Back to Journals » International Journal of Nanomedicine » Volume 18
A Review of in vivo Toxicity of Quantum Dots in Animal Models
Received 10 August 2023
Accepted for publication 15 December 2023
Published 29 December 2023 Volume 2023:18 Pages 8143—8168
DOI https://doi.org/10.2147/IJN.S434842
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Xiaotan Lin,1,2 Tingting Chen1
1School of Basic Medicine, Guangdong Medical University, DongGuan, People’s Republic of China; 2Department of Family Planning, Second Clinical Medical College of Jinan University, Shenzhen People’s Hospital, Shenzhen, People’s Republic of China
Correspondence: Tingting Chen, School of Basic Medicine, Guangdong Medical University, DongGuan, People’s Republic of China, Email [email protected]
Abstract: Tremendous research efforts have been devoted to nanoparticles for applications in optoelectronics and biomedicine. Over the past decade, quantum dots (QDs) have become one of the fastest growing areas of research in nanotechnology because of outstanding photophysical properties, including narrow and symmetrical emission spectrum, broad fluorescence excitation spectrum, the tenability of the emission wavelength with the particle size and composition, anti-photobleaching ability and stable fluorescence. These characteristics are suitable for optical imaging, drug delivery and other biomedical applications. Research on QDs toxicology has demonstrated QDs affect or damage the biological system to some extent, and this situation is generally caused by the metal ions and some special properties in QDs, which hinders the further application of QDs in the biomedical field. The toxicological mechanism mainly stems from the release of heavy metal ions and generation of reactive oxygen species (ROS). At the same time, the contact reaction with QDs also cause disorders in organelles and changes in gene expression profiles. In this review, we try to present an overview of the toxicity and related toxicity mechanisms of QDs in different target organs. It is believed that the evaluation of toxicity and the synthesis of environmentally friendly QDs are the primary issues to be addressed for future widespread applications. However, considering the many different types and potential modifications, this review on the potential toxicity of QDs is still not clearly elucidated, and further research is needed on this meaningful topic.
Keywords: quantum dots, nanotoxicology, nanoparticle, toxicity, cytotoxic
Graphical Abstract:
Introduction
As a new type of nanomaterials, quantum dots (QDs) have attracted wide attention all over the world due to their unique optical and electrical properties. QDs are semiconductor nanoparticles composed of II–VI elements (eg, CdTe, CdSe, CdS, ZnS, ZnSe, or ZnTe), group III–V elements (eg, InP or InAs), group I–III VI2 elements (eg, CuInS2 or AgInS2), group IV–VI elements (eg, PbSe, PbS, or PbTe) and group IV elements (eg, Si, C, or Ge).1 The structure of QDs usually consists of a semiconductor material and second semiconductor materials outside. Usually, the core determines the optical properties of QDs, while the shell enhances the optical stability of QDs. More importantly, QDs can also be coated with a special material for hydrophilic or functionalized modification in order to meet specific biomedical applications.2,3
In recent years, with its excellent fluorescence properties, QDs have been applied to many biotechnologies including immunofluorescence, cell tracking and in vivo imagine.4,5 The most successful biomedical application in the early days was cellular or tissue labeling. In 1998, Bruchez and his partners labeled 3T3 mouse fibroblast cells using two different size biotinylated CdSe/ZnS core-shell nanocrystals for the first time. Selection of fluorescent probe emission wavelengths in the near-infrared wavelength range of in vivo imaging can obtain the best tissue penetration and high-quality optical signal.6 In addition, QDs are also widely used for targeting tumor markers.7 The basic principle is that recognition of specific antigens on the surface of a tumor cell as a target. Antibody-conjugated QDs were injected into the animal body to achieve in vivo tumor targeting and imaging according to the specific bright fluorescent of QDs. These findings are of great significance for promoting complex, highly sensitive imaging and diagnosis of tumor targeting in vivo. In addition to the above studies, QDs have also been used for a variety of other purposes. So far, researchers have successfully described the multicolor and multi-strength fluorescent encoded spheres with different sizes of QDs encapsulated in polymer microspheres at different ratios,8 and successfully achieved the specific marking and discrimination of target DNA.9,10 QDs have also been used to track RNA interference11 and vasculature imaging.12
However, along with the increasing production and application of QDs, the risk of unintentional occupational or environmental exposure to QDs is growing. The potential harm of QDs to environment and human health has gradually attracted the attention of researchers.13,14 From 2004, many research teams have studied the toxicity of QDs and obtained some QDs toxicological data.15,16 Most toxicology is evaluated by using in vitro or in vivo models, and in vitro toxicity studies are evaluated using a variety of cell models.17 To some extent, these studies have made an evaluation of the toxicity of QDs. However, because of the diversity of QDs and the complex characteristics of biological samples, it is difficult for us to analyze and evaluate the obtained data. Current research results showed the toxicity of QDs is not only related to its physical and chemical properties, such as preparation methods and surface properties and particle size,18,19 but also influenced by cell type, treatment environment and administration route.20,21 At the same time, their toxicological research methods are not unified and systematic, making the work of evaluating and comparing the biosafety of QDs extremely complex and difficult. Many QDs emitting in the NIR limit their applications (for instance, in biomedical labeling) because of their toxic Cd (II), Hg (II), Pb (II), As (III) and other elements.22,23 Previous research evidence shows that even if the Cd2+ containing QDs are wrapped in a tight outer membrane that inhibits the release of Cd2+, the intracellular ROS content of QDs is still significantly increased.24 So QDs that do not contain Cd (non-Cd QDs) have been prepared as an alternative to the Cd-based QDs. These newly developed non-Cd QDs exhibit improved blood half-life and minimal reticuloendothelial system (RES) uptake.25 QDs toxicity has also been studied by using different animal models, such as zebrafish,26 xenopus,27 mouse,28 rat29,30 and macaque.31
The toxicity of QDs depends on multiple physicochemical factors including composition, size, shape, surface chemistry, dosage, and route of exposure.32 Cd2+ QDs can release toxic Cd2+ ions, while other QDs may induce toxicity through mechanisms like oxidative stress. Smaller QDs tend to have higher cellular uptake and toxicity compared to larger ones.33 Positively charged QDs are more toxic than neutral or negatively charged ones.34 Surface coatings like silica can reduce QD toxicity by preventing ion leaching and aggregation.35
Despite promising applications, concerns remain about potential health and environmental hazards of QDs, especially with long-term exposure. Most toxicity studies have been short-term in vitro evaluations. There is still limited understanding of QDs toxicity mechanisms, bioaccumulation, organ-specific effects, and impacts on organisms in real-world exposure scenarios.
This review summarizes current knowledge on the in vivo toxicity of QDs in animal models. A particular focus is QDs effects on major organs like liver, kidneys, lungs, brain, and the immune system. The review also examines parameters affecting QDs toxicity such as composition, size, surface modification, dose, exposure route, and duration. By reviewing recent literature, this paper aims to provide insights into QDs toxicity mechanisms, gaps in current understanding, and strategies to develop safer QDs for biomedical and environmental applications.
In vivo Imaging
QDs are semiconductor engineering nanomaterials (ENM) with tiny luminescent particles at the nanoscale (typically 2–10 nm in diameter) and are considered as an important choice for traditional fluorescent dyes in biomedical applications.36 The most classical and common QDs are mainly cadmium containing QDs, such as CdTe, CdS, and CdSe. However, the inherent toxicity of heavy metals Cd limits their practical applicability because they contain dangerous and expensive raw materials.37 Therefore, it is essential to develop environmentally friendly Cd2+ free QDs in practical applications.38 In the last two decades, a series of Cd2+ free QDs based on indium phosphide (InP),39 copper indium sulfide (CuInS2),40 silver indium sulfide (AgInS2),41 silver sulfide (Ag2S),42 doped Zn chalcogenides43 have been successfully developed. These QDs share a strong resemblance to the optical properties and colloidal stability of the conventional Cd-based QDs.44
QDs transmit fluorescence signals in a wavelength range by varying their sizes and composition, thus providing wide excitation profile and high absorption coefficient. Compared with traditional fluorescent dyes (1–10 ns), they have a narrow and symmetry emission spectrum, thus having longer excited state lifetimes (20–50 ns).45 Their quantum yield is 40% to 90% and has a high extinction coefficient. They are more stable than the traditional organic dyes. They can be coated and covered with hydrophilic materials to conjugate with biomolecules.46 In addition, the near-infrared (NIR) fluorescence (700–1000 nm) QDs have the characteristics of low optical damage to organisms, strong tissue penetration and low autofluorescence interference, so it has an important application prospect in the field of biological cell imaging, especially in the field of cancer diagnosis and cell tracking.47,48
Compared with conventional imaging techniques, such as magnetic resonance imaging (MRI), positron emission tomography (PET) and X-ray computed tomography (X-ray CT), in vivo animal imaging provides more cost-effective and high resolution in clinical diagnosis. However, compared with in vitro cell imaging, challenges arise with the increasing complexity of multicellular organisms. Unlike monolayer cells, the thickness of biological tissue is a major concern, because biological tissue can restrict the transmission of visible light and weaken the QDs signal for fluorescence imaging. Up to now, many in vivo animal imaging applications using functionalized QDs have been confirmed, such as in vivo cell tracking,49 tumor imaging,50 vascular system imaging,51 and so on. The greatest advantage of functionalized QDs in vivo animal imaging applications is that their emission spectra can be adjusted in the whole near-infrared wavelength range by changing their size and composition, thus producing light-stable fluorescent groups in the biological environment. For in vivo animal imaging using QDs, systemic intravenous delivery into the blood will be the main mode52. When QDs are exposed to blood, they may be rapidly absorbed by opsonin, leading to phagocytosis, platelet coagulation, stimulation of complement system and activation of immune system. In view of various factors that may affect the implementation of QDs, including particle size, charge, shape, surface ligands, the two most important parameters that may affect the biological distribution are particle size and preference for serum protein adsorption.53 Although many studies on the pharmacokinetics and biological distribution of QDs are still controversial, it has been considered that QDs are absorbed by reticuloendothelial system (RES), such as liver, spleen, kidney and lymphatic system.54,55
In vivo Toxicity of QDs
The evaluation of the in vitro toxicity test of QDs depends on the cytotoxicity of QDs. The biocompatibility and safety of QDs must be studied before QDs are applied in vivo. Because cytotoxicity research can pre-determine the potential toxicity of QDs and the existing mechanism of drug production before animal exposure, it is very important to study the cytotoxicity of QDs. Previous studies have found that QDs caused different degrees of cytotoxicity, such as decreasing cell viability56, altering cell morphology,57,58 inducing autophagy,59,60 and inducing cells to produce living oxygen substances61 and changing the expression of genes62 and so on.
In addition, the researchers also explored the cytotoxic mechanism of QDs using some immortalized cell lines. A number of research teams have reported the nonspecific intracellular uptake of QDs.63 Most QDs enter the cell through endocytosis, and cells regulate the endocytosis process through many ways including G protein coupled receptors. Although understanding the mechanism of QDs and cell reaction has a positive effect on inhibiting non-specific endocytosis, cell endocytosis is a complex process, which is regulated by multiple factors and levels. At present, the research on QDs endocytosis has just started, and researchers need to do more work in this area. The substance that enters the cell by endocytosis will be transported to different parts of the cell as needed to perform its function. The present results show that QDs are transported to the lysosome mainly through the early endosomes and late endosomes after entering the cell. Considering that the lysosome is an acidic organelle, QDs are highly likely to degrade under such low pH conditions, inducing cell organelle damage and cell function damage. Unfortunately, Experiments have proved that QDs caused a series of cell organ pressure, such as cell membrane defect, nuclear shrinkage,64 lysosome swelling,65 mitochondrial lipid oxidation and membrane potential decline.66,67 Although the cell has a certain self-repair function, when the organelle pressure exceeds a certain threshold, the cell will go to the irreversible process of death. The high concentration of QDs further activate the apoptosis pathway on the basis of organelle damage, leading to the cell apoptosis; or induce cells to trigger programmed death-autophagy.68 At the body level, QDs may induce adverse effects on some organs and systems, such as respiratory, cardiovascular, nervous, immune and endocrine systems.
Dynamics of QDs
With the continuous improvement of the preparation of functional QDs, multi-functional QDs with targeting, tracer, diagnosis and treatment are constantly emerging. It is necessary to carry out the commonness and individualized toxicological evaluation of the functional QDs. Due to a wide variety of functional QDs and different synthetic processes, there is a great difference in their toxicological evaluation, so there is a lack of comparability among the results of the toxicological studies in each experiment. Potential cytotoxicity mechanisms are still at the early exploration stage. It has been reported that QDs are able to disrupting cellular structures and attenuating cellular function in a number of different cell lines, including human HepG2 cell lines,69 lung epithelial A549 cells,70 and so on.
The toxicity of QDs in vitro cell cultures must be highly valued, but in vivo animal models, the QDs are not toxic or very weak in a short period of time. For toxicological testing and evaluation, information on the dynamics of QDs, including adsorption, distribution, metabolism and excretion, is essential.71 The pharmacokinetics of the QDs in vivo has been described in Figure 1. After QDs entering the physiological environment, whatever the entry routes (oral, injection, etc), they enter the fluid circulation through the blood flow, and then enter the tissue fluid and lymph through the internal circulation.72 QDs that enter the biological fluid are rapidly wrapped by natural organic substances, carbohydrates, lipids, especially proteins, thus forming the “protein corona”.73,74 On the one hand, the formation of protein corona changes the structure, dynamic behavior and functions of QDs, and further changes the distribution, accumulation, degradation, clearance, intracellular recognition, signal transduction and toxicity of QDs in vivo.75,76 On the other hand, the protein corona can be used to regulate the uptake of QDs in vivo and improve the biocompatibility between QDs and organisms.77
 |
Figure 1 The pharmacokinetics of the QDs in vivo. |
The distribution of QDs in vivo has a certain target and accumulates in some organs or tissues. As the QDs are very small, they can pass through the blood retinal barrier,78 the blood-brain barrier,79 it is possible to accumulate and cause toxic effects. However, the damage of tissues and organs is related to its special metabolic reaction and repair ability, and the mechanism of its damage remains to be discussed separately. Distribution and metabolism of QDs vary with the type, dose, particle size and surface coating.80 For example, Chan et al studied the short-term (<7 days) and long-term (>80 days) toxicity of different concentrations of QDs to SD mice. No tissue damage or inflammatory reaction related to QDs was observed during the experimental period and the tissue function and serum markers of liver, spleen, kidney and other organs in experimental animals were normal. However, the QDs accumulated in liver, spleen, kidney and other organs, and later had the trend of transferring from liver and spleen to kidney.81
Biodegradation and excretion are also key considerations for the use of QDs in vivo. Researchers have found that <5.5 nm in hydrodynamic diameter led to rapid and effective urinary excretion and elimination of QDs from the body, whereas those greater than 8 nm are retained in blood circulation.82 The renal pathway depends on glomerular filtration in the kidney, and the filtration threshold of glomerular capillary wall of globular particles is 6–8 nm (in this range, size, charge, and surface chemistry dependence), which indicates that renal excretion is limited to substances with ultra-small hydrodynamic diameter.83 Ge et al also demonstrated that Ag2Se QDs (<3 nm) could by rapidly removed from the kidneys of mice at 168 h without long-term organs accumulation.83 In addition to renal clearance, due to the binding of serum proteins, some hydrophobic enhanced QDs are excreted from the body through the liver pathway (including liver metabolism, bile excretion and feces excretion).84,85
However, so far, no QDs have been prepared which can be completely excreted from the living body.
