Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
A Retrospective Study on Predicting Recurrence of Intermediate-Stage Hepatocellular Carcinoma After Radical Therapy
Authors Han R , Gan L , Lang M, Li G, Chen L , Tian X, Zhu K, Sun L, Song T
Received 13 November 2023
Accepted for publication 5 January 2024
Published 12 January 2024 Volume 2024:11 Pages 51—64
DOI https://doi.org/10.2147/JHC.S449441
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Ruyu Han,* Leijuan Gan,* Mengran Lang,* Guangtao Li, Lu Chen, Xindi Tian, Kangwei Zhu, Liyu Sun, Tianqiang Song
Department of Hepatobiliary Cancer, Liver Cancer Center, Tianjin Medical University Cancer Institute & Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, Tianjin Key Laboratory of Digestive Cancer, Tianjin, 300060, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tianqiang Song, Department of Hepatobiliary Cancer, Liver Cancer Center, Tianjin Medical University Cancer Institute & Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, Tianjin Key Laboratory of Digestive Cancer, Tianjin, 300060, People’s Republic of China, Tel +86-022-23340123, Fax +86-022-23537796, Email [email protected]
Purpose: This study aimed to investigate the potential benefits of radical therapy in patients with stage B disease.
Patients and Methods: A retrospective analysis was conducted on a cohort of 437 patients diagnosed with stage B hepatocellular carcinoma, who underwent either hepatic resection (HR) or radiofrequency ablation (RFA) at the Cancer Institute and Hospital of Tianjin Medical University from May 2011 to May 2022. Multivariate COX regression analysis was performed to identify the independent prognostic factors related to recurrence-free survival (RFS). The performance of the developed nomogram was evaluated using various statistical measures, including the concordance index (C-index), receiver operating characteristic (ROC) curve, calibration curve, and decision curve analysis (DCA).
Results: Multivariate analysis revealed that tumor diameter, number of tumors, number of involved liver segments, alpha-fetoprotein (AFP), carbohydrate antigen 19– 9 (CA19-9), lactate dehydrogenase (LDH), and systemic immune inflammation index (SII) were independent prognostic factors influencing patients’ RFS, and these factors were incorporated into the nomogram. The C-index of the nomogram in the training cohort was 0.721, and the AUC at 2 and 3 years was 0.772 and 0.790, respectively. These values were appreciably higher than commonly used clinic staging systems and other predictive models. The calibration curve and DCA demonstrated good calibration and net benefit. Survival analysis comparing stage B patients who received radical treatment with stage A patients with multiple lesions did not reveal a significant difference in Kaplan-Meier survival curves (P=0.91).
Conclusion: The nomogram provided a precise prediction of the recurrence for stage B hepatocellular carcinoma patients undergoing radical treatment. Furthermore, certain stage B patients may benefit from radical treatment.
Keywords: nomogram, Barcelona clinic Liver cancer system, recurrence-free survival, hepatic resection, radiofrequency ablation
Introduction
Primary liver cancer is a highly prevalent malignancy worldwide, ranking sixth in terms of incidence and third in terms of cancer-related mortality. Hepatocellular carcinoma (HCC) accounts for a significant majority, approximately 75–85%, of primary liver cancer cases.1 Accurate assessment of patient prognosis is crucial for effective treatment strategies. In clinical practice, the BCLC staging system, originally proposed in 19992 and subsequently updated based on research involving both untreated and treated patients, is widely employed for staging HCC, determining prognosis, and guiding treatment options.3–6 The BCLC staging system has garnered endorsement from reputable organizations such as the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL).7,8 Liver transplantation (LT), RFA, and HR currently serve as potential radical treatment approaches for liver cancer. However, the limited availability of donor organs greatly impedes the widespread implementation of LT.9 As a result, HR and RFA emerge as the primary available options for radical treatment in patients diagnosed with HCC. Although the BCLC system suggests that HR and RFA should only be offered to patients in the early stages (BCLC stage 0/A) of HCC, there remains ongoing debate regarding therapeutic strategies for patients in the stage B, given their highly heterogeneous population.10 Recent studies have indicated the effectiveness and safety of HR in stage B patients,11–13with some reports even indicating its superiority over transarterial chemoembolization (TACE).14–16 The primary goal of a staging system is to divide patients into subgroups with significantly distinct prognoses. However, the wide range of reported survival rates for stage B patients suggests that the BCLC classification fails to fully achieve this objective. Consequently, there is an urgent need to further subdivide the intermediate-term patient population for a more accurate assessment of their prognostic performance. Additionally, the clarification of the potential benefits of hepatic resection (HR) or radiofrequency ablation (RFA) for stage B patients is of utmost importance. Therefore, we undertook an analysis to identify the relevant factors that impact the prognosis of stage B patients who undergo HR or RFA, and subsequently categorized the patients based on their risk factors. The aim was to screen out the patient population that could benefit from HR or RFA, and thus provide a reference for the selection of treatment options for stage B patients.
