Back to Journals » International Journal of General Medicine » Volume 16
A Retrospective Study of Anlotinib Combined with Anti-PD-1 Inhibitors in the 2nd or Later-Line Treatment of Advanced Solid Tumors
Authors Li SH, Li YW, Li YJ, Liu LB, Zhang Q, Lu D
Received 21 June 2023
Accepted for publication 19 September 2023
Published 4 October 2023 Volume 2023:16 Pages 4485—4498
DOI https://doi.org/10.2147/IJGM.S426590
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Shu-hui Li, Yi-Wen Li, Ying-Jue Li, Lin-Bo Liu, Qun Zhang, Dan Lu
Department of Oncology, The Second Affiliated Hospital of Harbin Medical University, Harbin Medical University, Harbin, 150086, People’s Republic of China
Correspondence: Dan Lu, Department of Oncology, The Second Affiliated Hospital of Harbin Medical University, Harbin Medical University, Harbin, 150086, People’s Republic of China, Tel +86 13845047493, Email [email protected]
Objective: To investigate the clinical efficacy and safety of anlotinib combined with anti-PD-1 inhibitors in the 2nd or later-line treatment of advanced solid tumors.
Patients and Methods: A total of 63 patients with advanced solid tumors who had failed or could not endure the adverse reactions after receiving first-line or more systematic treatment in the Second Affiliated Hospital of Harbin Medical University from March 2019 to April 2023 were treated with anlotinib Hydrochloride capsule combined with anti-PD-1 inhibitors. The efficacy and adverse reactions were evaluated according to RECIST1.1 and NCICTC4.0 standards.
Results: The percentage of overall response rate of 63 patients during the combination administration indicated that complete response was 1.6% (n=1), partial response was 23.8% (n=15), stable disease was 39.7% (n=25) and progressive disease was 34.9% (n=22), yielding objective response rate (ORR) of 25.4% and disease control rate (DCR) of 65.1%. Furthermore, the median PFS of 63 patients with advanced solid tumors was 7 months and the median OS was not reached, and the median follow-up time is 4.5 months. In subgroup analysis, there was no significant difference in PFS between first-line, second-line, third-line and above (p=0.631); there was no significant difference in PFS between PD-1 positive patients and PD-1 negative patients (p=0.094); there was no significant difference in PFS between patients who had previously used anti-PD-1 inhibitors and patients who had not used before (p=0.204). The most common adverse reactions were hypertension, hand-foot syndrome, and fatigue, with an incidence of 28.4% (18/63), 25.6% (14/63), and 25.6% (14/63), respectively. Most of the adverse reactions were grade 1– 2, and there were no grade 4 adverse reactions.
Conclusion: Anlotinib combined with anti-PD-1 inhibitors demonstrated promising efficacy and tolerable safety for patients with advanced solid tumors in the 2nd or later-line treatment.
Keywords: anlotinib, anti-PD-1 inhibitor, advanced solid tumors, adverse reactions
Introduction
In recent years, the overall incidence and mortality of cancer in China are increasing year by year, and cancer has become a major public health problem that seriously threatens the health of Chinese residents.1 Drug therapy is a very important treatment, including chemotherapy, immunotherapy, targeted therapy, endocrine therapy, and so on. Among them, anti-angiogenic drugs and immune checkpoint inhibitors (ICIs) play a more and more important role in the treatment of malignant tumors in recent years.2,3
Continuous and unregulated angiogenesis can be observed during tumor growth.4 It has been proved that angiogenesis is a key step in tumor growth, invasion, and metastasis. Anlotinib (AL3818) is a new type of oral multi-target receptor tyrosine kinase inhibitor independently developed in China, which can target vascular endothelial growth factor receptor-2 and 3 (VEGFR-2,3), fibroblast growth factor receptor-1-4 (FGFR1-4), Platelet-derived growth factor receptor-α, β (PDGFR-α, β), proto-oncogene tyrosine kinase receptor (c-Kit) and Ret to inhibit the transformation of tumor to capillaries and achieve the purpose of inhibiting tumor cell proliferation.5,6 At present, it has been approved for third-line non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), and some soft tissue sarcoma (STS).7 In addition, anlotinib has completed or is conducting a several Phase II/III clinical trials for various cancer types, including STS, renal cell carcinoma, hepatocellular carcinoma, ovarian cancer, esophageal squamous cell carcinoma, gastric cancer, CRC, nasopharyngeal carcinoma, etc.