Immunotoxicity
Immune system is an important system for the body to perform immune response and immune function, mainly containing innate and adaptive immune.86 It is the most effective weapon against the invasion of pathogens, which detects and removes factors such as foreign bodies and pathogenic microorganisms. As one of the important systems of the organism and the potential target organs of QDs, the immune toxicity of QDs has attracted the attention of some researchers. After the body is exposed to QDs, immune enhancement, immunosuppression and other immunotoxicity in the immune system often appear early.
The accumulation of QDs in the spleen may lead to inflammatory reaction, which may lead to unnecessary effects and toxicity. It has been confirmed that smaller QDs have stronger toxic effects on the immune system as they are more likely to pass through immune cells in vivo.87 It is also worth noting that surface coating is an effective strategy to change the toxicity of QDs in vivo. For example, after 6 weeks of oral exposure of adult Sprague Dawley rats with AgNPs, the spleen is one of the main tissues with significantly increased Ag and increased immune response.88 However, no harmful effects were found in treated animals when AgNPs were coated with polyethylene pyrrolidone (PVP-AgNPs).89 Spleen size and weight in Wistar rats increased significantly on the 28th day after intravenous injection of 20 nm and 100 nm silver QDs.90 The authors suggested that the increase in T, B and NK cell numbers might be the reason for the weight increase of spleen.
Cytokines are crucial to determining the type of immune response. The activation of Toll-like receptor 4 (TLR4) and subsequent stimulation of NF-κB and monocyte chemotactic protein-1 (MCP-1) pathway could ignite proinflammatory cytokines and simultaneously trigger immune responses after QDs exposure.91,92 It has been reported that after QDs enter biological environments, QDs gather “protein corona” to induce cytokines release.93 Our group has reported that exposure to PEGylated Cd-free CuInS2/ZnS QDs increased the levels of IL-4 on day 1 and enhanced the levels of IL-10 and IL-13 on day 28 in mice (Figure 2).94
Cells involved in the immune response or related to the immune response can be divided into a variety of cells, including lymphocytes, dendritic cells, mononuclear/macrophages, granulocytes, mast cells, and so on. However, the immunotoxicity of QDs to mast cells and bone marrow hematopoietic stem cells is rarely reported. The effect of QDs on the migration tendency of immune cells and the allergic reactions are not involved.
Hepatotoxicity
The liver is the largest and most important organ, which plays a major role in maintaining life and health. The functions of the liver include filtration of blood, biosynthesis of enzymes and bile, degradation of fat, storage of glycogen and regulation of blood sugar. After QDs absorbed through the gastrointestinal biofilm pass through the portal vein into the liver, some of them can be metabolized and inactivated, thus reducing the dose of systemic circulation. The process is called “first pass elimination”. On the other hand, the liver has detoxification function and detoxification-related enzymes, such as P450, so the liver can complete most of the organism’s metabolic process.95 Hepatocytes are the most abundant cells in the liver, which have metabolic, endocrine and secretory functions.96 Kupper cells are specialized macrophages, which exist in the liver and easy to absorb exogenous substances and cause liver damage.97 Therefore, the liver is one of the first organs to experience the effects of exogenous substances. When the high dose or high toxic QDs affect the body, the hepatocytes will be apoptotic or necrotic, and the body will lose the steady state. A large number of enzymes in the liver migrate to body fluids, resulting in significant changes in the function of amino acids, carbohydrates and hormones in the body fluids.98
The liver is the main enrichment organ of QDs, so the liver is very susceptible to toxic effects due to QD exposure.99 Furthermore, more than 60% of the Cd2+ administered to rats accumulates in the liver and liver is a major target of Cd injury.100
The main mechanism of liver damage caused by Cd2+ in vitro is the binding of sulfhydryl groups in key mitochondrial proteins, resulting in functional inactivation of these proteins, leading to oxidative stress, DNA impairment, mitochondrial dysfunction, autophagy induction, inflammatory cell infiltration and fibrosis.101,102 Alarifi et al demonstrated that after human hepatic carcinoma HuH-7 exposure to CdTe QDs, ROS content increased, intracellular antioxidant system damaged, intracellular glutathione (GSH) reduced and superoxide dismutase (SOD) activity increased.102 In addition, the latest research suggests that Cd2+ enhances mitochondrial autophagy and hepatotoxicity by upregulating mitochondrial calcium uniporter (MCU).103
Contrary to the cytotoxicity study of QDs, the reports on toxicity of QDs to organisms vary widely. Wei Liu et al found that mice exposed to CdSe QDs for 2 days (acute) and 6 weeks (chronic) caused hepatotoxicity.104 In contrast, Ken-Tye Yong et al injected phospholipid-micelle-encapsulated Cd-based QDs into macaques and observed the clinical features over 90 days without any abnormalities.105 Daily injection of Mn-doped ZnS QDs and polyethylene glycol-coated QDs via tail vein (1 mg/kg and 5 mg/kg) for 7 days did not cause obvious damage to the liver,106 which indicated that the toxicity of QDs in vivo was minimal under appropriate formulations and doses.
The underlying mechanism in vivo indicated that the QDs induced liver inflammation, pyroptosis and dysfunction via NLRP3 activation and mitochondrial reactive oxygen species (mtROS) production and Ca2+ mobilization in hepatocytes.107 Serban et al also reported the exposure of Si/SiO2 QDs caused oxidative stress in carp liver and was in the state of proinflammatory response. The oxidative stress marker recovered to normal level after 3 weeks, indicating that the liver of carp successfully counteracted the toxic effects of the accumulation of QDs. The study also showed that oxidative damage caused by QDs exposure to the liver is likely to promote inflammation.108 Moreover, METTL3-mediated m6a modification also plays a crucial role in hepatotoxicity caused by long-term exposure to Cd.109
Although the important mechanism of QDs induced hepatotoxicity is ROS production and metabolic enzyme activity damage, the effect of QDs on the metabolic function of normal drugs in the liver has not yet been studied.
Nephrotoxicity
Kidney plays an important role in maintaining the balance of body fluid and acid-base balance, so it is the main target organ for drug poisoning. Because of the large amount of kidney blood supply, large area of the endothelial surface, large area of contact with drugs, high concentration of drugs or excretion in kidney, high sensitivity of the kidney to drugs, high metabolic rate, high oxygen consumption and relatively insufficient blood supply in the medullary examination unit of renal tubulointerstitial area, the kidney is vulnerable to drug damage.110 When external substances reach the renal tubules, it is easy to cause high concentration accumulation by urine concentration. So as the main excretory organ of the exogenous chemicals in vivo, the QDs entering the body are easy to cause injury to the kidney.
It is generally believed that the particle size less than 5.5 nm QDs can be cleared through the kidneys. However, recent studies have shown that QDs within this size range accumulate in the kidneys for a long time without excreting urine, which may be attributed to differences in surface charges on QDs.111 In the study of pharmacokinetics and toxicology of heavy metal-free indium-based QDs in BALB/c mice and Wistar rats, Elnaz Yaghini et al found that after 5 mins to 3 months, the QDs of the liver, spleen and kidney gradually cleared without organ damage.112 An in vivo study showed that Balb/C mice injected with different surface modification of InP/ZnS QDs for 1, 3 7, 14, 28 d, resulting in the rapidly distribution in the liver and spleen.113
Cd2+ is a well-known nephrotoxicant.114 The proteinuria of renal tubules caused by Cd2+ is irreversible. Continued exposure to Cd2+ can lead to renal tubular dysfunction, resulting in glomerular damage and decreased glomerular filtration rates. Cd2+ has a long biological half-life and accumulates particularly in the kidney and bone.115 Pregnant CD-1 mice were inhaled 230 µg/m3 cadmium oxide (CdO) QDs to investigate the potential nephrotoxicity in mated and their newborn offspring by detecting the renal injury biomarkers, including kidney injury molecule-1 (Kim-1) and neutrophil gelatinase-associated lipocalin (NGAL).116 The results showed that Kim-1 and NGAL expression levels increased significantly both in maternal and in their offspring. Furthermore, histological analyses revealed proximal renal tubules pathology. Similarly, CdTe QDs induced time-dependent toxicity with elevated Cd2+ from the degradation of QDs and •OH production in the liver and kidneys in male ICR mice.117 Severe glomerular and tubular damage and apoptotic phenomena were observed in kidneys of male Sprague-Dawley rats exposed to cadmium-containing silica nanoparticles (Cd-SiNPs) and bare SiNPs, respectively.118
Therefore, it is of great significance to find a way to reduce Cd2+ accumulation in vivo. The previous results showed that betulinic acid (BA) pretreatment can reduce the residual level of Cd2+ in liver, kidney and testis, increase the excretion of Cd2+ in urine, and reduce the damage of cadmium chloride (CdCl2) to tissues. Therefore, BA can inhibit the apoptosis of kidney and liver cells induced by CdCl2.119
However, similarities and differences of excretion of different physical and chemical QDs in vivo, the pathway of QDs excretion under different exposure conditions and the effect of QDs on renal excretion have not yet been clearly reported.
Respiratory Toxicity
Respiratory system has the most frequent contact with external environment in various systems of human body, and the contact area is large. Respiratory system is firstly exposed directly to the outside world and attacked alongside the inhalation of particles. On one hand, respiratory system contacts with all the blood volume of the heart, the QDs entering the vein will be discharged into the lungs through the heart. On the other hand, pulmonary respiratory membrane is very important. Type I alveolar cells are the gas exchange sites, which are non-proliferative and therefore nonrenewable. Type II alveolar cells have the ability to bio transform and are easy to produce metabolic activation.120 The oxygen content in the lung is high, which is easy to cause oxidative damage.
The QDs that enter the organism through the respiratory tract are still easy to accumulate in respiratory system due to its small size effect. Its main toxicity on respiratory system is pulmonary inflammation and tissue damage. Several studies have shown that the deposition of QD in lung organs leads to lung injury.121,122 For example, cadmium oxide (CdO) QDs can stimulate lung inflammation, cell injury, tissue remodeling and other related pathways, and change the immune function.123 Philku Lee used Sprague-Dawley rats as the research object through inhalation exposure for 28 days. Soluble Ag QDs can be dissolved in Ag ions and transferred to extrapulmonary organs and can be quickly cleared from most organs except for the brain and olfactory bulb. However, insoluble Au QDs continue to migrate to extrapulmonary organs without rapid elimination, which may have toxic effects on some organs.124 In addition to the inherent toxicity of component materials, the toxicity of QDs also depends on other factors, including surface charge, concentration, size, outer coating as well as experimental conditions like mouse strains. Both C57BL/6J and A/J mice were exposed to 6 µg/kg Cd equivalents of amphiphilic polymer-coated Cd/Se core, ZnS shell QDs via pulmonary respiration. However, researchers have found that QDs induced acute changes in lung mechanics are mouse strain dependent. QDs induce lung inflammation in C57BL/6J and A/J mice, and A/J mice are more susceptible to the effects of QDs in lung mechanics.125 Tang et al observed that positively charged QDs using cationic polydiallydimethylammonium chloride (PDDA) as outer coatings were more toxic due to pulmonary embolism. Compared with positive or negative, PEGylated QDs showed the slightest chronic damages in the long-term toxicity.126 However, the effect of QDs on the function of lung-derived cells (such as type I cells), respiratory membrane function and the ability to penetrate blood gas barrier at different physical and chemical QDs needs to be further studied.
Neurotoxicity
Neurotoxicity refers to the toxic effects of foreign substances on the nervous system, including the central nervous system (brain and spinal cord) and the peripheral nervous system. It has been proved that QDs were able to cross the blood–brain barrier and entered the central nervous system.127 In addition, it was found that QDs are mainly transported from nose to brain through olfaction and transported through axons.128
Blood–brain barrier is highly selective and protective, which can prevent some foreign bodies from entering the central nervous system (CNS). However, because the diameter of QDs is very small, it is usually only 1–10 nm and has small size effect, it can cross the blood–brain barrier or move along neural pathways, and enter the CNS and cause certain toxicity. It is reported that QDs exposure can induce abnormal inflammatory responses, increase oxidative stress generation, alter neuronal function and morphological alterations, and elevate cytoplasmic Ca2+ levels and autophagy in adult animals.129 The common adverse effects of nervous system related to morphological changes include neuropathy, axonal lesion, myelin sheath lesion and gliopathy.
In vivo experiments are essential to investigate the neurotoxicity of QDs, as they allow the study of the entire organ system, which cannot be modeled in vitro. Up to now, most of the data in vivo have been produced in rodents to report neurobehavioral defects, neuroinflammation and some neurodegenerative changes. Wu et al have studied the toxic effects of different concentrations of 3-MPA-CdTe QDs on the hippocampus of Wistar rats. Low dose of QDs inhibited the excitation, but the inhibitory phosphorylation of AKT and ERK1/2 and the decrease of c-FOS transcription level in high-dose group indicated that QDs induced spatial recognition and memory impairment.130 What’ s more, CdTe QDs exposure could also alter genome-wide gene expression pattern in a size-dependent manner of rat hippocampus.131 CdTe QDs also cause neuroinflammatory responses in mice by activating the NLRP3 inflammasome through excessive ROS generation, resulting in proinflammatory factor release in the hippocampus.132 Mammalian experimental models are limited by logistics, finance and ethics. In recent years, Caenorhabditis elegans (C. elegans) has become a promising alternative for toxicological studies due to easy of cultivating, short life cycle, more offspring and so on.133 Most importantly, this model organism has a well conserved and fully described nervous system that makes it ideal for neurotoxicity assessment. 0.1–1 µg/L of CdTe caused neurotoxicity on both development and function of RMEs motor neurons due to oxidative stress, cell identity, and bioavailability. However, ZnS coating reduces the toxicity effects of CdTe QDs in C. elegans. Moreover, CdTe/ZnS could not be translocated into the RMEs motor neurons through the intestinal barrier.134
Both oxidative stress and neurotoxicity are huge challenges to human health, and effective methods and agents for resisting these adverse effects are limited, especially in vivo. QDs penetrate the blood–brain barrier mostly in vitro cell models and cannot simulate the process of QDs penetrating blood–brain barrier in vivo. The effect of QDs on blood–brain barrier permeability and function has not been clearly studied. Whether QDs in the brain could cause toxicity to other organs through body distribution has not been studied. Finally, yet importantly, the neurotoxicity of QDs has been mostly observed in experimental animals. Because of the difference between humans and animals, it is a great challenge to extrapolate these data to human beings.
Reproductive and Developmental Toxicity
With the continuous change of the industrial environment and the ecological environment, the problem of the decline of human reproductive health is becoming more and more prominent. The disease of reproductive system will be the third major social disease following the cardiovascular disease and malignant tumor. Reproductive and developmental dysfunction is one of the major public health problems that seriously affect human health at present. In recent years, it has been found that QDs, such as nano silica and nano silver, may accumulate in the testis through blood circulation and blood-testis and placental barriers, causing certain cytotoxicity to the Leydig cells and the damage of the reproductive system.135,136 The effect of some QDs on the female reproductive system may lead to ovarian dysplasia or infertility, increasing the spontaneous abortion rate, dysplasia of the progeny and decreasing fertility, etc.137 Therefore, it is of great significance to identify the QDs with reproductive toxicity and explore the mechanism of its toxicity, which is of great significance to the prevention and control of the reproductive system caused by the QDs.