Materials and Methods
Patients and Data Collection
We collected data retrospectively from patients diagnosed with multiple HCC who underwent HR or RFA at the Cancer Institute and Hospital of Tianjin Medical University between May 2011 and May 2022. The inclusion criteria involved: (A) individuals aged 18 years or older; (B) receiving HR,RFA or a combination of both; (C) postoperative pathological confirmation of HCC or clinical diagnosis following the latest clinical guideline when postoperative pathology was unavailable; (D) absence of large-vessel invasion or distant metastasis; (E) Child-Pugh class A or B hepatic function with a Performance Status score of 0; (F) preoperative CT or MRI scans indicating either 2–3 tumors with a maximum diameter exceeding 3 cm or 4 or more tumors. The exclusion criteria involved: (A) non-R0 grade hepatectomy or incomplete ablation; (B) History of other malignant tumors (C) incomplete clinical data; (D) death or loss to follow-up within one month post-surgery. Among the 437 patients enrolled in our institution, they were randomly allocated to a training cohort (n=305) and a validation cohort (n=132) in a 7:3 ratio. Figure 1 shows the flow chart of the patient selection process.
 |
Figure 1 Flowchart of patient selection. Abbreviation: BCLC, Barcelona Clinic Liver Cancer classification. |
Before undergoing surgical treatment, the feasibility of surgery for all patients was determined by two or more experienced radiologists and clinicians. HR is the preferred treatment option for patients presenting with tumors limited to a single liver segment or lobe, whereas RFA is favored with smaller lesions situated deeper within the liver parenchyma. The determination of the precise surgical approach is made by two or more experienced clinicians, who considered factors such as tumor size, location, and liver function.
All patients underwent thorough preoperative preparation and examination. Blood samples were collected within a three-day timeframe prior to the surgery. CT or MRI was performed for evaluation within one month prior to surgery. The collected information encompassed a variety of data points, such as age, gender, smoking history, alcohol consumption history, hepatitis status, tumor size, number of tumors, preoperative AFP levels, CA19-9 levels, postoperative pathology information, and the type of surgery performed, among other variables. The preoperative liver function of patients was assessed using Child-Pugh and ALBI classifications.17–19
During data collection, all researchers remained blind to clinical outcomes. In accordance with the Declaration of Helsinki (as revised in 2013), this study was conducted. Approval for the research was obtained from the ethics committee of Tianjin Medical University Cancer Institute & Hospital, and the requirement for informed consent was waived. The research adhered to Good Clinical Practice Guidelines and relevant local regulations. Data with the potential to reveal the identities of individuals were anonymized and de-identified prior to analysis.
Assessment and Follow-Up
Each patient underwent consistent surveillance one month post therapy, adopting tri-monthly assessments for the first two years post-procedure, with subsequent semiannual to annual evaluations thereafter. Evaluations encompassed customary hematological examinations, hepatic functionality assessments, oncologic biomarker profiling, as well as the utilization of abdominal ultrasound or contrast-enhanced CT or MRI scans. An independent review of all imaging examinations was conducted by two or more qualified radiologists.
Postoperative mortality in patients with HCC is primarily caused by recurrence. Thus, we compared the long-term outcomes of these cases by analyzing RFS. RFS was defined as the period between the surgical procedure and the identification of recurrence. For patients who did not visit the hospital, follow-up information was obtained via telephone.