Immune checkpoint inhibitor (ICI) is an important part of immunotherapy. Among all immune checkpoints, programmed cell death receptor 1 (PD-1)/programmed cell death ligand 1 (PD-L1) pathway stands out because of its value as a target for a large number of malignant tumors. By blocking the interaction between PD-1 and PD-L1, it can restore the anti-tumor activity of T lymphocytes, enhance the immune response, and reduce the proliferation and metastasis of tumor cells.8 So far, from later-line therapy to first-line treatment, from advanced tumors to early and middle tumors, ICIs have shown good anti-tumor efficacy in a variety of solid tumors, such as NSCLC, gastric cancer, esophageal cancer, head and neck squamous cell carcinoma, and have brought survival benefits to many patients, even long-term survival.9,10 The guidelines for multiple tumor species also have been rewritten. This therapy has not only become a new clinical standard, but also a pillar of combined therapy in the future. Despite these successes, only some patients initially responded to monotherapy ICIs, and most patients were inherently resistant to these treatments, in part because of the small number of infiltrating lymphocytes in the tumor bed.11 In addition, most patients eventually develop acquired resistance to ICIs, and the mechanism of drug resistance remains unclear.12
In recent years, some clinical evidence shows the potential of anti-angiogenesis and immune checkpoint inhibitor therapy.13 In a study of anlotinib and anlotinib combined with anti-PD-1 checkpoint inhibitors in the treatment of neuroblastoma (NB) mice, Su found that anlotinib remodeled tumor immune microenvironment through tumor vascular normalization mediated by CD4+ T (Th1) cells, transformed it from cold tumor to hot tumor, and finally activated T cells and treated neuroblastoma.14 The results of an experiment in vitro on gastric cancer showed that the combination of anlotinib and PD-1 monoclonal antibody could significantly improve efficacy.15 Therefore, the anti-angiogenic effect of normalizing tumor microenvironment may improve the effectiveness of immunotherapy.14,15 In addition, the addition of antiangiogenic drugs to immunotherapy can enhance the efficacy of the two drugs in delaying tumor growth by synergistically promoting the activation and release of antigen recognition. And the immunosuppressive tumor microenvironment can be transformed into the immune support microenvironment.12,16–18
At present, many clinical trials to study the efficacy of anti-angiogenic targeted drugs combined with immune checkpoint inhibitors in various solid tumors are also underway.16 This study is a single-center, retrospective study to research the clinical efficacy and safety of anlotinib combined with anti-PD-1 inhibitors in the 2nd or later-line treatment of advanced solid tumors.
Materials and Methods
Design of This Study and Eligibility Criteria
This study is a retrospective study of patients with advanced solid tumors who were treated with anlotinib and anti-PD-1 inhibitors at the second affiliated Hospital of Harbin Medical University from March 2019 to April 2023. The inclusion criteria were: (1) patients with advanced solid tumor confirmed by histology and they had received first-line or more systematic treatment; (2) aged 14 years or older; (3) there was at least one measurable target and showed drug response according to the response evaluation criteria (RECIST1.1) in the solid tumor; (4) patients received anlotinib plus anti-PD-1 inhibitors therapy; and (5) Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 score; Exclusion criteria: (1) previous use of anlotinib; (2) patients were diagnosed with clinically active or symptomatic brain metastases; (3) patients with active or uncontrolled autoimmune disease; (4) accompanied by another serious disease that may endanger the survival of patients, such as severe cerebrovascular diseases, acute myocardial infarction, and advanced liver cirrhosis; (5) curative effect evaluation data are not available. The study profile of the present study is illustrated in Figure 1. Finally, a total of 63 patients with advanced solid tumors were enrolled. All data came from follow-up and medical records.
 |
Figure 1 Flow chart of the retrospective study of anlotinib combined with PD-1 blockades in the treatment of patients with previously treated advanced solid tumors. |
Therapeutic Regimens
The selected patients were treated with anlotinib combined with anti-PD-1 inhibitors. The specific regimens were as follows: Anlotinib (Chia Tai Tianqing Pharmaceutical, China) was administered orally once daily (10 mg or 12mg) on Days 1–14 of a 21-day cycle. At the same time, ICIs were injected intravenously on the first day, once every 3 weeks, as a cycle. Anti-PD-1 inhibitors included sintilimab (200mg/times), toripalimab (240mg/time), camrelizumab (200mg/time), nivolumab (240mg/time), tislelizumab (200mg/time), pambrolizumab (200mg/time), penpulimab (200mg/time), zimberelimab (240mg/time), serplulimab (200mg/time). Treatment continues until the disease progresses or unbearable adverse reactions. Depending on the toxicity during the treatment, the dose of anlotinib is allowed to be adjusted to daily 10mg or 8mg.