In the development of Chinese rare minnow embryos in China, InP/ZnS QDs caused teratogenic effect and death, but InP/ZnS QDs did not cause significant genetic toxicity in the development.138 C. elegans owing to its short generation time and transparent body is becoming a well-studied model organism for toxicological problems. The short-term exposure of C. elegans at high concentrations of MPA-CdTe QDs was not significantly toxic, while long-term exposure could produce late toxicity, such as fertilization difficulties and injury of fertilized egg shells. At the same time, the toxicity of the positive QDs (immature larvae) was significantly greater than the QDs with negative electricity. Compared with mature nematodes treated with MPA-CdSe/ZnS and MPA-CdTe QDs, it was found that QDs toxicity with ZnS shell protection was significantly reduced.139 In addition, MPA-CdTe QDs (≥50 mg/L) affected C. elegans to reduce the cell number in the pachytene and terminal stages, leading to reproductive capacity defects, dysregulation of proliferation and differentiation, and oogenesis imbalance. The toxicity mechanism was mediated by SPO-11 and PCH-2, as well as the protective mechanism was mediated by GLP-1/Notch.140 In BALB/c mice, all the exposed mice had CdTe QDs accumulation in the testes, and there was no significant effect on the quantity of sperm. However, the high-dose group significantly decreased the sperm quality on day 60, the levels of the 3 major sex hormones (testosterone, Luteinizing hormone and follicle-stimulating hormone) in the serum were also disturbed.141 Amiri et al have studied the reproductive toxicity of different concentrations of CdSe/ZnS (10, 20, 40 mg/kg) on BALB/c mice by intraperitoneal exposure. Testicular histological studies showed that 40 mg/kg dose had a high toxic effect, reduced the lamina propria, damaged mesenchyme, produced seminiferous tubules, and reduced spermatogonia, spermatocytes and spermatids. The whole group (adult and embryo groups) of epididymal studies showed that the amount of sperm in the epididymis of the adult group at 40 mg/kg dose decreased considerably.142 Another study has demonstrated that high doses of PVP-AgNPs showed higher abnormalities in sperm morphology, while their effects on sperm motility and viability were not significant. These results indicated that oral sub-chronic doses of PVP-AgNPs have slight toxicological effects on rat sperm parameters.143 Our group has studied the reproductive toxicity of CdSe/ZnS QDs on the male reproductive system and offspring health in mice. We have found that high dose QDs induced apoptosis of Leydig cells in the testes at early timepoints, but did not cause significant histopathological changes overall. However, QDs did have effects on offspring from exposed males, including slower growth, changes in organ weights, and abnormalities in some blood parameters (Figure 3).144
In summary, most of the toxicological studies of QDs have toxic effects on cells or organisms, but their specific toxicity mechanisms are relatively slow. In future research, the research on the reproductive toxicity of QDs should be more specific, and more attention should be paid to the study of the toxicity of QDs. Its general direction is: A, in depth understanding of the physical and chemical properties of QDs, fully understand the absorption, distribution, metabolism and excretion process. B, the toxicity of QDs was refined, and the toxicity of each component (such as Cd, S, Si, Ti, Se) was studied. The toxicity of QDs to the reproductive system was also studied. C, Reproductive toxicity of QDs needs more animal experiments, especially for animals with chronic toxicity. D, reproductive toxicity should take into account the effects of QDs on the formation of eggs and sperm, and a series of effects on fertilized eggs, embryonic development, young growth, young offspring reproduction and so on. E, the toxicity mechanism of QDs and the toxic effects on reproductive system were comprehensively investigated from individual, cellular, molecular and genetic levels.
Genotoxicity
Genotoxicity refers to the ability of exogenous chemicals to damage genetic materials. Genotoxicity experiment is an essential part of toxicology safety evaluation, as it may lead to birth defects and malignancies.145 So in the past 20 years, scholars have preliminarily described and evaluated the genotoxicity of QDs through various experiments. The genotoxicity of QDs is mainly caused by DNA damage and chromosomal aberration caused by oxidative stress, while Ames test (gene mutation) is mostly negative. For example, Li Jin et al exposed the parents and offspring of rare minnow to ZnSe/ZnS QDs, DNA damage was still present in the F1 generation 96 hours after fertilization, and the toxicity may be due to the release of Zn2+ from QDs, leading to abnormal development of hatching glands.146 Katsumiti et al exposed mussel blood cells to CdS QDs, comet assay showed DNA damage.147 Aye et al treated rats with lipoamphiphile-coated CdSe/ZnS QDs by intraperitoneal injection and analyzed the comet assay with different organs. The results showed that significant DNA damage could be observed in brain, liver and testis, but not in kidney and lung.148 It indicates that QDs can accumulate in specific sites and produce corresponding toxic effects. Meanwhile, QDs can pass through biological barrier and damage target tissues after modification. Borm et al have noted that, to reach the threshold of genotoxic effect, at least 5 times of crystalline silica (CS) dose of pro-inflammatory effect in vivo is required.149 Thus, the main mechanism of CS genotoxicity is inflammation-driven secondary genotoxicity. Chromosome aberration (CA) is a serious genetic damage. CAs were analyzed in bone marrow cells of Sprague-Dawley rats treated through the oral route for once a day for five days with different doses. The results suggested Ag have the potential to induce genotoxicity.150
A series of studies on the toxicity of QDs in recent years are summarized in Table 1.
 |
Table 1 A Study of Some Reported QDs Toxicity |
Parameters Affecting the Toxicity of QDs
Chemical Composition
In fact, the fundamental factor of QDs toxicity is composition. From the classical metal QDs to the new inorganic non-metal QDs, it is the main component that determines the biocompatibility of QDs. By far, the most commonly used QDs are Cd-based QDs, such as CdS, CdTe, and CdSe. The composition of the Cd element raises concerns about the toxicity produced by the release of heavy metal Cd2+. Cd2+ has been identified as a carcinogen by the International Cancer Research Center and is recognized as a toxic heavy metal.159 Although the QDs have undergone surface modification and reduced their toxicity before entering the organism, after a long period of degradation and biological oxidation after entering the organism, the shell is likely to fall off to form the bare core structure and expose the core Cd2+. Su et al studied the relationship between Cd2+ and QDs toxicity. The control group was CdCl2 solution and the experimental group was CdTe QDs. The Cd2+ concentration in the cells of each group was the same after the experimental treatment. It was found that the toxicity of QDs exposed group was much greater than that of CdCl2 solution group, indicating that the toxic effect of QDs was indeed related to Cd.2+160 Some research groups have been dedicated to using new insights, such as flow cytometry or all-inclusive microscopy system, to investigate the relationship between Cd2+ and cytotoxicity.161 Data from Soenen et al showed that when QDs were exposed to the intracellular structure of a lower pH, it took endocytosis to result in partial degradation and release of Cd2+, thereby reducing its fluorescence intensity and increasing the toxicity of particles.162 To address this problem, environmental benign non-Cd QDs are recently developed, such as III–V InP,163 II–VI ZnSe,164 I-III-VI Cu-Ga-S (CGS) QDs165 and I-III-VI2 CuInS2.166
Particle Size
Because QDs are very small in size, once they are absorbed by the human body accidentally, they will enter blood circulation rapidly, distribute into most organs of the human body, and even penetrate various biological barriers, such as the blood-testosterone barrier and blood–brain barrier. Previously, it has been reported that the toxicity of QDs is dependent on their size. Normally, the smaller QDs in have the greater the toxicity.167 For example, Chang S et al have demonstrated that the MPA CdTe QDs of different sizes have obvious cytotoxicity. Under ultraviolet light, small size QDs show more obvious damage to the cells of larger size QDs.168 The possible reasons responsible for the greater toxicity caused by smaller QDs are: (A) the smaller the particle size of QDs, the larger the surface area, the more Cd exposed to the surface, and more opportunities to form the free Cd2+ to produce the toxicity; (B) many pathways in the body and cells are particle size dependent, allowing only small particle size QDs, such as the placental barrier, the nuclear pore, and so on. However, unlike in vitro cultured cells, Wang Q et al pointed out that the distribution of QDs in organs and tissues was related to the clearance time and the size of QDs. Small QDs can quickly be removed from the circulation system, while large QDs will take a long time to clear them. Therefore, large QDs generate more sever toxicity.169 Consistently, Su et al found that the QDs with larger sizes are more quickly to accumulate in the spleen; on the contrary, the smaller QDs are more easily to be absorbed by kidney.170
Different Surface Modification
Surface modification of QDs makes them better with controllable biocompatibility and stability. QDs commonly undergo different surface modifications that involve conjugating recognition biomolecules, resulting in specific biological functions. Previously, the surface modification of QDs has been proved to affect its toxicity to some extent. Firstly, core-shell QDs are normally less toxic than bare QDs.171 A layer of semiconductor shell is usually rewrapped on the surface of QDs to improve its fluorescence performance. This package can improve the fluorescence properties of QDs while preventing the Cd2+ from the medium in the environment, reducing the leakage of Cd2+ and the formation of free ions, and reducing the toxicity. Secondly, the appropriate ligands on the surface of QDs help to reduce toxicity. Jiang Z et al have encapsulated CdSe with water-soluble poly (ethylene glycol)-graft-chitosan (PEG-g-CS). The in vitro cytotoxicity studies showed that PEG-g-CS as ligand displayed very low cytotoxicity.172 Thirdly, different surface functional groups (eg COOH, NH2 or OH) also affect their biological behavior and toxic effects. Zheng N et al utilized zebrafish embryos to compare the toxicity in vivo of CdSe/ZnS QDs modified with carboxyl (-COOH) and amino (-NH2) groups. Both CdSe/ZnS QDs reduced the survival rate, hatching rate, and embryonic movement of zebrafish, ultimately leading to pericardial edema and cardiac dysfunction. This indicated that the properties of QDs and the release of Cd2+contribute to developmental toxicity. Moreover, two types of CdSe/ZnS QDs exhibited developmental toxicity and affected heart development, while carboxyl QDs exhibited greater toxicity, possibly due to their higher affinity and release to embryos and larvae.173 So many factors of surface modification are responsible for QDs toxicity, but their impact on toxicity varies. Rashi et al174 systematically investigated the toxicity of CdSe QDs with different particle size, functional group and surface charge on human bronchial epithelial cells. They rank the contribution of each property of QDs toxicity: charge > functionalization > size. Specifically: (A) positively charged QDs are more toxic than the negative QDs; (B) QDs with long ligands are more toxic than those functionalized with the shorter chain; (C) the toxicity of QDs with small particle size is larger than that of the large particle size.
Exposure Route
For the toxicity of QDs, the researchers are also concerned with its exposure route, which is of great importance to the target of understanding the toxicity of QDs. It is generally believed that the preparation of personnel, researchers and clinical patients may be exposed to QDs and other nanomaterials through environmental transmission, workplace exposure and disease diagnosis and treatment. We could divide the potential exposure routes of QDs into two different basic pathways: workplace and laboratory studies.175
At present, QDs as in vivo imaging probe or drug tracer are usually injected into the experimental animal by intravenous injection. Therefore, there is no doubt that QDs through the blood vessels are exposed to the human body, which follows is the potential damage to the blood vessels of QDs, and even because of the circulation of the blood, QDs will threaten more tissues and organs.176 In addition, QDs may also be in contact with the skin, the respiratory system, or the digestive tract, and other parts of the contact during the actual use.177–179 The skin is the largest organ which covers the whole body to prevent physical, chemical and pathogenic microorganisms from invading the body tissues and organs. The traditional idea is that the complete skin is a tight barrier, and the general material cannot penetrate it. The penetration of QDs to the skin only occurs when the skin is damaged or there is mechanical stress. However, Doge et al utilized a 2-photon microscope (2PM) and a total internal reflection microscope (TIRFM) to find that QDs aggregated in the upper part of the stratum corneum (SC) and villous hair follicles (HFs) through the skin barrier.180 Moreover, Research has also indicated that water-soluble CdSeS QDs could penetrate into the dermal layer through hair follicles and then get into blood circulation.181 It is indicated that QDs with different physical and chemical properties can penetrate intact skin into the body at working concentration. It also suggests that the skin may also be a major way for QDs to enter the human body. It should also be taken into consideration when investigating the health risks of QDs. QDs less than 100 nm have been confirmed to be distributed in nasal cavity, trachea and alveoli of experimental animals, and these QDs will also be transported to the extrapulmonary parts of liver and brain.182,183
Exposure Time and Dose
The toxicity of QDs is dependent on exposure time and dose.184,185 Basically, the longer is the exposure time, the higher is the dose, and the more obvious is the toxicity of QDs. For example, the activities of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) in the liver and kidneys of mice exposed to CdTe QDs increased in a dose-dependent manner. The time course study showed that the oxidative stress markers activities of SOD, CAT and GPx in the liver and kidneys in the exposure group increased on the first day, then decreased, and gradually returned to normal on the 28th day.186 In addition, Stan MS et al have found that Si/SiO2 QDs caused 2.2 and 2.6-fold higher compared to control for 25 µg/mL and 50 µg/mL treated group after 24 h incubation respectively, and 3.4-fold higher for the 200 µg/mL treated group. At longer exposure times, the increased ROS generation was 5.5 times (200 µg/mL, 48 h) and 7.0 times (200 µg/mL, 72 h) higher compared with control group.187
Although dosages are important for the evaluation of the toxicity of QDs, it is difficult to make effective comparison of the doses adopted by different researcher because of wide variety of QDs and the concentration of the QDs cannot be precisely quantified. The inconsistency of the concentration units used by each research groups leads to the incomparability of the mutual results, which has become one of the major obstacles to the study of the toxicity of QDs. In addition, the exposure time of QDs toxicity study also lacks a unified standard. Most toxicity studies on QDs are still focused on short-term acute toxicity effects, long-term toxicity of QDs toxicity is still very scarce and needed to be further investigated.
