ENG also inhibits the release of inflammatory cytokines in IDD models by preventing the activation of these inflammatory pathways. Based on these studies, ENG may be a promising treatment for arthritis because of its anti-inflammatory, anti-apoptotic, and antioxidant properties.
Antitumor Effect of ENG
ENG appears to play an antitumor role by regulating the post transcriptional translation of proteins, promoting autophagy in tumor cells, and inducing apoptosis in tumor cells. In 2011, Huang et al19 reported that ENG extracted from the leaves of Engelhardia roxburghiana inhibited the proliferation of human prostate cancer cells; however, they did not examine this mechanism in depth. In 2020, ENG was confirmed to induce tumor cell apoptosis, promote autophagy, and modify ubiquitination via the X-linked inhibitor apoptosis (XIAP)/ second mitochondria-derived activator of caspase (SMAC) signaling pathway in lung cancer cells.10 In addition to increasing ubiquitination and degradation of the anti-apoptotic protein XIAP via the ubiquitin–proteasome system at the translational level, ENG significantly increases the levels of the pro-apoptotic molecules t-BID and p53 upregulated modulator of apoptosis to induce apoptosis in lung cancer cells. The expression of the autophagy markers light chain-3B protein and autophagy-related gene-5 in lung cancer cells can be stimulated by ENG, thereby triggering autophagy and affecting the growth of lung cancer cells by inducing mitochondrial dysfunction.
Early distant metastasis in patients with cervical cancer is the major cause of death from this highly invasive cancer. ENG was studied by Bai et al9 to determine whether it affected the proliferation and metastasis of cervical cancer tumor cells. According to this study, ENG inhibits NF-κB signaling in cervical cancer tumors, interstitial epithelial transformation, vascular endothelial growth factor-A (VEGFA) and chemokine (CeC motif) ligand 2 (CCL2) expression, thereby restraining tumor migration, tumor invasion, tumor angiogenesis, and inflammatory progression.
Based on the current basic research, we can speculate that ENG is an antitumor agent. Despite the lack of clinical trials, it cannot be used in real-world settings. Further research into pharmacodynamics, pharmacokinetics, toxicology, and other areas will allow ENG to make progress as a potential antitumor active ingredient.
Summary and Outlook
ENG, the primary flavonoid compound found in liliaceous plants such as RSG and RSC, exhibits a wide range of beneficial properties including anti-inflammatory, antioxidant, anti-apoptotic, antitumor, antibacterial, and immunomodulatory effects, suggesting potential therapeutic applications across various diseases affecting the circulatory, respiratory, reproductive, endocrine, nervous, and motor systems (as outlined in Table 2). Numerous studies have indicated a positive correlation between the pharmacological activity of ENG and its concentration, with low cellular toxicity and a favorable safety profile. While its distinct antitumor activity has been demonstrated in lung and cervical cancers, its broader effectiveness as a broad-spectrum antitumor agent remains to be established. Despite ENG’s historical use as a therapeutic agent, its pharmacological effects are still in the early stages of development. For instance, its impact on the cardiovascular system has been relatively understudied, necessitating further research to determine its potential role in cardiac ischemia-reperfusion injury (IRI) and septic cardiomyopathy.
Future investigations should thoroughly explore ENG’s effects on different systems and establish its safe dosage. Furthermore, all current studies regarding ENG are preclinical, with no clinical trials conducted. Consequently, subsequent clinical drug trials and randomized clinical trials are imperative to verify its efficacy and safety. The following studies should focus on various perspectives, including mechanism investigation, compound optimization, drug development, and clinical research. Delving deeper into the mechanism of action and exploring the targets of ENG, including its cellular and molecular-level activity, as well as its interactions with other drugs or therapies, will provide a more robust scientific basis for drug development and clinical application. Optimizing the chemical structure of ENG is paramount for enhancing its pharmacological activity and bioavailability, and drug development is pivotal in translating research findings into tangible medications, offering new prospects for the development of novel drugs and treatment regimens. Conducting clinical studies is a vital step in translating the potential value of ENG into clinical applications, validating its effectiveness and safety in treating specific diseases. A comprehensive exploration of these research directions will help unleash the full potential pharmacological value of ENG and contribute to promoting human health.
Author Contributions
All authors contributed significantly to the work reported, including theme design, literature search and screening, and graphics production, drafting, reviewing, and revising of articles, or all of these areas. All authors have participated in the submission of the article to the journal and agreed to be accountable for all aspects of the work.