Statistical Analyses
R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS version 25.0 (SPSS, Chicago, IL, USA) were used for all statistical analyses. X-tile software was used to calculate optimal thresholds for preoperative serum AFP, CA19-9, LDH, and SII data based on RFS.
Continuous variables were expressed as median values with interquartile ranges (IQR). Student’s t-tests were employed when the data met the normality hypothesis and the variances were homogeneous. Otherwise, the Wilcoxon rank-sum test was used. Categorical variables were presented as numbers and percentages. The chi-square test or Fisher exact test was utilized for intergroup comparisons of categorical variables. Using Kaplan-Meier (K–M) analysis, we estimated the RFS rate and compared survival curves using Log rank tests. All reported p-values were two-sided, with significance set at p < 0.05.
Results
Basic Characteristics
Among the entire cohort of 437 patients, a total of 319 patients underwent HR, 24 patients underwent RFA, and 94 patients received a combination of both interventions. The outcome variable used for analysis was RFS. Notably, there were no statistically significant differences between the training and validation cohorts in clinicopathological baseline characteristics, as indicated in Table 1.
 |
Table 1 Clinicopathologic Characteristics of Patients in Two Cohorts |
Development of the Prognostic Nomogram
Initially, the training cohort was analyzed using univariate and multivariate Cox regressions, using RFS as the outcome variable. It was decided to include in the multivariate analysis those variables that showed significant results in the univariate analysis. A total of 7 factors, including tumor diameter, number of tumors, number of involved liver segments, AFP, CA19-9, LDH, and SII, were ultimately selected for inclusion in the final model (Table 2). These 7 factors were utilized to develop nomograms for predicting RFS (Figure 2). The scores assigned on the nomogram reflect the predictive probability associated with each factor, and the cumulative score of all factors indicates the anticipated likelihood of recurrence for the patient at 2 and 3 years.
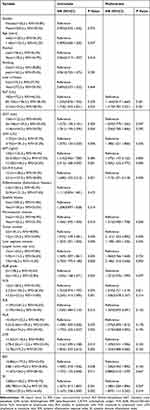 |
Table 2 Univariate and multivariate analyses of prognostic factors based on RFS |
Validation of the Prognostic Nomogram
In the training cohort, the nomogram demonstrated a C-index of 0.721 (95% CI, 0.702–0.740). Similarly, in the validation cohort, the C-index reached 0.720 (95% CI, 0.694–0.747). The calibration curves for the 2- and 3-year RFS of both the training and validation cohorts illustrated excellent concordance between the predictions provided by the nomogram and the actual observed outcomes. (Figure 3A–D). ROC curves plotting the 2- and 3-year RFS in both cohorts indicated that the AUC values were 0.772 (95% CI, 0.713–0.832) and 0.790 (95% CI, 0.724–0.857), respectively, in the training cohort and 0.750 (95% CI, 0.688–0.812) and 0.758 (95% CI, 0.686–0.830) in the validation cohort (Figure 4A–D).
Comparisons were made with commonly used clinic staging systems and other predictive models via nomogram. In the training cohort, the C-index for TNM, CLIP,20 Tokyo,21 HKLC,22 CNLC, Bolondi,23 Kinki,24 and MICAN.25 subgroup staging were as follows: 0.569 (95% CI, 0.549–0.588), 0.564 (95% CI, 0.544–0.583), 0.576 (95% CI, 0.554–0.597), 0.593 (95% CI, 0.573–0.613), 0.543 (95% CI, 0.528–0.559), 0.580 (95% CI. 0.571–0.599), 0.583 (95% CI, 0.564–0.603), and 0.590 (95% CI, 0.570–0.610). Notably, these scores were significantly lower than that of the nomogram at 0.721. The nomogram showed significantly higher AUC values than other staging systems and predictive models when analyzing time-dependent ROC curves. (Figure 5A). Furthermore, plotting the DCA curves for 2- and 3-year RFS revealed that nomogram provided more favorable predictions compared to other staging systems and prediction models (Figure 5B and C).