Toxicity Mechanism
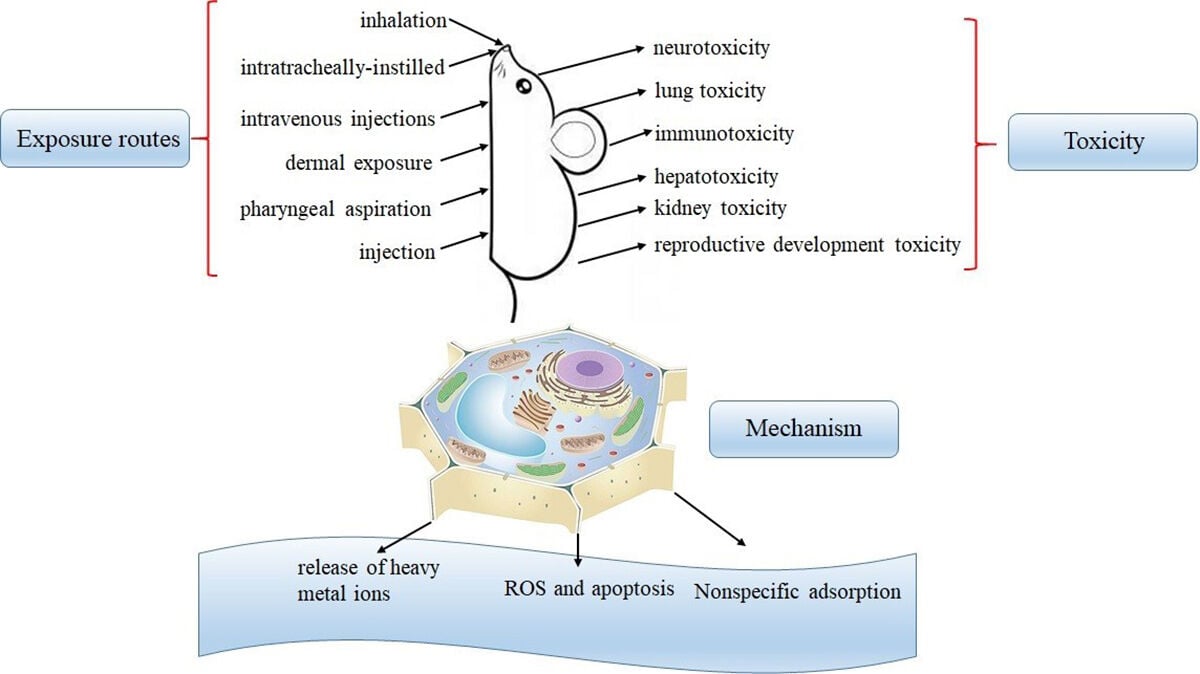
QDs are widely used as fluorescent nanomaterials with superior performance, but the toxic problems are also concerned. It is known that the toxicity studies of QDs begin at the cellular level. At present, a large number of experiments have been conducted to observe the effects of QDs on cell growth, viability, structure and survival. Most results showed that the QDs are toxic in a dose-dependent manner,188,189 but it was also found that QDs did not affect the behavior of stem cells and were suitable for in vivo fluorescence tracking and diagnosis.190,191 The toxicity of QDs is a complex process, including the interaction with body, cells and molecules. The toxicity is determined by itself and environmental factors. In recent years, some research groups have discussed the mechanism of QDs from different perspectives. Its cytotoxic mechanism can be summed up as the following three points (Figure 4):192,193 (A) The core of QDs interacts with the medium to degrade and release of heavy metal ions such as Cd2+194 and Ag.+195 (B) Increase intracellular oxidative stress by producing ROS and induce apoptosis.196 (C) Nonspecific adsorption to the surface of the cell, binding with the protein, destroying the structure, and affecting the normal function of the cell.197,198 It is well known that toxicity of QDs is largely dependent on many physical and chemical characteristics, such as their chemical composition, size, surface charge, the presence or absence of a shell and active groups on the surface. In addition, the toxicity of QDs is also determined by exposure conditions, including time, dose and administration route.
ROS Generation
Oxidative stress (OS) is recognized as a major mechanism triggered by QDs, as it can intrinsically produce ROS or induce the intracellular production of ROS and anti-oxidant depletion upon interacting with cells.199 OS refers to the imbalance between oxidation and antioxidant system. The body tends to oxidation state due to excessive accumulation of ROS. ROS usually have adverse effects on cell functions, such as oxidative damage of DNA and protein, damage of biomembrane, etc.200,201 At the same time, there are also protective antioxidant systems, including antioxidant enzymes, catalase, Superoxide dismutase (SOD) and some non-enzyme molecules such as thioredoxin, glutathione (GSH), vitamin C and vitamin E.202 In the physiological environment, the oxidation and antioxidant systems are in a dynamic equilibrium state.203 When the active oxygen species continuously stimulated by foreign organisms exceed the speed of internal clearance, the imbalance between oxidation and antioxidant system in vivo has influence on the tissue damage and pathogenesis of serious diseases.204 For example, Si/SiQ2 QDs can increase the ROS and malondialdehyde (MDA) levers with lower glutathione content, which leads to the disorder of redox system in cells, thus affecting the sensitive signal pathway of redox system.187 At present, the research on ROS produced by QDs mainly focuses on DNA damage,205 apoptosis206 and autophagy.207
Heavy Metal Ions Release
The release of QDs core can explain the toxic mechanism of QDs to some extent. Among them, Cd-based QDs are the most widely used, as they have higher toxicity than others. Due to the complex internal environment of the organism, QDs are corroded, degraded or oxidized by the microenvironment of the organism after entering organism. For example, under the condition of low pH, such as gastric juice, the core-shell dissociation occurs after the connection of the protonation, which makes the QDs reduce stably, release metal ions, and produce damage to the cells. Derfus et al found that the CdSe QDs coated with tri-n-octylphosphine oxide (TOPO), reacted with the cells 30 minutes after oxidation in the air and then modified with mercaptoacetic acid (MAA). The cell activity decreased significantly from 98% to 21%.208 It can be concluded that the cytotoxicity may be caused by the oxidation reaction on the surface of CdSe. In order to test this hypothesis, the researchers used UV-light source to accelerate the oxidation process of CdSe. The results showed that the cell activity decreased significantly with the increase of oxidation time, and there was a significant time effect relationship. Through the release of free Cd2+ concentration in the system, it is found that the Cd2+ concentration of non-oxidized MAA-QDs was 6 ppm under the same conditions; the concentration of Cd2+ of air-oxidized QDs system was 126 ppm; the concentration of Cd2+ in the QDs system was 82 ppm after UV catalytic oxidation. Therefore, the toxicity mechanism of QDs is proposed: the oxidation of CdSe nuclear surface leads to the release of Cd2+. Kirchner et al discussed the effect of different surface modifications on cell absorption and further studied the relationship between the toxicity of CdSe and CdSe/ZnS QDs and the release of free Cd2+ ions. They evaluated the proliferation toxicity of QDs by the number of adherent cells before and after exposure and determined the concentration of free dissolved Cd2+ in the system by plasma optical emission spectroscopy (ICP-OES). The results showed that the cytotoxicity increased with the increase of Cd2+ concentration in the system, which was consistent with the conclusion of Derfus.209 After that, researchers focused on the toxicity caused by the release of Cd2+.173,210 Cd2+ can inactivate mitochondrial protein and affect mitochondrial biochemical function through interaction with sulfhydryl group.211 Cd2+ can react directly with DNA, such as binding with phosphoric acid and base in DNA, which is very harmful to organism.212 The release of Cd2+ ion in the system is an important but not the only factor causing cell damage, and other mechanisms affecting its toxicity need further study.
Disturbance of Organelles
After QDs treatment, it interacts with cells and enters the cell, which mainly causes mitochondrial dysfunction, endoplasmic reticulum (ER) stress and lysosomal disruption, thus affecting cell growth, inducing apoptosis and necrosis and ferroptosis. The QDs are small in size, high in surface activity and easy to pass through the cell membrane. On the one hand, it may interfere with the hydrophobic interaction or double bond oxidation of polyunsaturated fatty acids in the lipid bilayer of the cell membrane, thereby disturbing fluidity, permeability and ion gradient distribution of the membrane, causing damage to the cell membrane; on the other hand, it may interact with the organelle and affect the structure and function of the organelle.213
The main function of cell membrane is to prevent extracellular substances from entering the cell freely, maintain the relative stability of the cell environment, and make various biochemical reactions orderly. The toxic effect of QDs on the cell membrane is mainly manifested in the damage of the integrity of the cell membrane and the change of the permeability of the cell membrane.214,215 The amount of LDH in cell culture medium can reflect the damage degree of cell membrane. Zhang et al incubated L929 cells with 2.2 nm CdSe QDs of different concentrations (0–14 μg/mL) for 24 hours. The results showed that the amount of LDH in the experimental group was significantly higher than that in the control group with a dose-dependent relationship.216 Wang et al217 incubated human umbilical vein endothelial cells (HUVECs) with 0–100 μg/mL silver nanorods (AgNR) for 24 hours, and the results showed that oxidative stress, intracellular MDA levels and proinflammatory cytokine secretion increased, suggesting cell membrane structure disruption.
The mitochondrial toxicity caused by QDs includes morphological change, increase of ROS production, change of calcium level, loss of mitochondrial membrane potential, inhibition of enzyme activity, inhibition of electron transport chain, inhibition of cell respiration, reduction of ATP synthesis, etc., resulting in insufficient function and affecting cell activity.98,218 Dong et al found that the silver metal nanocluster coated with bovine serum albumin (Ag-BSA NC) caused mitochondrial dysfunction mainly by inducing the mitochondrial membrane permeability transition (MPT) and ROS production, damaging mitochondrial respiratory chain, damaging mitochondrial respiratory function, causing oxidative phosphorylation disorder, insufficient ATP synthesis, and decreased cell viability.219 Paesano et al found that low-dose CdS QDs did not cause significant damage to mitochondrial DNA, and stress caused an increase in ROS production, activated mitochondrial mediated endogenous apoptosis pathway, and induced cell death, which was mainly cell apoptosis.220 In this process, mitochondrial membrane potential lost mitochondrial ATP synthesis dysfunction, resulting in insufficient cell function.221 It has been shown that the mitochondrial autophagy induced by cadmium exposure is the main cause of mitochondrial reduction in human normal hepatocyte lines and mouse liver, while the mitochondrial autophagy induced by cadmium is initiated by the mitosis dependent on dynamic 1-like.222 At the same time, some studies have also shown that cadmium induces mitochondrial autophagy through the activation of PINK1/parkin pathway in the kidney and brain of mice. In this process, ROS induced by cadmium plays a role in the upstream of PINK1/parkin pathway and regulates mitochondrial autophagy.223
In addition to mitochondria, endoplasmic reticulum (ER) is also very sensitive to QDs. The changes of ER caused by QDs include morphological changes, functional disorders, ER stress, such as ER swelling, metabolic disorders, protein misfolding, protein synthesis increase or decrease, etc. Yu et al showed that TiO2 induced the swelling and rupture of ER, the increase of expression level of ER stress-related proteins such as GRP78/BiP, chop and ire-1a in mouse lung cells in a TiO2 dose-dependent manner.224 ER stress is considered to be one of the early sensitive indexes of QDs induced cytotoxicity. Chen et al found that when human umbilical vein endothelial cells (HUVECs) were incubated with 120 μM ZnO, the CCK-8 test results showed that there was no significant cytotoxicity. The level of ER stress was determined by the detection of xbp-1s mRNA. Under this dose measurement, the expression of xbp-1s mRNA in 8 hours was higher than that in 4 hours and 12 hours, which means that ER stress was at the highest level at this time point, but there was no significant cytotoxicity at this time.225 ER stress also participates in the autophagy process induced by QDs,60,226 and the damage of QDs to target organs through ER stress pathway may be permanent.227
QDs enter lysosome mainly through passive diffusion or endocytosis, causing lysosomal structure change. Condello et al showed that ZnO enters lysosome mainly through endocytosis. In the process of interaction with acid environment, it causes lysosome morphological damage, releases a large amount of Zn2+, and the part of Zn2+ entering the cytoplasm is captured by mitochondria, triggers ROS production, and causes mitochondrial dysfunction and apoptosis.228
Gene Expression Profile Alterations
QDs can reach the nucleus through the cell membrane.229 The influence on the nucleus is mainly the influence on genetic material, such as destroying the shape of nucleus, damaging DNA, affecting gene expression, etc.230 Kumer et al showed that TiO2 can cause chromosome and DNA damage and induce apoptosis by up regulating Bax/Bcl-2 ratio, mitochondrial membrane potential loss and cytochrome c release.231 Wu Z et al showed that FeAuNPs induced up regulation of Hsp70 protein expression, DNA double-strand break and apoptosis, which is consistent with the above conclusions.232 Pasquali et al showed that QDs enter the cell by regulating several key nuclear genes, such as TOM5 and FSKI, resulting in an increase in the level of intracellular ROS, a decrease in the ratio of reduced vs oxidized glutathione (GSH/GSSG), a decrease in oxygen consumption, an impact on the morphology and function of mitochondria and cell growth.233
At present, the mechanism of gene expression-level change caused by QDs is that histone is an alkaline protein binding to DNA in eukaryote nucleus, and its role in epigenetic regulation, such as gene expression regulation, DNA damage repair, chromatin state regulation, cell differentiation and so on, has been paid more and more attention. The surface of the unmodified QDs is negatively charged, which can accumulate rapidly in the nuclei and nucleoli of living human cells, and preferentially combine with the positively charged core histone to form QD/protein adduct. The formation of adducts may affect the regulation of histones, thus activating, inhibiting genes or changing gene expression level.234,235
Summary and Outlook
At present, QDs have been widely used in in vitro labeling and have a wide range of applications in the field of biomedicine. However, the application of QDs in vivo is still a long process. How to solve the QDs, especially the biocompatibility of the most efficient Cd2+ QDs, has become the bottleneck restricting the clinical application of the nanomaterials, and its research has attracted wide attention. Due to the wide variety of QDs, cell lines, and analytical methods, it is difficult to draw a consistent conclusion from the present study. The main reasons are: (1) The synthesis and surface modification of QDs will affect their physical and chemical properties to a great extent and affect their interaction with the cell membrane and the degree of entry into the cells; (2) The sensitivity of different kinds of cells to QDs is different, so the threshold of cytotoxicity is different; (3) At present, cytotoxicity is carried out by detecting cell number, growth curve, degree of apoptosis, cell morphology or metabolic activity. (4) There are many kinds of QDs. At present, the toxicity research is still focused on a few QDs, especially Cd2+ QDs. The toxicity study of non-cadmium QDs, such as ternary QDs CuInS2, is still in its infancy. (5) Data on toxicity of susceptible persons, such as elderly and small, are not complete. (6) A little study on the toxicity of QDs under the disease model. (7) In vivo metabolism and excretory path of QDs are unclear. (8) The influence of QDs on specific biological barrier function needs further study. It also causes great difficulties for different research results, making QDs toxicity research more complicated.
Above summaries have shown that the difference of QDs surface modification, particle size, exposure time and concentration, and the amount of cell intake and the changes in the external environment will lead to the change of QDs toxicity. Therefore, the biological safety evaluation of QDs is needed to consider many factors, and cannot simply be defined as “toxic” or “non-toxic”.
Here is a detailed summary of the current improvements and drawbacks related to the toxicity of quantum dots (QDs), based on recent publications. Improvements: (1) Development of non-toxic QDs: There has been progress in developing heavy metal-free QDs such as carbon dots, graphene dots, silicon dots, and indium phosphide dots, which show lower toxicity. These provide alternatives to toxic Cd- and Pb-based QDs. (2) Surface modifications: Coating QDs with biocompatible polymers, lipids, proteins, or silica can reduce toxicity by preventing leaching of metals, reducing aggregation, and improving stability. Strategies like PEGylation have shown promise. (3) Lower doses: Using lower doses of QDs for bioimaging has helped reduce toxicity. However, the long-term impacts of lower chronic exposures need further study. (4) Organ targeting: Functionalizing QDs to target specific organs or tissues may lower systemic toxicity. But organ clearance and accumulation require more research. Drawbacks: (1) Toxicity mechanisms: The mechanisms underlying QDs toxicity, whether from metal release, reactive oxygen species, aggregation, etc. are still not fully elucidated. (2) Biopersistence: Most current QDs are not biodegradable and remain in the body long-term. Their long-term fate and chronic toxicity remain concerns. (3) Organ accumulation: QDs accumulate in organs like liver, spleen and kidneys. The impacts of long-term retention in organs require further investigation. (4) Variability: QDs toxicity depends on many variables like composition, size, shape, coating, dose and exposure route. More systematic toxicity analyses are needed. (5) Extrapolation to humans: Since most toxicity data come from cell cultures and animal models, extrapolating QD toxicity to humans remains a challenge.