Funding
This work was funded by the National Natural Science Foundation of China (No.82160072, No.82360085), the Natural Science Foundation of Hubei Province (No.2023AFD073) and the Science and Technology Support Project of Enshi Prefecture Science and Technology Bureau (No. D20210024).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Chan K. Some aspects of toxic contaminants in herbal medicines. Chemosphere. 2003;52(9):1361–1371. doi:10.1016/S0045-6535(03)00471-5
2. El-Nashar HAS, El-Labbad EM, Al-Azzawi MA. Ashmawy NS: a New Xanthone Glycoside from Mangifera indica L.: physicochemical Properties and In Vitro Anti-Skin Aging Activities. Molecules. 2022;27(9):2609. doi:10.3390/molecules27092609
3. El-Nashar HAS, Abbas H, Zewail M, et al. Neuroprotective Effect of Artichoke-Based Nanoformulation in Sporadic Alzheimer’s Disease Mouse Model: focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceuticals (Basel). 2022;15(10):1202. doi:10.3390/ph15101202
4. Abdelghffar EAR, El-Nashar HAS, Fayez S, Obaid WA, Eldahshan OA. Ameliorative effect of oregano (Origanum vulgare) versus silymarin in experimentally induced hepatic encephalopathy. Sci Rep. 2022;12(1):17854. doi:10.1038/s41598-022-20412-3
5. Jing Xu XL, Zhang P, Zhan-Lin L, Wang Y. Antiinflammatory Constituents from the Roots of Smilax bockii warb. Arch Pharm Res. 2005;28:395.
6. Feng H, He Y, La L, et al. The flavonoid-enriched extract from the root of Smilax China L. inhibits inflammatory responses via the TLR-4-mediated signaling pathway. J Ethnopharmacol. 2020;256:112785.
7. Zhao X, Chen R, Shi Y, Zhang X, Tian C, Xia D. Antioxidant and Anti-Inflammatory Activities of Six Flavonoids from Smilax glabra Roxb. Molecules. 2020;25(22):5295. doi:10.3390/molecules25225295
8. Pushkala VP, Sulekha SMP, Mathukumar S, Ragavi B, Sowmiya U. Molecular Docking Analysis of Siddha Formulation Parangipattai Chooranam Against Vaginal Candidiasis. Appl Biochem Biotechnol. 2022;194(3):1039–1050. doi:10.1007/s12010-022-03813-y
9. Bai H, Yin H. Engeletin suppresses cervical carcinogenesis in vitro and in vivo by reducing NF-kappaB-dependent signaling. Biochem Biophys Res Commun. 2020;526(2):497–504. doi:10.1016/j.bbrc.2020.03.091
10. Liu T, Li Y, Sun J, Tian G, Shi Z. Engeletin suppresses lung cancer progression by inducing apoptotic cell death through modulating the XIAP signaling pathway: a molecular mechanism involving ER stress. Biomed Pharmacother. 2020;128:110221. doi:10.1016/j.biopha.2020.110221
11. Chen T, Li J, Cao J, Xu Q, Komatsu K, Namba T. A new flavanone isolated from rhizoma smilacis glabrae and the structural requirements of its derivatives for preventing immunological hepatocyte damage. Planta Med. 1999;65(1):56–59. doi:10.1055/s-1999-13963
12. Wirasathien L, Pengsuparp T, Suttisri R, Ueda H, Moriyasu M, Kawanishi K. Inhibitors of aldose reductase and advanced glycation end-products formation from the leaves of Stelechocarpus cauliflorus R.E. Fr. Phytomedicine. 2007;14(7–8):546–550. doi:10.1016/j.phymed.2006.09.001
13. Zhang QF, Guo YX, Zheng G, Wang WJ. Chemical constituents comparison between Rhizoma Smilacis Glabrae and Rhizoma Smilacis Chinae by HPLC-DAD-MS/MS. Nat Prod Res. 2013;27(3):277–281. doi:10.1080/14786419.2012.666747
14. Su BN, Cuendet M, Hawthorne ME, et al. Constituents of the bark and twigs of Artocarpus dadah with cyclooxygenase inhibitory activity. J Nat Prod. 2002;65(2):163–169. doi:10.1021/np010451c
15. Li YP, Li YH, Zhong JD, Li RT. Antioxidant phenolic glycoside and flavonoids from Pieris japonica. J Asian Nat Prod Res. 2013;15(8):875–879. doi:10.1080/10286020.2013.803475
16. Negm WA, El-Seoud KA A, Kabbash A, Kassab AA, El-Aasr M. Hepatoprotective, cytotoxic, antimicrobial and antioxidant activities of Dioon spinulosum leaves Dyer Ex Eichler and its isolated secondary metabolites. Nat Prod Res. 2021;35(23):5166–5176. doi:10.1080/14786419.2020.1789636
17. Chamkha M, Cathala B, Cheynier V, Douillard R. Phenolic composition of champagnes from Chardonnay and Pinot Noir vintages. J Agric Food Chem. 2003;51(10):3179–3184. doi:10.1021/jf021105j
18. Xu J, Li X, Zhang P, Li ZL, Wang Y. Antiinflammatory constituents from the roots of Smilax bockii warb. Arch Pharm Res. 2005;28(4):395–399. doi:10.1007/BF02977667
19. Huang H, Cheng Z, Shi H, Xin W, Wang TT, Yu LL. Isolation and characterization of two flavonoids, engeletin and astilbin, from the leaves of Engelhardia roxburghiana and their potential anti-inflammatory properties. J Agric Food Chem. 2011;59(9):4562–4569. doi:10.1021/jf2002969
20. Ruangnoo S, Jaiaree N, Makchuchit S, Panthong S, Thongdeeying P, Itharat A. An in vitro inhibitory effect on RAW 264.7 cells by anti-inflammatory compounds from Smilax corbularia Kunth. Asian Pac J Allergy Immunol. 2012;30(4):268–274.