Evaluation of Efficacy
The primary endpoint of this study was progression-free survival (PFS), and the secondary endpoint was overall survival (OS), objective response rate (ORR), disease control rate (DCR), and safety of the combination regimen. The tumor was evaluated according to the evaluation standard of solid tumor response (RECIST1.1 version).19 The short-term efficacy was selected to evaluate the therapeutic effect, which was usually defined as 2 cycles after the establishment of combined therapy. Clinical reactions can be divided into complete response (CR), partial response (PR), stable disease (SD) or, progressive disease (PD). Objective response rate (ORR) was defined as the percentage of CR and PR in all patients. DCR is defined as the percentage of CR, PR, and SD in all patients. We call patients with the disease control rate (CR+PR+SD) as responders and patients with PD as non-responders. PFS was the time from the beginning of treatment with anti-PD-1 inhibitors combined with anlotinib to the last visit, including disease progression, death, and no progression of the disease. OS is the time from the beginning of treatment with anti-PD-1 inhibitors combined with anlotinib to death.
Follow-Up and Safety Assessment
When patients are treated in the hospital, the clinical characteristics, adverse reactions, and disease progression status of each patient are collected through the electronic medical record system; follow-up is carried out by phone, text message, WeChat, email, and so on. In addition, adverse reactions during treatment were evaluated and recorded according to the General terminology Standard for adverse events version 4.0 (CTC4.0). It is recommended that patients with grade 1 or 2 adverse events continue to take medication and follow up in the outpatient department. Patients with grade 3 or more adverse events should stop taking medication and, if necessary, be hospitalized for symptomatic treatment until the adverse events are less than grade 2. If the patient is tolerant, continue to take the drug, and stop taking the drug if the patient’s safety is threatened or serious sequelae are suspected.
Statistical Analysis
Statistical analysis was carried out by R.4.1.3 and SPSS statistical software (version 26.0; SPSS, IBM Corporation). The counting data is expressed in the number of cases and the rate (%). If the measurement data are in line with the normal distribution, it will be expressed by (x±s), and if it does not conform to the normal distribution, it will be expressed by the median. PFS was analyzed by the Kaplan-Meier method, and subgroups were compared with the Log rank test for the total number of patients.
Results
Patient Characteristics
From March 2019 to April 2023, 63 patients with advanced solid tumors were enrolled, including 37 males (58.7%) and 26 females (41.3%), with a median age of 62 (15–82) years. According to the ECOG score, 0–1 score was found in 46 cases (73%) and 2 score in 17 cases (27%). The histopathology types included non-small cell lung cancer (n=21, 33.3%), sarcoma (osteosarcoma, shoulder, and back myofibrosarcoma, hepatic angiosarcoma, pleomorphic rhabdomyosarcoma, renal sarcoma, pulmonary sarcoma, uterine leiomyosarcoma, pelvic liposarcoma) (n=8, 12.7%), small cell lung cancer (n=7, 11.1%), ovarian cancer (n=4, 6.3%), cervical squamous cell carcinoma (n=3, 4.8%), breast cancer (n=3, 4.8%), pancreatic cancer (n=3, 4.8%), renal pelvis carcinoma (n=2, 3.2%), liver cancer (n=2, 3.2%), colorectal cancer (n=2, 3.2%), thoracic adenocarcinoma (n=2, 3.2%), unknown primary tumor (n=2, 3.2%), gastric cancer (n=1, 1.6%), renal cell carcinoma (n=1, 1.6%), esophageal cancer (n=1, 1.6%), pelvic mucinous carcinoma (n=1, 1.6%). Among the patients who used anti-PD-1 inhibitors, 27 cases (28.6%) were treated with sintilimab, 13 cases (20.6%) with toripalimab, 8 cases (12.7%) with camrelizumab, 5 cases (7.9%) with tislelizumab, 3 cases (4.8%) with penpulimab, 3 cases (4.8%) with Pabolizumab, and 2 cases (3.2%) with zimberelimab, 1 case (1.6%) with nivolumab and 1 case (1.6%) with serplulimab. In addition, 18 cases (28.6%) had received first-line treatment, 13 cases (20.6%) had second-line treatment, and 32 cases (50.8%) had third-line and above. Among them, 17 cases (27.0%) were positive for PD-L1 detection, 18 cases (28.6%) were negative, and 28 cases (44.4%) were not tested. Anti-PD-1 inhibitors were used in 20 patients (31.7%) and unused in 43 patients (68.3%). All patients could evaluate the efficacy and adverse reactions. The baseline characteristics of the patients in the group are shown in Table 1.