In summary, important progress has been made in improving QD toxicity, but limitations around biopersistence, organ accumulation, variability, and human health impacts mean further research is needed for safer QD-based technologies. The applications versus toxicity are summarized in Table 2.
 |
Table 2 QDs Applications versus Toxicity: Pros and Cons |
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sobhanan J, Rival JV, Anas A, et al. Luminescent quantum dots: synthesis, optical properties, bioimaging and toxicity. Adv Drug Deliv Rev. 2023;197:114830. doi:10.1016/j.addr.2023.114830
2. Pham XH, Park SM, Ham KM, et al. Synthesis and Application of Silica-Coated Quantum Dots in Biomedicine. Int J Mol Sci. 2021;22(18):10116. doi:10.3390/ijms221810116
3. Han Z, Sarkar S, Smith AM. Zwitterion and Oligo(ethylene glycol) Synergy Minimizes Nonspecific Binding of Compact Quantum Dots. ACS Nano. 2020;14(3):3227–3241. doi:10.1021/acsnano.9b08658
4. Wang W, van Niekerk EA, Zhang Y, et al. Compact, “Clickable” Quantum Dots Photoligated with Multifunctional Zwitterionic Polymers for Immunofluorescence and In Vivo Imaging. Bioconjug Chem. 2020;31(5):1497–1509. doi:10.1021/acs.bioconjchem.0c00169
5. Zhang MQ, Wang ZG, Fu DD, et al. Quantum Dots Tracking Endocytosis and Transport of Proteins Displayed by Mammalian Cells. Anal Chem. 2022;94(21):7567–7575. doi:10.1021/acs.analchem.2c00411
6. Chen LL, Zhao L, Wang ZG, et al. Near-Infrared-II Quantum Dots for In Vivo Imaging and Cancer Therapy. Small. 2022;18(8):e2104567.
7. Tang WS, Zhang B, Xu LD, et al. CdSe/ZnS quantum dot-encoded maleic anhydride-grafted PLA microspheres prepared through membrane emulsification for multiplexed immunoassays of tumor markers. Analyst. 2022;147(9):1873–1880. doi:10.1039/D2AN00350C
8. Liu Y, Liu L, He Y, et al. Quantum-dots-encoded-microbeads based molecularly imprinted polymer. Biosens Bioelectron. 2016;77:886–893. doi:10.1016/j.bios.2015.10.024
9. Tang T, Deng J, Zhang M, et al. Quantum dot-DNA aptamer conjugates coupled with capillary electrophoresis: a universal strategy for ratiometric detection of organophosphorus pesticides. Talanta. 2016;146:55–61. doi:10.1016/j.talanta.2015.08.023
10. Rostam Gohari S, Yazdanparast R. A simplified globally affordable experimental setup for monitoring DNA diagnosis by a QD-based technique. Folia Microbiol (Praha). 2018;63(2):229–235. doi:10.1007/s12223-017-0554-3
11. Chandler M, Minevich B, Roark B, et al. Controlled Organization of Inorganic Materials Using Biological Molecules for Activating Therapeutic Functionalities. ACS Appl Mater Interfaces. 2021;13(33):39030–39041. doi:10.1021/acsami.1c09230
12. Tong S, Zhong J, Chen X, et al. In Vivo Deep-Brain 3- and 4-Photon Fluorescence Imaging of Subcortical Structures Labeled by Quantum Dots Excited at the 2200 nm Window. ACS Nano. 2023;17(4):3686–3695. doi:10.1021/acsnano.2c10724
13. Pavlicek A, Neubauer S, Zafiu C, et al. The use and detection of quantum dots as nanotracers in environmental fate studies of engineered nanoparticles. Environ Pollut. 2023;317:120461. doi:10.1016/j.envpol.2022.120461
14. Bai C, Tang M. Progress on the toxicity of quantum dots to model organism-zebrafish. J Appl Toxicol. 2023;43(1):89–106. doi:10.1002/jat.4333
15. Shiohara A, Hoshino A, Hanaki K, et al. On the cyto-toxicity caused by quantum dots. Microbiol Immunol. 2004;48(9):669–675. doi:10.1111/j.1348-0421.2004.tb03478.x
16. Yao Y, Zhang T, Tang M. The DNA damage potential of quantum dots: toxicity, mechanism and challenge. Environ Pollut. 2023;317:120676. doi:10.1016/j.envpol.2022.120676
17. Manshian BB, Soenen SJ, Al-Ali A, et al. Cell type-dependent changes in CdSe/ZnS quantum dot uptake and toxic endpoints. Toxicol Sci. 2015;144(2):246–258. doi:10.1093/toxsci/kfv002
18. Wang Y, Pang S, Chen Z, et al. Surface Modification Determines the Distribution and Toxicity of Quantum Dots during the Development of Early Staged Zebrafish. Environ Sci Technol. 2023;57(29):10574–10581. doi:10.1021/acs.est.3c01949
19. Almeida G, van der Poll L, Evers WH, et al. Size-Dependent Optical Properties of InP Colloidal Quantum Dots. Nano Lett. 2023;23(18):8697–8703. doi:10.1021/acs.nanolett.3c02630
20. Yang Y, Xia R, Zhang X, et al. Effects of Oral Exposure to Mn-Doped ZnS Quantum Dots on Intestinal Tract and Gut Microbiota in Mice. Front Physiol. 2021;12:657266. doi:10.3389/fphys.2021.657266
21. Kominkova M, Milosavljevic V, Vitek P, et al. Comparative study on toxicity of extracellularly biosynthesized and laboratory synthesized CdTe quantum dots. J Biotechnol. 2017;241:193–200. doi:10.1016/j.jbiotec.2016.10.024
22. Hong S, Yang Z, Mou Q, et al. Monitoring leaching of Cd(2+) from cadmium-based quantum dots by an Cd aptamer fluorescence sensor. Biosens Bioelectron. 2023;220:114880. doi:10.1016/j.bios.2022.114880
23. Gao Y, Xu S, Liu Z, et al. Dual-Emission Fluorescence Probe Based on CdTe Quantum Dots and Rhodamine B for Visual Detection of Mercury and Its Logic Gate Behavior. Micromachines (Basel). 2021;12(6):713.
24. Kauffer FA, Merlin C, Balan L, et al. Incidence of the core composition on the stability, the ROS production and the toxicity of CdSe quantum dots. J Hazard Mater. 2014;268:246–255. doi:10.1016/j.jhazmat.2014.01.029
25. Xie R, Chen K, Chen X, et al. InAs/InP/ZnSe Core/Shell/Shell Quantum Dots as Near-Infrared Emitters: bright, Narrow-Band, Non-Cadmium Containing, and Biocompatible. Nano Res. 2008;1(6):457–464. doi:10.1007/s12274-008-8048-x
26. Bosch S, Botha TL, Wepener V. Influence of different functionalized CdTe quantum dots on the accumulation of metals, developmental toxicity and respiration in different development stages of the zebrafish (Danio rerio). Front Toxicol. 2023;5:1176172. doi:10.3389/ftox.2023.1176172
27. Galdiero E, Falanga A, Siciliano A, et al. Daphnia magna and Xenopus laevis as in vivo models to probe toxicity and uptake of quantum dots functionalized with gH625. Int J Nanomed. 2017;12:2717–2731. doi:10.2147/IJN.S127226
28. Li B, Wang G, Tong Y, et al. Noninvasive Gastrointestinal Tract Imaging Using BSA-Ag(2)Te Quantum Dots as a CT/NIR-II Fluorescence Dual-Modal Imaging Probe in Vivo. ACS Biomater Sci Eng. 2023;9(1):449–457. doi:10.1021/acsbiomaterials.2c00886
29. Das K, Meena R, Gaharwar US, et al. Bioaccumulation of CdSe Quantum Dots Show Biochemical and Oxidative Damage in Wistar Rats. Oxid Med Cell Longev. 2023;2023:7707452. doi:10.1155/2023/7707452
30. Wani MR, Maheshwari N, Shadab G. Eugenol attenuates TiO(2) nanoparticles-induced oxidative damage, biochemical toxicity and DNA damage in Wistar rats: an in vivo study. Environ Sci Pollut Res Int. 2021;28(18):22664–22678. doi:10.1007/s11356-020-12139-3
31. Ye L, Hu R, Liu L, et al. Comparing Semiconductor Nanocrystal Toxicity in Pregnant Mice and Non-Human Primates. Nanotheranostics. 2019;3(1):54–65. doi:10.7150/ntno.27452
32. Wang Y, Tang M. Review of in vitro toxicological research of quantum dot and potentially involved mechanisms. Sci Total Environ. 2018;625:940–962. doi:10.1016/j.scitotenv.2017.12.334
33. Li JY, Huang PL. Effects of CdTe quantum dots on the content of cytochrome P450 in rat liver. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2018;36(3):174–178. doi:10.3760/cma.j.issn.1001-9391.2018.03.004
34. Wang X, He K, Hu Y, et al. A review of pulmonary toxicity of different types of quantum dots in environmental and biological systems. Chem Biol Interact. 2022;368:110247. doi:10.1016/j.cbi.2022.110247
35. Auclair J, Turcotte P, Gagnon C, et al. The influence of surface coatings on the toxicity of silver nanoparticle in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol. 2019;226:108623. doi:10.1016/j.cbpc.2019.108623
36. Le N, Kim K. Current Advances in the Biomedical Applications of Quantum Dots: promises and Challenges. Int J Mol Sci. 2023;24(16):12682. doi:10.3390/ijms241612682
37. Han X, Lei J, Chen K, et al. Cytotoxicity of CdTe quantum dots with different surface coatings against yeast Saccharomyces cerevisiae. Ecotoxicol Environ Saf. 2019;174:467–474. doi:10.1016/j.ecoenv.2019.03.013
38. Babkin IA, Udepurkar AP, Van Avermaet H, et al. Encapsulation of Cadmium-Free InP/ZnSe/ZnS Quantum Dots in Poly(LMA-co-EGDMA) Microparticles via Co-flow Droplet Microfluidics. Small Methods. 2023;7(7):e2201454. doi:10.1002/smtd.202201454
39. Chen H, Chen J, Wu Y, et al. A study on the mechanism of Indium phosphide/zinc sulfide core/shell quantum dots influencing embryo incubation of rare minnow (Gobiocypris rarus). Aquat Toxicol. 2023;261:106593. doi:10.1016/j.aquatox.2023.106593
40. Zhang J, Bifulco A, Amato P, et al. Copper indium sulfide quantum dots in photocatalysis. J Colloid Interface Sci. 2023;638:193–219. doi:10.1016/j.jcis.2023.01.107
41. Hashemkhani M, Loizidou M, MacRobert AJ, et al. One-Step Aqueous Synthesis of Anionic and Cationic AgInS(2) Quantum Dots and Their Utility in Improving the Efficacy of ALA-Based Photodynamic Therapy. Inorg Chem. 2022;61(6):2846–2863. doi:10.1021/acs.inorgchem.1c03298
42. Hou J, Zhao Y, Sun L, et al. Enzyme/GSH/pH-responsive hyaluronic acid grafted porous silica nanocarriers bearing Ag(2)S QDs for fluorescence imaging and combined therapy. Carbohydr Polym. 2023;305:120547. doi:10.1016/j.carbpol.2023.120547
43. Li F, Benetti D, Zhang M, et al. Modulating the 0D/2D Interface of Hybrid Semiconductors for Enhanced Photoelectrochemical Performances. Small Methods. 2021;5(8):e2100109. doi:10.1002/smtd.202100109
44. Mrad M, Ben Chaabane T, Rinnert H, et al. Aqueous Synthesis for Highly Emissive 3-Mercaptopropionic Acid-Capped AIZS Quantum Dots. Inorg Chem. 2020;59(9):6220–6231. doi:10.1021/acs.inorgchem.0c00347
45. Chen T, Chen Y, Li Y, et al. A Review on Multiple I-III-VI Quantum Dots: preparation and Enhanced Luminescence Properties. Materials (Basel). 2023;16(14):5039.
46. Kovacova M, Kleinova A, Vajdak J, et al. Photodynamic-active smart biocompatible material for an antibacterial surface coating. J Photochem Photobiol B. 2020;211:112012. doi:10.1016/j.jphotobiol.2020.112012
47. Li W-Y, Yin S, Huang S-W, et al. The trajectory patterns of single HIV-1 virus-like particle in live CD4 cells: a real time three-dimensional multi-resolution microscopy study using encapsulated nonblinking giant quantum dot. J Microbiol Immunol Infect. 2023;56(2):257–266. doi:10.1016/j.jmii.2022.08.011
48. Ren F, Wang F, Baghdasaryan A, et al. Shortwave-infrared-light-emitting probes for the in vivo tracking of cancer vaccines and the elicited immune responses. Nat Biomed Eng. 2023. doi:10.1038/s41551-023-01083-5
49. Fath-Bayati L, Vasei M, Sharif-Paghaleh E. Optical fluorescence imaging with shortwave infrared light emitter nanomaterials for in vivo cell tracking in regenerative medicine. J Cell Mol Med. 2019;23(12):7905–7918. doi:10.1111/jcmm.14670
50. Hamidu A, Pitt WG, Husseini GA. Recent Breakthroughs in Using Quantum Dots for Cancer Imaging and Drug Delivery Purposes. Nanomaterials (Basel). 2023;13(18):2566.
51. Wu T, Liang X, He K, et al. The role of NLRP3 inflammasome activation in the neuroinflammatory responses to Ag(2)Se quantum dots in microglia. Nanoscale. 2019;11(43):20820–20836.
52. Wang H, Yang H, P XZ, et al. Anionic Long-Circulating Quantum Dots for Long-Term Intravital Vascular Imaging. Pharmaceutics. 2018;10(4):56.
53. Foroozandeh P, Aziz AA. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res Lett. 2018;13(1):339. doi:10.1186/s11671-018-2728-6
54. Thovhogi N, Sibuyi NR, Onani MO, et al. Peptide-functionalized quantum dots for potential applications in the imaging and treatment of obesity. Int J Nanomed. 2018;13:2551–2559. doi:10.2147/IJN.S158687
55. Meng X, Li H, Chen Y, et al. In Vivo Precision Evaluation of Lymphatic Function by SWIR Luminescence Imaging with PbS Quantum Dots. Adv Sci (Weinh). 2023;10(7):e2206579.