21. Ye W, Chen R, Sun W, et al. Determination and pharmacokinetics of engeletin in rat plasma by ultra-high performance liquid chromatography with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1060:144–149. doi:10.1016/j.jchromb.2017.06.018
22. Xie J, Li L, Shi Y, et al. Simultaneous ultra-performance liquid chromatography-tandem mass spectrometry determination of six components in rat plasma after oral administration of Smilacis glabrae Roxb. extract. Biomed Chromatogr. 2019;33(12):e4680. doi:10.1002/bmc.4680
23. Chen L, Chen H, Lu Y, et al. Decoding active components in a formulation of multiple herbs for treatment of psoriasis based on three cell lines fishing and liquid chromatography-mass spectrometry analysis. J Pharm Biomed Anal. 2020;186:113331. doi:10.1016/j.jpba.2020.113331
24. Huang Z, Ji H, Shi J, Zhu X, Zhi Z. Engeletin Attenuates Abeta1-42-Induced Oxidative Stress and Neuroinflammation by Keap1/Nrf2 Pathway. Inflammation. 2020;43(5):1759–1771. doi:10.1007/s10753-020-01250-9
25. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089. doi:10.1161/STR.0b013e318296aeca
26. Liu H, Li S, Xu Y, et al. Engeletin protects against cerebral ischemia/reperfusion injury by modulating the VEGF/vasohibin and Ang-1/Tie-2 pathways. Braz J Med Biol Res. 2021;54(10):e11028. doi:10.1590/1414-431x2020e11028
27. Hossain R, Kim KI, Li X, Lee HJ, Lee CJ. Involvement of IKK/IkBalpha/NF-kB p65 Signaling into the Regulative Effect of Engeletin on MUC5AC Mucin Gene Expression in Human Airway Epithelial Cells. Biomol Ther (Seoul). 2022;30(5):473–478. doi:10.4062/biomolther.2022.088
28. Zhang J, Chen X, Chen H, et al. Engeletin ameliorates pulmonary fibrosis through endoplasmic reticulum stress depending on lnc949-mediated TGF-beta1-Smad2/3 and JNK signalling pathways. Pharm Biol. 2020;58(1):1105–1114. doi:10.1080/13880209.2020.1834590
29. Jiang X, Chen L, Zhang Z, Sun Y, Wang X, Wei J. Protective and Therapeutic Effects of Engeletin on LPS-Induced Acute Lung Injury. Inflammation. 2018;41(4):1259–1265. doi:10.1007/s10753-018-0773-z
30. Shen K, Li R, Zhang X, et al. Acetyl oxygen benzoate engeletin ester promotes KLF4 degradation leading to the attenuation of pulmonary fibrosis via inhibiting TGFbeta1-smad/p38MAPK-lnc865/lnc556-miR-29b-2-5p-STAT3 signal pathway. Aging (Albany NY). 2021;13(10):13807–13821. doi:10.18632/aging.202975
31. Fang Z, Liu Z, Tao B, Jiang X. Engeletin mediates antiarrhythmic effects in mice with isoproterenol-induced cardiac remodeling. Biomed Pharmacother. 2023;161:114439. doi:10.1016/j.biopha.2023.114439
32. Tian Q, Wang G, Zhang Y, et al. Engeletin inhibits Lipopolysaccharide/d-galactosamine-induced liver injury in mice through activating PPAR-gamma. J Pharmacol Sci. 2019;140(3):218–222. doi:10.1016/j.jphs.2019.06.011
33. Wei J, Zhang Y, Li D, et al. Integrating Network Pharmacology and Component Analysis Study on Anti-Atherosclerotic Mechanisms of Total Flavonoids of Engelhardia roxburghiana Leaves in Mice. Chem Biodivers. 2020;17(3):e1900629. doi:10.1002/cbdv.201900629
34. Libby P. The changing landscape of atherosclerosis. Nature. 2021;592:7855):524–533. doi:10.1038/s41586-021-03392-8
35. Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104(4):503–516. doi:10.1016/S0092-8674(01)00238-0
36. Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi:10.1038/nature01323
37. Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47(8 Suppl):C7–C12. doi:10.1016/j.jacc.2005.09.068