 |
Table 1 Demographics and Baseline Characteristics of Patients |
Efficacy and Survival
As of April 2023, 31 patients (49.2%) with advanced solid tumors had completed at least 6 cycles of treatment, the median follow-up time is 4.5 months, and 15 cases (23.8%) had received treatment for less than 3 cycles due to disease progression or intolerance, with a median of 6 (1–43) cycles. According to the clinical efficacy evaluation criteria of RECIST1.1 solid tumor, 1 case (1.6%) of CR was observed, 15 cases (23.8%) reached PR, 25 cases (39.7%) had SD, 22 cases (34.9%) developed PD, of which 10 cases (15.9%) died. ORR was 25.4% and DCR was 65.1% (Table 2). 4 patients stopped treatment due to adverse reactions, but all of them were evaluated as PR or SD during treatment. The therapeutic effect is shown in Table 3. The median PFS of all patients was 7 months (Figure 4). The swimmer plot, waterfall plot, and PFS survival curve function are shown in Figures 2, 3 and 4, respectively. The median OS has not been reached. In the statistics of different cohorts, there was a significant difference in PFS between male and female patients in terms of gender (p=0.007). In terms of age, there was no significant difference in PFS between patients ≤ 60 years old and > 60 years old (p= 0.356). In terms of ECOG score, there was no significant difference in PFS between ECOG scores 0–1 and 2 (p=0.434). In terms of drug types, we compared sintilimab with the PFS of other anti-PD-1 inhibitors. The results showed that there was no significant difference in PFS between patients with sintilimab and other anti-PD-1 inhibitors (p=0.861). In the subgroup analysis, there was no significant difference in PFS between the patients with first-line, second-line, third-line, and above (p=0.631), as shown in Figure 5A. There was no significant difference in PFS between the patients with positive and negative PD-1 detection in Figure 5B (p= 0.094), and there was no significant difference in PFS between patients who had used anti-PD-1 inhibitors before and those who had not used before (p=0.204), as shown in Figure 5C.
 |
Table 2 Validity Analysis |
 |
Table 3 mPFS, 95% Confidence Interval (CI) and p value of Different Baseline Characteristics in Patients with Advanced Solid Tumor |
 |
Figure 4 Kaplan-Meier estimates of progression-free survival in all patients. |
 |
Figure 5 Subgroup analysis of PFS in patients with solid tumors according to treatment line (A), PD-L1(+)/PD-L1(-) (B), history of PD-1 inhibitors (C). |
Adverse Reaction
Common treatment emergent adverse events (TEAEs) are shown in Table 4. The most common adverse events were hypertension (28.6%) and hand-foot syndrome (25.4%). Most of the adverse reactions were in grade 1–2, and no grade 4 adverse reactions occurred. 4 patients stopped treatment due to adverse reactions, but their condition was evaluated and all reached PR or SD.