56. Ku T-H, Shen W-T, Hsieh C-T, et al. Specific Forms of Graphene Quantum Dots Induce Apoptosis and Cell Cycle Arrest in Breast Cancer Cells. Int J Mol Sci. 2023;24(4). doi:10.3390/ijms24044046
57. Guan X, Wang B, Zhang Y, et al. Monitoring Stress Response Difference in Nucleolus Morphology and ATP Content Changes during Hyperthermia Cell Apoptosis with Plasmonic Fluorescent Nanoprobes. Anal Chem. 2022;94(40):13842–13851. doi:10.1021/acs.analchem.2c02464
58. Zheng W, Xu YM, Wu DD, et al. Acute and chronic cadmium telluride quantum dots-exposed human bronchial epithelial cells: the effects of particle sizes on their cytotoxicity and carcinogenicity. Biochem Biophys Res Commun. 2018;495(1):899–903. doi:10.1016/j.bbrc.2017.11.074
59. Liang Q, Yu F, Cai H, et al. Photo-activated autophagy-associated tumour cell death by lysosome impairment based on manganese-doped graphene quantum dots. J Mater Chem B. 2023;11(11):2466–2477. doi:10.1039/D2TB02761E
60. Wang L, Lin M, Hou X, et al. Black phosphorus quantum dots induce autophagy and apoptosis of human bronchial epithelial cells via endoplasmic reticulum stress. Chemosphere. 2023;327:138463. doi:10.1016/j.chemosphere.2023.138463
61. Hu A, Zhang JW, Yang LY, et al. Curcumin-loaded graphene oxide quantum dots enhance otoprotective effects via blocking cuproptosis. Front Bioeng Biotechnol. 2023;11:1183197. doi:10.3389/fbioe.2023.1183197
62. Alothaid H, Al-Anazi MR, Al-Qahtani AA, et al. Exposure to Cadmium Telluride Quantum Dots and Gene Expression Profile of Huh-7 Hepatocellular Carcinoma Cell Line. Dose Response. 2023;21(3):15593258231185457. doi:10.1177/15593258231185457
63. Zhang M, Kim DS, Patel R, et al. Intracellular Trafficking and Distribution of Cd and InP Quantum Dots in HeLa and ML-1 Thyroid Cancer Cells. Nanomaterials (Basel). 2022;12(9):1517.
64. Wang Y, Tang M. Dysfunction of various organelles provokes multiple cell death after quantum dot exposure. Int J Nanomed. 2018;13:2729–2742. doi:10.2147/IJN.S157135
65. Liu J, Huang Z, Yin S, et al. The lysosome-mitochondrion crosstalk engaged in silver nanoparticles-disturbed mitochondrial homeostasis. Sci Total Environ. 2023;889:164078. doi:10.1016/j.scitotenv.2023.164078
66. Fang Y, Zhuo L, Yuan H, et al. Construction of graphene quantum dot-based dissolving microneedle patches for the treatment of bacterial keratitis. Int J Pharm. 2023;639:122945. doi:10.1016/j.ijpharm.2023.122945
67. Chen H, Wu Y, Xie W, et al. InP/ZnS quantum dots cause liver damage in rare minnow (Gobiocypris rarus) larvae. Comp Biochem Physiol C Toxicol Pharmacol. 2023;266:109546. doi:10.1016/j.cbpc.2023.109546
68. Zhou Y, Wang Q, Song B, et al. A real-time documentation and mechanistic investigation of quantum dots-induced autophagy in live Caenorhabditis elegans. Biomaterials. 2015;72:38–48. doi:10.1016/j.biomaterials.2015.08.044
69. Wu D, Lu J, Ma Y, et al. Mitochondrial dynamics and mitophagy involved in MPA-capped CdTe quantum dots-induced toxicity in the human liver carcinoma (HepG2) cell line. Environ Pollut. 2021;274:115681. doi:10.1016/j.envpol.2020.115681
70. Litvinov I, Salova A, Aksenov N, et al. Microenvironmental Impact on InP/ZnS-Based Quantum Dots in In Vitro Models and in Living Cells: spectrally- and Time-Resolved Luminescence Analysis. Int J Mol Sci. 2023;24(3):2699. doi:10.3390/ijms24032699
71. Sobhanan J, Anas A, Biju V. Nanomaterials for Fluorescence and Multimodal Bioimaging. Chem Rec. 2023;23(3):e202200253. doi:10.1002/tcr.202200253
72. Vu MN, Kelly HG, Wheatley AK, et al. Cellular Interactions of Liposomes and PISA Nanoparticles during Human Blood Flow in a Microvascular Network. Small. 2020;16(33):e2002861. doi:10.1002/smll.202002861
73. Gao L-X, Hao H, Yu Y-Q, et al. Protein Labeling Facilitates the Understanding of Protein Corona Formation via Fluorescence Resonance Energy Transfer and Fluorescence Correlation Spectroscopy. Langmuir. 2023;39(43):15275–15284. doi:10.1021/acs.langmuir.3c01986
74. Qu S, Qiao Z, Zhong W, et al. Chirality-Dependent Dynamic Evolution of the Protein Corona on the Surface of Quantum Dots. ACS Appl Mater Interfaces. 2022;14(39):44147–44157. doi:10.1021/acsami.2c11874
75. Liu N, Liang Y, Wei T, et al. Protein Corona mitigated the cytotoxicity of CdTe QDs to macrophages by targeting mitochondria. NanoImpact. 2022;25:100367. doi:10.1016/j.impact.2021.100367
76. Wang J, Yang B, Yu X, et al. The impact of Zn doping on CdTe quantum dots-protein Corona formation and the subsequent toxicity at the molecular and cellular level. Chem Biol Interact. 2023;373:110370. doi:10.1016/j.cbi.2023.110370
77. Zhang P, Qiao Y, Zhu L, et al. Nanoprobe Based on Biominerals in Protein Corona for Dual-Modality MR Imaging and Therapy of Tumors. ACS Nano. 2023;17(1):184–196. doi:10.1021/acsnano.2c05917
78. Tawfik M, Hadlak S, Gotze C, et al. Live In-Vivo Neuroimaging Reveals the Transport of Lipophilic Cargo Through the Blood-Retina Barrier with Modified Amphiphilic Poly-N-Vinylpyrrolidone Nanoparticles. J Biomed Nanotechnol. 2021;17(5):846–858. doi:10.1166/jbn.2021.3073
79. Wang Y, Wang X, Xie R, et al. Overcoming the Blood-Brain Barrier for Gene Therapy via Systemic Administration of GSH-Responsive Silica Nanocapsules. Adv Mater. 2023;35(6):e2208018.
80. Javidi J, Haeri A, Nowroozi F, et al. Pharmacokinetics, Tissue Distribution and Excretion of Ag(2)S Quantum Dots in Mice and Rats: the Effects of Injection Dose, Particle Size and Surface Charge. Pharm Res. 2019;36(3):46. doi:10.1007/s11095-019-2571-1
81. Hauck TS, Anderson RE, Fischer HC, et al. In vivo quantum-dot toxicity assessment. Small. 2010;6(1):138–144. doi:10.1002/smll.200900626
82. Jiang D, Rosenkrans ZT, Ni D, et al. Nanomedicines for Renal Management: from Imaging to Treatment. Acc Chem Res. 2020;53(9):1869–1880. doi:10.1021/acs.accounts.0c00323
83. Du B, Jiang X, Das A, et al. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Nat Nanotechnol. 2017;12(11):1096–1102. doi:10.1038/nnano.2017.170
84. Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine. 2008;3(5):703–717. doi:10.2217/17435889.3.5.703
85. Kim W, Ly NK, He Y, et al. Protein Corona: friend or foe? Co-opting serum proteins for nanoparticle delivery. Adv Drug Deliv Rev. 2023;192:114635. doi:10.1016/j.addr.2022.114635
86. Sng IS, Xavier YX, Lau CM, et al. Infection induces tissue-resident memory NK cells that safeguard tissue health. Immunity. 2023;56(9):2173–2174. doi:10.1016/j.immuni.2023.08.004
87. Bi J, Mo C, Li S, et al. Immunotoxicity of metal and metal oxide nanoparticles: from toxic mechanisms to metabolism and outcomes. Biomater Sci. 2023;11(12):4151–4183. doi:10.1039/d3bm00271c
88. Ryan J, Jacob P, Lee A, et al. Biodistribution and toxicity of antimicrobial ionic silver (Ag(+)) and silver nanoparticle (AgNP(+)) species after oral exposure, in Sprague-Dawley rats. Food Chem Toxicol. 2022;166:113228. doi:10.1016/j.fct.2022.113228
89. Beus M, Pongrac IM, Capjak I, et al. Particle surface functionalization affects mechanism of endocytosis and adverse effects of silver nanoparticles in mammalian kidney cells. J Appl Toxicol. 2023;43(3):416–430. doi:10.1002/jat.4392
90. De Jong WH, Van Der Ven LT, Sleijffers A, et al. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials. 2013;34(33):8333–8343. doi:10.1016/j.biomaterials.2013.06.048
91. Chen Y, Su W, Tie S, et al. Orally deliverable sequence-targeted astaxanthin nanoparticles for colitis alleviation. Biomaterials. 2023;293:121976. doi:10.1016/j.biomaterials.2022.121976
92. Lim J-O, I W-I, Pak S-W, et al. Toll-like receptor 4 is a key regulator of asthma exacerbation caused by aluminum oxide nanoparticles via regulation of NF-kappaB phosphorylation. J Hazard Mater. 2023;448:130884. doi:10.1016/j.jhazmat.2023.130884
93. Du H, Wang X, Zhang H, et al. Serum protein coating enhances the antisepsis efficacy of silver nanoparticles against multidrug-resistant Escherichia coli infections in mice. Front Microbiol. 2023;14:1153147. doi:10.3389/fmicb.2023.1153147
94. Chen T, Li L, Lin X, et al. In vitro and in vivo immunotoxicity of PEGylated Cd-free CuInS(2)/ZnS quantum dots. Nanotoxicology. 2020;14(3):372–387. doi:10.1080/17435390.2019.1708495
95. Sasaki E, Yokoi T. Role of cytochrome P450-mediated metabolism and involvement of reactive metabolite formations on antiepileptic drug-induced liver injuries. J Toxicol Sci. 2018;43(2):75–87. doi:10.2131/jts.43.75
96. Wang H, Thorling CA, Liang X, et al. Diagnostic imaging and therapeutic application of nanoparticles targeting the liver. J Mater Chem B. 2015;3(6):939–958. doi:10.1039/C4TB01611D
97. Zheng L, Wu J, Hu H, et al. Single-cell RNA transcriptome landscape of murine liver following systemic administration of mesoporous silica nanoparticles. J Control Release. 2023;361:427–442. doi:10.1016/j.jconrel.2023.07.037
98. Nguyen KC, Rippstein P, Tayabali AF, et al. Mitochondrial Toxicity of Cadmium Telluride Quantum Dot Nanoparticles in Mammalian Hepatocytes. Toxicol Sci. 2015;146(1):31–42. doi:10.1093/toxsci/kfv068
99. Lu J, Tang M, Zhang T. Review of toxicological effect of quantum dots on the liver. J Appl Toxicol. 2019;39(1):72–86. doi:10.1002/jat.3660
100. Winiarska-Mieczan A, Kwiecien M. The Effect of Exposure to Cd and Pb in the Form of a Drinking Water or Feed on the Accumulation and Distribution of These Metals in the Organs of Growing Wistar Rats. Biol Trace Elem Res. 2016;169(2):230–236. doi:10.1007/s12011-015-0414-4
101. Zhang Y, Liang R, Chen Y, et al. HSF1 protects cells from cadmium toxicity by governing proteome integrity. Ecotoxicol Environ Saf. 2023;266:115571. doi:10.1016/j.ecoenv.2023.115571
102. Sun J, Qu H, Ali W, et al. Co-exposure to cadmium and microplastics promotes liver fibrosis through the hemichannels -ATP-P2X7 pathway. Chemosphere. 2023;344:140372. doi:10.1016/j.chemosphere.2023.140372
103. Liu C, Li HJ, Duan WX, et al. MCU Upregulation Overactivates Mitophagy by Promoting VDAC1 Dimerization and Ubiquitination in the Hepatotoxicity of Cadmium. Adv Sci (Weinh). 2023;10(7):e2203869.
104. Liu W, Zhang S, Wang L, et al. CdSe quantum dot (QD)-induced morphological and functional impairments to liver in mice. PLoS One. 2011;6(9):e24406.