38. Wu H, Zhao G, Jiang K, Li C, Qiu C, Deng G. Engeletin Alleviates Lipopolysaccharide-Induced Endometritis in Mice by Inhibiting TLR4-mediated NF-kappaB Activation. J Agric Food Chem. 2016;64(31):6171–6178. doi:10.1021/acs.jafc.6b02304
39. Zou W, Zhou H, Hu J, et al. Rhizoma Smilacis Glabrae inhibits pathogen-induced upper genital tract inflammation in rats through suppression of NF-kappaB pathway. J Ethnopharmacol. 2017;202:103–113. doi:10.1016/j.jep.2017.02.034
40. Wang C, La L, Feng H, et al. Aldose Reductase Inhibitor Engeletin Suppresses Pelvic Inflammatory Disease by Blocking the Phospholipase C/Protein Kinase C-Dependent/NF-kappaB and MAPK Cascades. J Agric Food Chem. 2020;68(42):11747–11757. doi:10.1021/acs.jafc.0c05102
41. De Bernardis F, Agatensi L, Ross IK, Emerson GW, Lorenzini R, Sullivan PA. Cassone A: evidence for a role for secreted aspartate proteinase of Candida albicans in vulvovaginal candidiasis. J Infect Dis. 1990;161(6):1276–1283. doi:10.1093/infdis/161.6.1276
42. Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23(2):253–273. doi:10.1128/CMR.00076-09
43. Schumacher HR. Crystal-induced arthritis: an overview. Am J Med. 1996;100(2A):46S–52S. doi:10.1016/s0002-9343(97)89546-0
44. Terkeltaub RA, Ginsberg MH. The inflammatory reaction to crystals. Rheum Dis Clin North Am. 1988;14(2):353–364. doi:10.1016/S0889-857X(21)00969-8
45. Liang G, Nie Y, Chang Y, et al. Protective effects of Rhizoma smilacis glabrae extracts on potassium oxonate- and monosodium urate-induced hyperuricemia and gout in mice. Phytomedicine. 2019;59:152772. doi:10.1016/j.phymed.2018.11.032
46. Piche ME, Tchernof A, Despres JP. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ Res. 2020;126(11):1477–1500. doi:10.1161/CIRCRESAHA.120.316101
47. Afinogenova Y, Danve A, Neogi T. Update on gout management: what is old and what is new. Curr Opin Rheumatol. 2022;34(2):118–124. doi:10.1097/BOR.0000000000000861
48. Li Y, Wang D, Ping X, et al. Local hyperthermia therapy induces browning of white fat and treats obesity. Cell. 2022;185(6):949–966 e919. doi:10.1016/j.cell.2022.02.004
49. Kong L, Zhang W, Liu S, Zhong Z, Zheng G. Quercetin, Engelitin and Caffeic Acid of Smilax China L. Polyphenols, Stimulate 3T3-L1 Adipocytes to Brown-like Adipocytes Via beta3-AR/AMPK Signaling Pathway. Plant Foods Hum Nutr. 2022;77(4):529–537. doi:10.1007/s11130-022-00996-x
50. Nguyen KH, Ta TN, Pham TH, et al. Nuciferine stimulates insulin secretion from beta cells-an in vitro comparison with glibenclamide. J Ethnopharmacol. 2012;142(2):488–495. doi:10.1016/j.jep.2012.05.024
51. Cogan DG, Kinoshita JH, Kador PF, et al. NIH conference. Aldose reductase and complications of diabetes. Ann Intern Med. 1984;101(1):82–91. doi:10.7326/0003-4819-101-1-82
52. Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5(5):415–418. doi:10.1023/A:1009616228304
53. Wang H, Jiang Z, Pang Z, et al. Engeletin Protects Against TNF-alpha-Induced Apoptosis and Reactive Oxygen Species Generation in Chondrocytes and Alleviates Osteoarthritis in vivo. J Inflamm Res. 2021;14:745–760. doi:10.2147/JIR.S297166
54. Li B, Yang X, Zhang P, et al. Engeletin Alleviates the Inflammation and Apoptosis in Intervertebral Disc Degeneration via Inhibiting the NF-kappaB and MAPK Pathways. J Inflamm Res. 2022;15:5767–5783. doi:10.2147/JIR.S371809
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