Prognostic Stratification Based on the Nomogram
The optimal threshold for prognostic stratification in the training cohort was determined by employing X-tile software, considering the nomogram scores. The resulting optimal threshold was 274, classifying the training cohort and validation cohort into the high recurrence risk (HRR) group (total score ≥274) and the low recurrence risk (LRR) group (total score <274). K-M survival curves were generated to demonstrate a notable disparity in prognosis between HRR and LRR groups in both the training (P<0.0001) and validation cohorts (P<0.0001) (Figure 6A and B).
We gathered data from 178 patients with multiple stage A hepatocellular carcinomas who underwent HR or RFA in our center from May 2011 to May 2022. We conducted a survival analysis by comparing this cohort with individuals in the stage B LRR group. The results revealed that patients in the stage B LRR group exhibited a significantly better prognosis compared to those in stage A (P=0.0051) (Figure 6C). Furthermore, when examining the survival analysis encompassing the entire stage B cohort and stage A patients, we observed no notable difference in prognosis between the two cohorts (P=0.91) (Figure 6D).
Discussion
Intermediate stage (stage B) HCC encompasses a highly heterogeneous population with diverse clinicopathological features and tumor biological behaviors, resulting in varying prognosis.26 Therefore, stage B patients should be treated individually based on their specific clinicopathological characteristics. In 2012, Bolondi et al introduced a subcategorization of intermediate grade HCC.23 This subcategorization has been recognized by many scholars.27,28 For patients meeting the “up-to-7” criteria, liver transplantation is suggested as a possible treatment option, offering better life expectancy than TACE.29 In 2015, Kudo et al developed the “Kinki Criteria”, which recommends HR as an alternative treatment for stage B patients with a Child-Pugh score of 5–6 and who meet the “within up-to-7” criteria.24 Hiraoka et al developed the MICAN criteria in 2016, considering the “albumin-bilirubin (ALBI) grade” and “within up-to-7” criteria.25 Kim et al proposed Kim’s criteria in 2017 for stage B patients undergoing TACE.30 Although studies on stage B patients continue, most have focused on populations undergoing TACE. Several recent studies have reported that radical therapy may provide a better prognosis for certain stage B patients compared to TACE.14–16 The 2022 update of the BCLC staging criteria includes liver transplantation as an alternative treatment for stage B BCLC patients based on tumor size and AFP levels.6 However, it does not mention the feasibility of HR and RFA in stage B patients. Although numerous studies have demonstrated the effectiveness and safety of HR and RFA in stage B patients, it is still unclear which specific subgroup of the population is more appropriate for HR or RFA.11,12 In this study, we specifically analyzed stage B patients who underwent HR or RFA, excluding patients with clinically unresectable disease. This controlled for variables that are difficult to collect and quantify, such as the proximity of tumor to large blood vessels and inadequate residual liver volume.
The aim of this research was to analyze factors influencing the prognosis of stage B patients, stratify patients based on prognosis, provide recommendations for clinical decision-making, and therefore focused on selecting variables that are easy to obtain preoperatively as predictors. A nomogram was finally constructed based on a total of 7 variables that can be easily obtained preoperatively, including tumor diameter, tumor number, number of liver segments involved in the tumor, AFP, CA19-9, LDH and SII. In addition to tumor diameter, number of tumors and AFP, which have long been proven in many studies to have a significant impact on prognosis, the number of liver segments involved in the tumor,31 CA19-9,32 LDH,33,34 and SII,35 have also been studied to prove their impact on prognosis. The nomogram demonstrated reliable predictive performance in both the training and validation cohorts during the subsequent validation process. When compared with other staging systems and prediction models commonly used in clinical practice, our model demonstrated superior predictive efficacy for RFS in stage B patients. In order to categorize patients based on their prognoses, we classified them into two groups, namely HRR and LRR, based on their nomogram scores. Significant differences in prognostic outcomes between the HRR and LRR groups were observed in both the training and validation cohorts. To determine if stage B patients could benefit from radical treatment, we further collected data on BCLC stage A patients with multiple HCC who underwent radical treatment, while excluding patients with single tumors in stage A. The analysis indicated that the prognosis of the LRR group was even better than stage A patients. Moreover, there was no significant difference in prognosis between all patients in stage B and patients in stage A who underwent radical treatment. This suggests that stage B patients, with adequate hepatic functional reserve and resectable tumors, can attain a prognosis comparable to that of stage A patients following radical treatment. However, by excluding patients with a solitary tumor in stage A, the study’s stage A population became unrepresentative of all stage A patients, potentially contributing to the absence of a prognostic difference between stage A and stage B patients. Nonetheless, it partially controlled for the variable of tumor number. To further validate the study’s results, a follow-up propensity score matching study could be conducted comparing stage A and stage B patients who received radical therapy. In addition, patients in the HRR group had a significantly worse prognosis than those in the LRR group. However, since there was no comparison of different treatment modalities for patients in the HRR group, it is not yet possible to conclude that patients in the HRR group are not suitable for surgical treatment. We will follow up with a propensity score matching analysis of patients treated with surgery versus TACE to further investigate the optimal treatment options for patients.