105. Yong K-T, Law W-C, Hu R, et al. Nanotoxicity assessment of quantum dots: from cellular to primate studies. Chem Soc Rev. 2013;42(3):1236–1250. doi:10.1039/C2CS35392J
106. Yang Y, Lv SY, Yu B, et al. Hepatotoxicity assessment of Mn-doped ZnS quantum dots after repeated administration in mice. Int J Nanomed. 2015;10:5787–5796. doi:10.2147/IJN.S88789
107. Lu Y, Xu S, Chen H, et al. CdSe/ZnS quantum dots induce hepatocyte pyroptosis and liver inflammation via NLRP3 inflammasome activation. Biomaterials. 2016;90:27–39. doi:10.1016/j.biomaterials.2016.03.003
108. Serban AI, Stanca L, Sima C, et al. Complex responses to Si quantum dots accumulation in carp liver tissue: beyond oxidative stress. Chem Biol Interact. 2015;239:56–66. doi:10.1016/j.cbi.2015.06.015
109. Li W, Tan M, Wang H, et al. METTL3-mediated m6A mRNA modification was involved in cadmium-induced liver injury. Environ Pollut. 2023;331(Pt 2):121887. doi:10.1016/j.envpol.2023.121887
110. Perazella MA. Drug-induced acute kidney injury: diverse mechanisms of tubular injury. Curr Opin Crit Care. 2019;25(6):550–557. doi:10.1097/MCC.0000000000000653
111. Liang X, Wang H, Zhu Y, et al. Short- and Long-Term Tracking of Anionic Ultrasmall Nanoparticles in Kidney. ACS Nano. 2016;10(1):387–395. doi:10.1021/acsnano.5b05066
112. Yaghini E, Tacconi E, Pilling A, et al. Population pharmacokinetic modelling of indium-based quantum dot nanoparticles: preclinical in vivo studies. Eur J Pharm Sci. 2021;157:105639. doi:10.1016/j.ejps.2020.105639
113. Li L, Chen Y, Xu G, et al. In vivo Comparison of the Biodistribution and Toxicity of InP/ZnS Quantum Dots with Different Surface Modifications. Int J Nanomed. 2020;15:1951–1965. doi:10.2147/IJN.S241332
114. Lin P-ID, Cardenas A, Rifas-Shiman SL, et al. Non-essential and essential trace element mixtures and kidney function in early pregnancy - A cross-sectional analysis in project viva. Environ Res. 2023;216(Pt 4):114846. doi:10.1016/j.envres.2022.114846
115. E CE, Gattu M, Camilli S, et al. The Cd/Zn Axis: emerging Concepts in Cellular Fate and Cytotoxicity. Biomolecules. 2023;13(2). doi:10.3390/biom13020316
116. Blum JL, Edwards JR, Prozialeck WC, et al. Effects of Maternal Exposure to Cadmium Oxide Nanoparticles During Pregnancy on Maternal and Offspring Kidney Injury Markers Using a Murine Model. J Toxicol Environ Health A. 2015;78(12):711–724. doi:10.1080/15287394.2015.1026622
117. Wang M, Wang J, Sun H, et al. Time-dependent toxicity of cadmium telluride quantum dots on liver and kidneys in mice: histopathological changes with elevated free cadmium ions and hydroxyl radicals. Int J Nanomed. 2016;11:2319–2328. doi:10.2147/IJN.S103489
118. Coccini T, Barni S, Manzo L, et al. Apoptosis induction and histological changes in rat kidney following Cd-doped silica nanoparticle exposure: evidence of persisting effects. Toxicol Mech Methods. 2013;23(8):566–575. doi:10.3109/15376516.2013.803270
119. Fan R, Hu PC, Wang Y, et al. Betulinic acid protects mice from cadmium chloride-induced toxicity by inhibiting cadmium-induced apoptosis in kidney and liver. Toxicol Lett. 2018;299:56–66. doi:10.1016/j.toxlet.2018.09.003
120. Raredon MSB, Adams TS, Suhail Y, et al. Single-cell connectomic analysis of adult mammalian lungs. Sci Adv. 2019;5(12):eaaw3851. doi:10.1126/sciadv.aaw3851
121. Scoville DK, White CC, Botta D, et al. Susceptibility to quantum dot induced lung inflammation differs widely among the Collaborative Cross founder mouse strains. Toxicol Appl Pharmacol. 2015;289(2):240–250. doi:10.1016/j.taap.2015.09.019
122. Stan MS, Sima C, Cinteza LO, et al. Silicon-based quantum dots induce inflammation in human lung cells and disrupt extracellular matrix homeostasis. FEBS J. 2015;282(15):2914–2929. doi:10.1111/febs.13330
123. Blum JL, Rosenblum LK, Grunig G, et al. Short-term inhalation of cadmium oxide nanoparticles alters pulmonary dynamics associated with lung injury, inflammation, and repair in a mouse model. Inhal Toxicol. 2014;26(1):48–58. doi:10.3109/08958378.2013.851746
124. Lee P, K JK, Jo MS, et al. Biokinetics of subacutely co-inhaled same size gold and silver nanoparticles. Part Fibre Toxicol. 2023;20(1):9. doi:10.1186/s12989-023-00515-z
125. Scoville DK, White CC, Botta D, et al. Quantum dot induced acute changes in lung mechanics are mouse strain dependent. Inhal Toxicol. 2018;30(9–10):397–403. doi:10.1080/08958378.2018.1542046
126. Tang Y, Han S, Liu H, et al. The role of surface chemistry in determining in vivo biodistribution and toxicity of CdSe/ZnS core-shell quantum dots. Biomaterials. 2013;34(34):8741–8755. doi:10.1016/j.biomaterials.2013.07.087
127. Hasannejad-Asl B, Pooresmaeil F, Choupani E, et al. Nanoparticles as Powerful Tools for Crossing the Blood-brain Barrier. CNS Neurol Disord Drug Targets. 2023;22(1):18–26. doi:10.2174/1871527321666220222092655
128. Bencsik A, Lestaevel P, Guseva Canu I. Nano- and neurotoxicology: an emerging discipline. Prog Neurobiol. 2018;160:45–63. doi:10.1016/j.pneurobio.2017.10.003
129. Wu T, Zhang T, Chen Y, et al. Research advances on potential neurotoxicity of quantum dots. J Appl Toxicol. 2016;36(3):345–351. doi:10.1002/jat.3229
130. Wu T, He K, Ang S, et al. Impairments of spatial learning and memory following intrahippocampal injection in rats of 3-mercaptopropionic acid-modified CdTe quantum dots and molecular mechanisms. Int J Nanomed. 2016;11:2737–2755. doi:10.2147/IJN.S104985
131. Wu T, Liang X, He K, et al. Transcriptome analysis of different sizes of 3-mercaptopropionic acid-modified cadmium telluride quantum dot-induced toxic effects reveals immune response in rat hippocampus. J Appl Toxicol. 2018;38(9):1177–1194. doi:10.1002/jat.3629
132. Wu T, Liang X, He K, et al. The NLRP3-Mediated Neuroinflammatory Responses to CdTe Quantum Dots and the Protection of ZnS Shell. Int J Nanomed. 2020;15:3217–3233. doi:10.2147/IJN.S246578
133. Galdiero E, Siciliano A, Lombardi L, et al. Quantum dots functionalized with gH625 attenuate QDs oxidative stress and lethality in Caenorhabditis elegans: a model system. Ecotoxicology. 2020;29(2):156–162. doi:10.1007/s10646-019-02158-3
134. Zhao Y, Wang X, Wu Q, et al. Translocation and neurotoxicity of CdTe quantum dots in RMEs motor neurons in nematode Caenorhabditis elegans. J Hazard Mater. 2015;283:480–489. doi:10.1016/j.jhazmat.2014.09.063
135. Mohammadi E, Behnam B, Mokhtarzadeh A, et al. Reproductive Toxicity of Nanoparticles: a Comprehensive Review. Curr Med Chem. 2023. doi:10.2174/0929867331666230815101141
136. Zhang X-D, Luo Z, Chen J, et al. Storage of gold nanoclusters in muscle leads to their biphasic in vivo clearance. Small. 2015;11(14):1683–1690. doi:10.1002/smll.201402233
137. Zhang W, Yang L, Kuang H, et al. Acute toxicity of quantum dots on late pregnancy mice: effects of nanoscale size and surface coating. J Hazard Mater. 2016;318:61–69. doi:10.1016/j.jhazmat.2016.06.048
138. Chen Y, Yang Y, Ou F, et al. InP/ZnS QDs exposure induces developmental toxicity in rare minnow (Gobiocypris rarus) embryos. Environ Toxicol Pharmacol. 2018;60:28–36. doi:10.1016/j.etap.2018.04.005
139. Qu Y, Li W, Zhou Y, et al. Full assessment of fate and physiological behavior of quantum dots utilizing Caenorhabditis elegans as a model organism. Nano Lett. 2011;11(8):3174–3183. doi:10.1021/nl201391e
140. Qu M, Qiu Y, Lv R, et al. Exposure to MPA-capped CdTe quantum dots causes reproductive toxicity effects by affecting oogenesis in nematode Caenorhabditis elegans. Ecotoxicol Environ Saf. 2019;173:54–62. doi:10.1016/j.ecoenv.2019.02.018
141. Li X, Yang X, Yuwen L, et al. Evaluation of toxic effects of CdTe quantum dots on the reproductive system in adult male mice. Biomaterials. 2016;96:24–32. doi:10.1016/j.biomaterials.2016.04.014
142. Amiri G, Valipoor A, Parivar K, et al. Comparison of Toxicity of CdSe: znS Quantum Dots on Male Reproductive System in Different Stages of Development in Mice. Int J Fertil Steril. 2016;9(4):512–520. doi:10.22074/ijfs.2015.4610
143. Lafuente D, Garcia T, Blanco J, et al. Effects of oral exposure to silver nanoparticles on the sperm of rats. Reprod Toxicol. 2016;60:133–139. doi:10.1016/j.reprotox.2016.02.007
144. Li L, Lin X, Chen T, et al. Systematic evaluation of CdSe/ZnS quantum dots toxicity on the reproduction and offspring health in male BALB/c mice. Ecotoxicol Environ Saf. 2021;211:111946. doi:10.1016/j.ecoenv.2021.111946
145. Samadian H, Salami MS, Jaymand M, et al. Genotoxicity assessment of carbon-based nanomaterials; Have their unique physicochemical properties made them double-edged swords? Mutat Res Rev Mutat Res. 2020;783:108296. doi:10.1016/j.mrrev.2020.108296
146. Ding Y, Yang Y, Chen J, et al. Toxic effects of ZnSe/ZnS quantum dots on the reproduction and genotoxiticy of rare minnow (Gobiocypris rarus). Comp Biochem Physiol C Toxicol Pharmacol. 2021;247:109065. doi:10.1016/j.cbpc.2021.109065
147. Katsumiti A, Gilliland D, Arostegui I, et al. Cytotoxicity and cellular mechanisms involved in the toxicity of CdS quantum dots in hemocytes and gill cells of the mussel Mytilus galloprovincialis. Aquat Toxicol. 2014;153:39–52. doi:10.1016/j.aquatox.2014.02.003
148. Aye M, Di Giorgio C, Mekaouche M, et al. Genotoxicity of intraperitoneal injection of lipoamphiphile CdSe/ZnS quantum dots in rats. Mutat Res Genet Toxicol Environ Mutagen. 2013;758(1–2):48–55. doi:10.1016/j.mrgentox.2013.09.004
149. Borm PJ, Tran L, Donaldson K. The carcinogenic action of crystalline silica: a review of the evidence supporting secondary inflammation-driven genotoxicity as a principal mechanism. Crit Rev Toxicol. 2011;41(9):756–770. doi:10.3109/10408444.2011.576008
150. Patlolla AK, Hackett D, Tchounwou PB. Genotoxicity study of silver nanoparticles in bone marrow cells of Sprague-Dawley rats. Food Chem Toxicol. 2015;85:52–60. doi:10.1016/j.fct.2015.05.005
151. Wang M, Wang Y, Wang S, et al. Selenium alleviates cadmium-induced oxidative stress, endoplasmic reticulum stress and programmed necrosis in chicken testes. Sci Total Environ. 2023;863:160601. doi:10.1016/j.scitotenv.2022.160601
152. He J, Wang -Z-Z, Li C-H, et al. Metabolic alteration of Tetrahymena thermophila exposed to CdSe/ZnS quantum dots to respond to oxidative stress and lipid damage. Biochim Biophys Acta Gen Subj. 2023;1867(1):130251. doi:10.1016/j.bbagen.2022.130251
153. Skrodenyte-Arbaciauskiene V, Butrimiene R, Kalnaityte-Vengeliene A, et al. A multiscale study of the effects of a diet containing CdSe/ZnS-COOH quantum dots on Salmo trutta fario L.: potential feed-related nanotoxicity. Sci Total Environ. 2024;906:167696. doi:10.1016/j.scitotenv.2023.167696
154. Liu N, Liang Y, Wei T, et al. The role of ferroptosis mediated by NRF2/ERK-regulated ferritinophagy in CdTe QDs-induced inflammation in macrophage. J Hazard Mater. 2022;436:129043. doi:10.1016/j.jhazmat.2022.129043
155. Zhang T, Lu J, Yao Y, et al. MPA-capped CdTequantum dots induces endoplasmic reticulum stress-mediated autophagy and apoptosis through generation of reactive oxygen species in human liver normal cell and liver tumor cell. Environ Pollut. 2023;326:121397. doi:10.1016/j.envpol.2023.121397
156. Paithankar JG, Kushalan S, S N, et al. Systematic toxicity assessment of CdTe quantum dots in Drosophila melanogaster. Chemosphere. 2022;295:133836. doi:10.1016/j.chemosphere.2022.133836
157. Chen J, Ding Y, Chen H, et al. Reproductive toxicity of InP/ZnS QDs in male rare minnow (Gobiocypris rarus). Comp Biochem Physiol C Toxicol Pharmacol. 2022;259:109392. doi:10.1016/j.cbpc.2022.109392
158. de Carvalho SM, Mansur AAP, Mansur HS, et al. In vitro and in vivo assessment of nanotoxicity of CdS quantum dot/aminopolysaccharide bionanoconjugates. Mater Sci Eng C Mater Biol Appl. 2017;71:412–424. doi:10.1016/j.msec.2016.10.023
159. Grioni S, Agnoli C, Krogh V, et al. Dietary cadmium and risk of breast cancer subtypes defined by hormone receptor status: a prospective cohort study. Int, J, Cancer. 2019;144(9):2153–2160. doi:10.1002/ijc.32039
160. Su Y, Hu M, Fan C, et al. The cytotoxicity of CdTe quantum dots and the relative contributions from released cadmium ions and nanoparticle properties. Biomaterials. 2010;31(18):4829–4834. doi:10.1016/j.biomaterials.2010.02.074
161. Zhao L, Guo Z, Wu H, et al. New insights into the release mechanism of Cd(2+) from CdTe quantum dots within single cells in situ. Ecotoxicol Environ Saf. 2020;196:110569. doi:10.1016/j.ecoenv.2020.110569
162. Soenen SJ, Montenegro J-M, Abdelmonem AM, et al. The effect of nanoparticle degradation on amphiphilic polymer-coated quantum dot toxicity: the importance of particle functionality assessment in toxicology [corrected]. Acta Biomater. 2014;10(2):732–741. doi:10.1016/j.actbio.2013.09.041
163. Dussert F, Sarret G, Wegner KD, et al. Physico-Chemical Transformation and Toxicity of Multi-Shell InP Quantum Dots under Simulated Sunlight Irradiation, in an Environmentally Realistic Scenario. Nanomaterials (Basel). 2022;12(20):3703. doi:10.3390/nano12203703
164. Nie C, Lin X, Zhao G, et al. Low-Toxicity ZnSe/ZnS Quantum Dots as Potent Photoreductants and Triplet Sensitizers for Organic Transformations. Angew Chem Int Ed Engl. 2022;61(49):e202213065. doi:10.1002/anie.202213065
165. Kim J-H, Y S-Y, Kim K-H, et al. Electroluminescence from two I-III-VI quantum dots of A-Ga-S (A=Cu, Ag). Opt Lett. 2018;43(21):5287–5290. doi:10.1364/OL.43.005287
166. Lim LJ, Zhao X, Tan ZK. Non-Toxic CuInS(2) /ZnS Colloidal Quantum Dots for Near-Infrared Light-Emitting Diodes. Adv Mater. 2023;35(28):e2301887. doi:10.1002/adma.202301887
167. Huang G, Wang L, Zhang X. Involvement of ABC transporters in the efflux and toxicity of MPA-COOH-CdTe quantum dots in human breast cancer SK-BR-3 cells. J Biochem Mol Toxicol. 2019;33(8):e22343. doi:10.1002/jbt.22343
168. Chang S, Chen D, Kang B, et al. UV-enhanced cytotoxicity of CdTe quantum dots in PANC-1 cells depend on their size distribution and surface modification. J Nanosci Nanotechnol. 2013;13(2):751–754. doi:10.1166/jnn.2013.6085