 |
Table 4 Treatment-Related Adverse Events in the Total Treated Patients |
Discuss
At present, treatment options for advanced cancer are still limited, and later-line treatment for advanced cancer is usually based on the experience of each doctor. Therefore, there is an urgent need for new treatments. The development of targeted therapy and immunotherapy, including anlotinib, has promoted a new era of personalized cancer treatment. Anti-angiogenesis therapy targeting tumor vascular endothelial cells is a promising treatment in tumor later-line therapy.6,20 Hanet et al ALTER0302 study21 and subsequent ALTER0303 study22 included Phase II and III clinical trials, respectively, to determine the efficacy and safety of anlotinib in the treatment of recurrent advanced NSCLC. The results showed that the median PFS of anlotinib group was prolonged by 3.6 months and 4.0 months, respectively. The comparisons between ORR and the placebo group in two clinical trials were 10.0% vs 0% and 9.2% vs 0.7%, respectively, which were all significantly better than those in the placebo group. There were significant differences in median OS (9.6 months vs 6.3 months) and DCR (81.0% vs 37.1%) in the ALTER0303 study, and the most common grade 3 adverse reactions were hypertension and hyponatremia. Similarly, in the ALTER1202 study,23 anlotinib prolonged the PFS of SCLC patients by 3.4 months, reducing the risk of disease progression or death by 81%. Anlotinib later-line therapy can significantly improve the PFS of patients with SCLC. However, anlotinib monotherapy inevitably faces the problem of drug resistance in the later stage of cancer treatment.
Similarly, ICIs monotherapy also faces the problem of drug resistance in the later-line of tumor treatment. Recent studies have shown that the combination of ICIs and antiangiogenic drugs may be a promising therapeutic strategy that can overcome the low efficacy of ICIs. Anti-angiogenic inhibitors, such as VEGF inhibitors, such as bevacizumab, can promote T-cell infiltration into solid tumors and improve the efficacy of immunotherapy.24 Preclinical studies have shown that mouse models using VEGFR inhibitors combined with adoptive T-cell transfer have shown that down-regulation of VEGF is the cause of increased T-cell infiltration.25 In addition, through the direct anticancer effect of inhibiting tumor growth and metastasis, anti-angiogenic drugs reprogram the tumor environment from immunosuppression to the immune-permissible microenvironment. Because both anti-angiogenesis and immune checkpoint blocking focus on targeting the tumor microenvironment, the combination of ICIs and antiangiogenic agents has a potential synergistic antitumor effect.26 A multicenter, single-group, prospective II phase trial to evaluate the efficacy and safety of sintilimab combined with anlotinib in second-line or follow-up treatment of recurrent or metastatic cervical cancer with PD-L1 positive (comprehensive positive score≥1),27 forty-two patients were recruited. Among the 39 patients who could evaluate the curative effect, the objective remission rate was 59.0%; the disease control rate was 94.9%. The median progression-free survival time was 9.4 months. The median OS was not reached. 85.8% of the patients experienced treatment-related side effects. The most common treatment-related side effects were hypothyroidism (33.3%), elevated levels of aspartate aminotransferase (21.4%), and hypertension (19.0%). This study increased ORR by 46.8% compared to a study of a single drug pembrolizumab in the treatment of recurrent or metastatic cervical cancer (KEYNOTE-158).28 This study shows that sintilimab combined with anlotinib is effective and safe as second-line or later-line therapy for patients with advanced cervical cancer who have previously failed chemotherapy. In some patients with previously treated small-cell lung cancer, anlotinib combined with PD-1 inhibitors showed more significant efficacy and safety than anlotinib or immune checkpoint inhibitors alone.29–31
However, there are relatively few real-world studies of anlotinib combined with PD-1 inhibitors in the 2nd or later-line treatment of patients with advanced solid tumors. Therefore, we further explore this issue through a retrospective analysis of clinical data. In this study, anlotinib combined with anti-PD-1 inhibitors demonstrated promising antitumor activity with favorable response rate, durable response, and tolerable toxicity profile in patients with advanced solid tumors, of which 1 (1.6%) achieved CR, 15 (23.8%) achieved PR, 25 (39.7%) achieved SD and 22 (34.9%) achieved PD. ORR was 25.4%, and DCR was 65.1%. The media PFS was 7 months (95% CI:3.7~10.2), and media OS has not yet been achieved. Compared with a recent study32 of anlotinib combined with PD-1 inhibitors in the treatment of advanced solid tumors, mPFS increased tumor for 2.3 months and ORR increased by 8.7%. DCR was lower than that in this study (65.1% vs 80.8%). The grade 4 or 5 adverse effects were also not observed, which is similar to our study.