While staging systems commonly used in clinical practice primarily consider morphological aspects like tumor number and size, this study expanded upon the traditional staging system by incorporating serologic indicators such as LDH and SII. This addition increased the complexity of the staging system but also improved prediction accuracy.
Nevertheless, our study has certain limitations. Primarily, it primarily focused on stage B patients who underwent radical treatment, which may not fully represent the entire stage B population. Therefore, it needs to be applied in combination with experienced clinicians after evaluating the patients’ tumor loads and residual liver volume. Secondly, due to its retrospective design, this study inevitably received the effect of selection bias. Lastly, given the single-center design of this study, it is essential to conduct a multicenter study to validate our findings.
Conclusion
We have developed a nomogram that accurately predicts the prognosis of patients with stage B hepatocellular carcinoma who undergo radical treatment. This nomogram aids in patient monitoring and prognosis prediction. Additionally, subsequent research has revealed that stage B patients who have undergone radical surgical treatment can achieve a comparable prognosis to that of stage A patients with multiple lesions.
Acknowledgments
This work is supported by the grant from the National Natural Science Foundation of China (82173317).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338. doi:10.1055/s-2007-1007122
3. Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30(1):61–74. doi:10.1055/s-0030-1247133
4. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. doi:10.1016/S0140-6736(11)61347-0
5. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
6. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
7. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatol. 2018;68(2):723–750. doi:10.1002/hep.29913
8. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatol. 2018;67(1):358–380. doi:10.1002/hep.29086
9. De Carlis L, Di Sandro S, Centonze L, et al. Liver-allocation policies for patients affected by HCC in Europe. Curr Transplant Rep. 2016;3(4):313–318. doi:10.1007/s40472-016-0117-6
10. Chang WT, Kao WY, Chau GY, et al. Hepatic resection can provide long-term survival of patients with non-early-stage hepatocellular carcinoma: extending the indication for resection? Surgery. 2012;152(5):809–820. doi:10.1016/j.surg.2012.03.024
11. Garancini M, Pinotti E, Nespoli S, Romano F, Gianotti L, Giardini V. Hepatic resection beyond Barcelona clinic liver cancer indication: when and how. World J Hepatol. 2016;8(11):513–519. doi:10.4254/wjh.v8.i11.513
12. Roayaie S, Jibara G, Tabrizian P, et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatol. 2015;62(2):440–451. doi:10.1002/hep.27745
13. Vitale A, Burra P, Frigo AC, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona clinic liver cancer stages: a multicentre study. J Hepatol. 2015;62(3):617–624. doi:10.1016/j.jhep.2014.10.037
14. Kapitanov T, Neumann UP, Schmeding M. Hepatocellular carcinoma in liver cirrhosis: surgical resection versus transarterial chemoembolization-a meta-analysis. Gastroenterol Res Pract. 2015;2015:696120. doi:10.1155/2015/696120
15. Lin CT, Hsu KF, Chen TW, et al. Comparing hepatic resection and transarterial chemoembolization for Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma: change for treatment of choice? World J Surg. 2010;34(9):2155–2161. doi:10.1007/s00268-010-0598-x
16. Yin L, Li H, Li AJ, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan criteria: a RCT. J Hepatol. 2014;61(1):82–88. doi:10.1016/j.jhep.2014.03.012
17. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
18. Hiraoka A, Kumada T, Tsuji K, et al. Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: a multicenter analysis. Liver Cancer. 2019;8(2):121–129. doi:10.1159/000488778
19. Hiraoka A, Kumada T, Michitaka K, et al. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31(5):1031–1036. doi:10.1111/jgh.13250
20. Daniele B, Elba S, Capuano G, et al. The CLIP score: a new prognostic system for hepatocellular carcinoma. Hepatol. 1998;28(4):1.