169. Wang Q, Zhou Y, Song B, et al. Linking Subcellular Disturbance to Physiological Behavior and Toxicity Induced by Quantum Dots in Caenorhabditis elegans. Small. 2016;12(23):3143–3154. doi:10.1002/smll.201600766
170. Su Y, Peng F, Jiang Z, et al. In vivo distribution, pharmacokinetics, and toxicity of aqueous synthesized cadmium-containing quantum dots. Biomaterials. 2011;32(25):5855–5862. doi:10.1016/j.biomaterials.2011.04.063
171. Silva BF, Andreani T, Gavina A, et al. Toxicological impact of cadmium-based quantum dots towards aquatic biota: effect of natural sunlight exposure. Aquat Toxicol. 2016;176:197–207. doi:10.1016/j.aquatox.2016.05.001
172. Jiang Z, Zhao C, Liu X. Synthesis of poly(ethylene glycol)-graft-chitosan and using as ligand for fabrication of water-soluble quantum dots. Colloids Surf B Biointerfaces. 2014;115:260–266. doi:10.1016/j.colsurfb.2013.12.001
173. Zheng N, Yan J, Qian W, et al. Comparison of developmental toxicity of different surface modified CdSe/ZnS QDs in zebrafish embryos. J Environ Sci (China). 2021;100:240–249. doi:10.1016/j.jes.2020.07.019
174. Nagy A, Steinbruck A, Gao J, et al. Comprehensive analysis of the effects of CdSe quantum dot size, surface charge, and functionalization on primary human lung cells. ACS Nano. 2012;6(6):4748–4762. doi:10.1021/nn204886b
175. Andreu I, Ngo TM, Perez V, et al. Contact transfer of engineered nanomaterials in the workplace. R Soc Open Sci. 2021;8(8):210141. doi:10.1098/rsos.210141
176. Jiang X-Y, Sarsons CD, Gomez-Garcia MJ, et al. Quantum dot interactions and flow effects in angiogenic zebrafish (Danio rerio) vessels and human endothelial cells. Nanomedicine. 2017;13(3):999–1010. doi:10.1016/j.nano.2016.12.008
177. Wu T, Tang M. Toxicity of quantum dots on respiratory system. Inhal Toxicol. 2014;26(2):128–139. doi:10.3109/08958378.2013.871762
178. Zhang JH, Niu A, Li J, et al. In vivo characterization of hair and skin derived carbon quantum dots with high quantum yield as long-term bioprobes in zebrafish. Sci Rep. 2016;6:37860. doi:10.1038/srep37860
179. Wang Y, Zhao Y, Cui Y, et al. Overcoming multiple gastrointestinal barriers by bilayer modified hollow mesoporous silica nanocarriers. Acta Biomater. 2018;65:405–416. doi:10.1016/j.actbio.2017.10.025
180. Doge N, Hadam S, Volz P, et al. Identification of polystyrene nanoparticle penetration across intact skin barrier as rare event at sites of focal particle aggregations. J Biophotonics. 2018;11(4):e201700169. doi:10.1002/jbio.201700169
181. Tang L, Zhang C, Song G, et al. In vivo skin penetration and metabolic path of quantum dots. Sci China Life Sci. 2013;56(2):181–188. doi:10.1007/s11427-012-4404-x
182. Lin G, Chen T, Pan Y, et al. Biodistribution and acute toxicity of cadmium-free quantum dots with different surface functional groups in mice following intratracheal inhalation. Nanotheranostics. 2020;4(3):173–183. doi:10.7150/ntno.42786
183. Varmazyari A, Taghizadehghalehjoughi A, Sevim C, et al. Cadmium sulfide-induced toxicity in the cortex and cerebellum: in vitro and in vivo studies. Toxicol Rep. 2020;7:637–648. doi:10.1016/j.toxrep.2020.04.011
184. Zhang T, Qu J, Yao Y, et al. N-doped carbon dots triggered the induction of ROS-mediated cytoprotective autophagy in Hepa1-6 cells. Chemosphere. 2020;251:126440. doi:10.1016/j.chemosphere.2020.126440
185. Aldughaim MS, Al-Anazi MR, Bohol MFF, et al. Gene Expression and Transcriptome Profiling of Changes in a Cancer Cell Line Post-Exposure to Cadmium Telluride Quantum Dots: possible Implications in Oncogenesis. Dose Response. 2021;19(2):15593258211019880. doi:10.1177/15593258211019880
186. Wang J, Sun H, Meng P, et al. Dose and time effect of CdTe quantum dots on antioxidant capacities of the liver and kidneys in mice. Int J Nanomed. 2017;12:6425–6435. doi:10.2147/IJN.S142008
187. Stan MS, Memet I, Sima C, et al. Si/SiO2 quantum dots cause cytotoxicity in lung cells through redox homeostasis imbalance. Chem Biol Interact. 2014;220:102–115. doi:10.1016/j.cbi.2014.06.020
188. Ruan F, Liu R, Wang K, et al. Cytotoxicity of black phosphorus quantum dots on lung-derived cells and the underlying mechanisms. J Hazard Mater. 2021;402:122875. doi:10.1016/j.jhazmat.2020.122875
189. Cao X, Pan X, Couvillion SP, et al. Fate, cytotoxicity and cellular metabolomic impact of ingested nanoscale carbon dots using simulated digestion and a triculture small intestinal epithelial model. NanoImpact. 2021;23:100349. doi:10.1016/j.impact.2021.100349
190. Rak-Raszewska A, Marcello M, Kenny S, et al. Quantum dots do not affect the behaviour of mouse embryonic stem cells and kidney stem cells and are suitable for short-term tracking. PLoS One. 2012;7(3):e32650. doi:10.1371/journal.pone.0032650
191. Yukawa H, Baba Y. In Vivo Fluorescence Imaging and the Diagnosis of Stem Cells Using Quantum Dots for Regenerative Medicine. Anal Chem. 2017;89(5):2671–2681. doi:10.1021/acs.analchem.6b04763
192. Tsoi KM, Dai Q, Alman BA, et al. Are Quantum Dots Toxic? Exploring the Discrepancy Between Cell Culture and Animal Studies. Acc. Chem. Res. 2013;46(3):662–671. doi:10.1021/ar300040z
193. Nel AE, Madler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–557. doi:10.1038/nmat2442
194. Lai Y, Dong L, Sheng X, et al. Monitoring the Cd(2+) release from Cd-containing quantum dots in simulated body fluids by size exclusion chromatography coupled with ICP-MS. Anal Bioanal Chem. 2022;414(18):5529–5536. doi:10.1007/s00216-022-03976-x
195. Wang C, Zhu S, Liang Y, et al. Flexible free-standing antibacterial nanoporous Ag ribbon. J Colloid Interface Sci. 2023;645:287–296. doi:10.1016/j.jcis.2023.04.153
196. Chen T, Li L, Xu G, et al. Cytotoxicity of InP/ZnS Quantum Dots With Different Surface Functional Groups Toward Two Lung-Derived Cell Lines. Front Pharmacol. 2018;9:763. doi:10.3389/fphar.2018.00763
197. Anastasiadis SH, Chrissopoulou K, Stratakis E, et al. How the Physicochemical Properties of Manufactured Nanomaterials Affect Their Performance in Dispersion and Their Applications in Biomedicine: a Review. Nanomaterials (Basel). 2022;12(3):552. doi:10.3390/nano12030552
198. Sukhanova A, Bozrova S, Sokolov P, et al. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res Lett. 2018;13(1):44. doi:10.1186/s11671-018-2457-x
199. Sarkar A, Ghosh M, Sil PC. Nanotoxicity: oxidative stress mediated toxicity of metal and metal oxide nanoparticles. J Nanosci Nanotechnol. 2014;14(1):730–743. doi:10.1166/jnn.2014.8752
200. Yuan LQ, Wang C, Lu DF, et al. Induction of apoptosis and ferroptosis by a tumor suppressing magnetic field through ROS-mediated DNA damage. Aging (Albany NY). 2020;12(4):3662–3681. doi:10.18632/aging.102836
201. Seyedjavadi SS, Khani S, Eslamifar A, et al. The Antifungal Peptide MCh-AMP1 Derived From Matricaria chamomilla Inhibits Candida albicans Growth via Inducing ROS Generation and Altering Fungal Cell Membrane Permeability. Front Microbiol. 2019;10:3150. doi:10.3389/fmicb.2019.03150
202. Sun H, Cui E, Liu R. Molecular mechanism of copper-zinc superoxide dismutase activity change exposed to N-acetyl-L-cysteine-capped CdTe quantum dots-induced oxidative damage in mouse primary hepatocytes and nephrocytes. Environ Sci Pollut Res Int. 2015;22(22):18267–18277. doi:10.1007/s11356-015-5035-0
203. Nocella C, Cammisotto V, Pigozzi F, et al. Impairment between Oxidant and Antioxidant Systems: short- and Long-term Implications for Athletes’ Health. Nutrients. 2019;11(6):1353. doi:10.3390/nu11061353
204. Mohamed HRH. Estimation of genomic instability and mitochondrial DNA damage induction by acute oral administration of calcium hydroxide normal- and nano- particles in mice. Toxicol Lett. 2019;304:1–12. doi:10.1016/j.toxlet.2018.12.012
205. Phatvej W, Datta HK, Wilkinson SC, et al. Endocytosis and Lack of Cytotoxicity of Alkyl-Capped Silicon Quantum Dots Prepared from Porous Silicon. Materials (Basel). 2019;12(10):1702. doi:10.3390/ma12101702
206. Zhan Q, Tang M. Research advances on apoptosis caused by quantum dots. Biol Trace Elem Res. 2014;161(1):3–12. doi:10.1007/s12011-014-0068-7
207. Manshian BB, Martens TF, Kantner K, et al. The role of intracellular trafficking of CdSe/ZnS QDs on their consequent toxicity profile. J Nanobiotechnology. 2017;15(1):45. doi:10.1186/s12951-017-0279-0
208. Derfus AM, Chan WCW, Bhatia SN. Probing the Cytotoxicity Of Semiconductor Quantum Dots. Nano Lett. 2004;4(1):11–18. doi:10.1021/nl0347334
209. Kirchner C, Liedl T, Kudera S, et al. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005;5(2):331–338. doi:10.1021/nl047996m
210. Jiaxin S, Shengchen W, Yirong C, et al. Cadmium exposure induces apoptosis, inflammation and immunosuppression through CYPs activation and antioxidant dysfunction in common carp neutrophils. Fish Shellfish Immunol. 2020;99:284–290. doi:10.1016/j.fsi.2020.02.015
211. Chwalba A, Orlowska J, Slota M, et al. Effect of Cadmium on Oxidative Stress Indices and Vitamin D Concentrations in Children. J Clin Med. 2023;12(4):1572. doi:10.3390/jcm12041572
212. Nagaraju R, Kalahasthi R, Balachandar R, et al. Cadmium exposure and DNA damage (genotoxicity): a systematic review and meta-analysis. Crit Rev Toxicol. 2022;52(10):786–798. doi:10.1080/10408444.2023.2173557
213. Havrdova M, Urbancic I, Barton Tomankova K, et al. Self-Targeting of Carbon Dots into the Cell Nucleus: diverse Mechanisms of Toxicity in NIH/3T3 and L929 Cells. Int J Mol Sci. 2021;22(11):5608. doi:10.3390/ijms22115608
214. Soriano GB, da Silva Oliveira R, Camilo FF, et al. Interaction of non-aqueous dispersions of silver nanoparticles with cellular membrane models. J Colloid Interface Sci. 2017;496:111–117. doi:10.1016/j.jcis.2017.02.017
215. McShan D, Ray PC, Yu H. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 2014;22(1):116–127. doi:10.1016/j.jfda.2014.01.010
216. Zhang T, Wang Y, Kong L, et al. Threshold Dose of Three Types of Quantum Dots (QDs) Induces Oxidative Stress Triggers DNA Damage and Apoptosis in Mouse Fibroblast L929 Cells. Int J Environ Res Public Health. 2015;12(10):13435–13454. doi:10.3390/ijerph121013435
217. Wang J, Jiang M, Wan G, et al. Exposure to ZnO nanoparticles induced blood-milk barrier dysfunction by disrupting tight junctions and cell injury. Toxicol Lett. 2023;384:63–72. doi:10.1016/j.toxlet.2023.07.004
218. Bai C, Wei T, Zou L, et al. The apoptosis induced by CdTe quantum dots through the mitochondrial pathway in dorsal root ganglion cell line ND7/23. J Appl Toxicol. 2022;42(7):1218–1229. doi:10.1002/jat.4291
219. Dong P, Li JH, Xu SP, et al. Mitochondrial dysfunction induced by ultra-small silver nanoclusters with a distinct toxic mechanism. J Hazard Mater. 2016;308:139–148. doi:10.1016/j.jhazmat.2016.01.017
220. Paesano L, Perotti A, Buschini A, et al. Markers for toxicity to HepG2 exposed to cadmium sulphide quantum dots; damage to mitochondria. Toxicology. 2016;374:18–28. doi:10.1016/j.tox.2016.11.012
221. Song B, Zhou T, Yang W, et al. Contribution of oxidative stress to TiO(2) nanoparticle-induced toxicity. Environ Toxicol Pharmacol. 2016;48:130–140. doi:10.1016/j.etap.2016.10.013
222. Pi H, Xu S, Zhang L, et al. Dynamin 1-like-dependent mitochondrial fission initiates overactive mitophagy in the hepatotoxicity of cadmium. Autophagy. 2013;9(11):1780–1800. doi:10.4161/auto.25665
223. Wei X, Qi Y, Zhang X, et al. Cadmium induces mitophagy through ROS-mediated PINK1/Parkin pathway. Toxicol Mech Methods. 2014;24(7):504–511. doi:10.3109/15376516.2014.943444
224. Yu KN, Sung JH, Lee S, et al. Inhalation of titanium dioxide induces endoplasmic reticulum stress-mediated autophagy and inflammation in mice. Food Chem Toxicol. 2015;85:106–113. doi:10.1016/j.fct.2015.08.001
225. Chen R, Huo L, Shi X, et al. Endoplasmic reticulum stress induced by zinc oxide nanoparticles is an earlier biomarker for nanotoxicological evaluation. ACS Nano. 2014;8(3):2562–2574. doi:10.1021/nn406184r
226. Wei F, Xie Q, Huang Z, et al. Induction of autophagy and endoplasmic reticulum autophagy caused by cadmium telluride quantum dots are protective mechanisms of yeast cell. J Appl Toxicol. 2022;42(7):1146–1158. doi:10.1002/jat.4282
227. Simard J-C, Vallieres F, de Liz R, et al. Silver nanoparticles induce degradation of the endoplasmic reticulum stress sensor activating transcription factor-6 leading to activation of the NLRP-3 inflammasome. J Biol Chem. 2015;290(9):5926–5939. doi:10.1074/jbc.M114.610899
228. Condello M, De Berardis B, Ammendolia MG, et al. ZnO nanoparticle tracking from uptake to genotoxic damage in human colon carcinoma cells. Toxicol In Vitro. 2016;35:169–179. doi:10.1016/j.tiv.2016.06.005
229. Wu K, Zhou Q, Ouyang S. Direct and Indirect Genotoxicity of Graphene Family Nanomaterials on DNA-A Review. Nanomaterials (Basel). 2021;11(11):2889. doi:10.3390/nano11112889
230. Tian J, Hu J, Liu G, et al. Altered Gene expression of ABC transporters, nuclear receptors and oxidative stress signaling in zebrafish embryos exposed to CdTe quantum dots. Environ Pollut. 2019;244:588–599. doi:10.1016/j.envpol.2018.10.092
231. Kumar S, Meena R, Paulraj R. Role of Macrophage (M1 and M2) in Titanium-Dioxide Nanoparticle-Induced Oxidative Stress and Inflammatory Response in Rat. Appl Biochem Biotechnol. 2016;180(7):1257–1275. doi:10.1007/s12010-016-2165-x
232. Wu Z, Stangl S, Hernandez-Schnelzer A, et al. Functionalized Hybrid Iron Oxide-Gold Nanoparticles Targeting Membrane Hsp70 Radiosensitize Triple-Negative Breast Cancer Cells by ROS-Mediated Apoptosis. Cancers (Basel). 2023;15(4):1167.
233. Pasquali F, Agrimonti C, Pagano L, et al. Nucleo-mitochondrial interaction of yeast in response to cadmium sulfide quantum dot exposure. J Hazard Mater. 2017;324(Pt B):744–752. doi:10.1016/j.jhazmat.2016.11.053
234. Conroy J, Byrne SJ, Gun’ko YK, et al. CdTe nanoparticles display tropism to core histones and histone-rich cell organelles. Small. 2008;4(11):2006–2015. doi:10.1002/smll.200800088
235. Indra R, Cerna T, Heger Z, et al. Ellipticine-loaded apoferritin nanocarrier retains DNA adduct-based cytochrome P450-facilitated toxicity in neuroblastoma cells. Toxicology. 2019;419:40–54. doi:10.1016/j.tox.2019.03.009
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