21. Tateishi R, Yoshida H, Shiina S, et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54(3):419–425. doi:10.1136/gut.2003.035055
22. Yau T, Tang VYF, Yao T-J, Fan S-T, Lo C-M, Poon RTP. Development of Hong Kong liver cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146(7):1691–1700.e1693. doi:10.1053/j.gastro.2014.02.032
23. Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32(4):348–359. doi:10.1055/s-0032-1329906
24. Kudo M, Arizumi T, Ueshima K, Sakurai T, Kitano M, Nishida N. Subclassification of BCLC B stage hepatocellular carcinoma and treatment strategies: proposal of modified bolondi’s subclassification (kinki criteria). Dig Dis. 2015;33(6):751–758. doi:10.1159/000439290
25. Hiraoka A, Kumada T, Nouso K, et al. Proposed new sub-grouping for intermediate-stage hepatocellular carcinoma using albumin-bilirubin grade. Oncology. 2016;91(3):153–161. doi:10.1159/000447061
26. Mathew EN, Nadkarni N, Choo SP, et al. BCLC subclassification and tumour characteristics to provide prognostication of outcomes in an Asian population of locally advanced hepatocellular carcinoma treated using selective internal radiation therapy with yttrium-90. J clin oncol. 2018;36(4):443. doi:10.1200/JCO.2018.36.4_suppl.443
27. Giannini EG, Moscatelli A, Pellegatta G, et al. Application of the intermediate-stage subclassification to patients with untreated hepatocellular carcinoma. Am J Gastroenterol. 2016;111(1):70–77. doi:10.1038/ajg.2015.389
28. Scaffaro LA. Survival rates according to Barcelona clinic liver cancer sub-staging system after transarterial embolization for intermediate hepatocellular carcinoma. World J Hepatol. 2015;7(3):628–632. doi:10.4254/wjh.v7.i3.628
29. Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35–43. doi:10.1016/S1470-2045(08)70284-5
30. Kim JH, Shim JH, Lee HC, et al. New intermediate-stage subclassification for patients with hepatocellular carcinoma treated with transarterial chemoembolization. Liver Int. 2017;37(12):1861–1868. doi:10.1111/liv.13487
31. Wang XH, Liu QB, Xiang CL, et al. Multi-institutional validation of novel models for predicting the prognosis of patients with huge hepatocellular carcinoma. Int J Cancer Res. 2021;149(1):127–138. doi:10.1002/ijc.33516
32. Zhang W, Wang Y, Dong X, et al. Elevated serum CA19-9 indicates severe liver inflammation and worse survival after curative resection in hepatitis B-related hepatocellular carcinoma. Biosci Trends. 2022;15(6):397–405. doi:10.5582/bst.2021.01517
33. Wang X, Mao M, He Z, et al. Development and validation of a prognostic nomogram in AFP-negative hepatocellular carcinoma. Int J Bio Sci. 2019;15(1):221–228. doi:10.7150/ijbs.28720
34. Su K, Huang W, Li X, et al. Evaluation of lactate dehydrogenase and alkaline phosphatase as predictive biomarkers in the prognosis of hepatocellular carcinoma and development of a new nomogram. J Hepatocell Carcinoma. 2023;10:69–79. doi:10.2147/JHC.S398632
35. Wang D, Hu X, Xiao L, et al. Prognostic nutritional index and systemic immune-inflammation index predict the prognosis of patients with HCC. World J Gastrointest Surg. 2021;25(2):421–427. doi:10.1007/s11605-019-04492-7
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