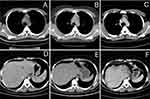
In our study, three groups of subgroups were analyzed, that is, the number of treatment lines, the results of the PD-L1 status test, and whether or not PD-1 inhibitors were used in the past. There was no significant difference in PFS between each subgroup. In PD-L1 status, several meta-analysis33,34 had demonstrated that PD-1/PD-L1 inhibitors significantly improved OS in both PD-L1 positive and PD-L1 negative patients, because of the limitation of the clinical value that PD-L1 was used as biomarker, the conclusion may offer a treatment option for the patients who are negative in PD-L1 status. There was a significant difference in PFS between different genders (p=0.007), the baseline characteristics of the different genders of patients in the group are shown in Table 5, but there was no significant difference in different ages, ECOG scores, and the type of anti-PD-1 inhibitors (sintilimab compared with other anti-PD-1 inhibitors). The reason for the significant difference in PFS between different genders may be due to the high proportion of lung cancer in male patients in this study. The proportion of later-line therapy is more than that of male patients, and the sample size of this study is smaller, so there is a significant difference in PFS between females and males. Among them, 2 patients were treated with anlotinib combined with toripalimab. One was a 14-year-old boy who was diagnosed with unresectable advanced thymic lymphoepithelioma-like carcinoma (LELC), with liver metastasis. Relapsed after receiving chemotherapy with paclitaxel and carboplatin for six cycles, the boy developed bone marrow metastasis. As an alternative treatment, the patient was treated with anlotinib combined with toripalimab, and there was no disease progression for 43 months. The condition was evaluated as PR (Figure 6). The other was a 58-year-old man who was initially diagnosed with advanced gastric adenocarcinoma. After diagnosed, the metastatic lesion was enlarged after 5 cycles of docetaxel combined with raltitrexed chemotherapy, so the patient was treated with anlotinib combined with toripalimab. After 7 cycles, the gastric lesions were completely relieved, and the metastatic lesions were stable (Figure 7). There was no disease progression for 42 months, and the overall condition was evaluated as PR. In this study, it was found that the adverse reactions were tolerable for the vast majority of patients, and most of the adverse reactions were grade 1–2. After the occurrence of grade 3 adverse reactions, some patients could continue to be treated after reducing medication or stopping 1–2 cycles of treatment. There were no grade 4 adverse reactions and no death caused by adverse reactions. 4 patients stopped treatment due to adverse reactions because of follow-up safety considerations, and did not reach grade 4 adverse reactions.
 |
Table 5 Demographics and Baseline Characteristics of Different Genders of Patients |
Limitations were observed in the present study, inevitably. First of all, this study was retrospective, involving only one center, and there was no comparative study in the control group. Secondly, because of the treatment of patients during the spread of novel coronavirus and regional reasons, the compliance of patients was relatively lower than during the non-epidemic period, so the treatment outcome could be different. In addition, this was a small sample study, and there were many kinds of tumors, and the efficacy of statistics of each kind of tumor was less convincing. Give the above limitations, our conclusion may need a larger sample size to further confirm.
Conclusion
In conclusion, anlotinib combined with PD-1 inhibitor is effective and well tolerated in the 2nd or later-line treatment of advanced solid tumors, which can be used as a choice for the treatment of advanced solid tumors, but a randomized controlled study is still needed to further confirm its efficacy and safety.
Ethical Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ethic Committee (EC) from the Second Affiliated Hospital of Harbin Medical University (KY2023-032). In this research, informed consent was obtained from all participants over the age of 18 years, and for those under the age of 18 years of age, parental/legal guardian consent was obtained.
Acknowledgments
We would like to thank all members in our group.
Disclosure
The authors report no potential conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Harper J, Moses MA. Molecular regulation of tumor angiogenesis: mechanisms and therapeutic implications. EXS. 2006;96:223–268. doi:10.1007/3-7643-7378-4_10
3. Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi:10.1038/nm0603-653
4. Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333(26):1757–1763.
5. Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11(1):120. PMID: 30231931; PMCID: PMC6146601. doi:10.1186/s13045-018-0664-7
6. Kerbel RS. A decade of experience in developing preclinical models of advanced- or early-stage spontaneous metastasis to study antiangiogenic drugs, metronomic chemotherapy, and the tumor microenvironment. Cancer J. 2015;21(4):274–283. PMID: 26222079. doi:10.1097/PPO.0000000000000134
7. Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol. 2019;12(1):47. PMID: 31046803; PMCID: PMC6498593. doi:10.1186/s13045-019-0736-3
8. Desai J, Deva S, Lee JS, et al. Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors. J Immunother Cancer. 2020;8(1):e000453. PMID: 32540858; PMCID: PMC7295442. doi:10.1136/jitc-2019-000453
9. Ghahremanloo A, Soltani A, Modaresi SMS, Hashemy SI. Recent advances in the clinical development of immune checkpoint blockade therapy. Cell Oncol. 2019;42(5):609–626. PMID: 31201647. doi:10.1007/s13402-019-00456-w
10. Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity. 2018;48(3):434–452. PMID: 29562194; PMCID: PMC7116507. doi:10.1016/j.immuni.2018.03.014
11. Fucà G, De Braud F, Di Nicola M. Immunotherapy-based combinations: an update. Curr Opin Oncol. 2018;30(5):345–351. PMID: 29994900. doi:10.1097/CCO.0000000000000466
12. Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18(1):60. PMID: 30925919; PMCID: PMC6441150. doi:10.1186/s12943-019-0974-6
13. Chu T, Zhang W, Zhang B, et al. Efficacy and safety of first-line anlotinib-based combinations for advanced non-small cell lung cancer: a three-armed prospective study. Transl Lung Cancer Res. 2022;11(7):1394–1404. PMID: 35958322; PMCID: PMC9359953. doi:10.21037/tlcr-22-438
14. Su Y, Luo B, Lu Y, et al. Anlotinib Induces a T cell-inflamed tumor microenvironment by facilitating vessel normalization and enhances the efficacy of PD-1 checkpoint blockade in neuroblastoma. Clin Cancer Res. 2022;28(4):793–809. PMID: 34844980; PMCID: PMC9377760. doi:10.1158/1078-0432.CCR-21-2241
15. Zheng W, Sun G, Li Z, et al. The effect of anlotinib combined with anti-PD-1 in the Treatment of gastric cancer. Front Surg. 2022;9:895982. PMID: 35495754; PMCID: PMC9039330. doi:10.3389/fsurg.2022.895982
16. Manegold C, Dingemans AC, Gray JE, et al. Low-dose apatinib optimizes tumor microenvironment and potentiates antitumor effect of PD-1/PD-L1 blockade in lung cancer. Cancer Immunol Res. 2019;7(4):630–643. PMID: 30755403. doi:10.1158/2326-6066.CIR-17-0640
17. Wang Q, Gao J, Di W, Wu X. Anti-angiogenesis therapy overcomes the innate resistance to PD-1/PD-L1 blockade in VEGFA-overexpressed mouse tumor models. Cancer Immunol Immunother. 2020;69(9):1781–1799. PMID: 32347357. doi:10.1007/s00262-020-02576-x
18. Allen E, Jabouille A, Rivera LB, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017;9(385):eaak9679. PMID: 28404866; PMCID: PMC5554432. doi:10.1126/scitranslmed.aak9679
19. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
20. Al-Husein B, Abdalla M, Trepte M, Deremer DL, Somanath PR. Antiangiogenic therapy for cancer: an update. Pharmacotherapy. 2012;32(12):1095–1111. PMID: 23208836; PMCID: PMC3555403. doi:10.1002/phar.1147
21. Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer. 2018;118(5):654–661. PMID: 29438373; PMCID: PMC5846072. doi:10.1038/bjc.2017.478
22. Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 Phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–1575. PMID: 30098152; PMCID: PMC6248083. doi:10.1001/jamaoncol.2018.3039
23. Shi J, Cheng Y, Wang Q, et al. Anlotinib as third- or further-line therapy for short-term relapsed small-cell lung cancer: subgroup analysis of a randomized Phase 2 study (ALTER1202). Front Med. 2022;16(5):766–772. PMID: 35844026. doi:10.1007/s11684-021-0916-8
24. Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70(15):6171–6180. PMID: 20631075; PMCID: PMC2912959. doi:10.1158/0008-5472.CAN-10-0153
25. Liu C, Peng W, Xu C, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res. 2013;19(2):393–403. PMID: 23204132; PMCID: PMC4120472. doi:10.1158/1078-0432.CCR-12-1626
26. Georganaki M, Van Hooren L, Dimberg A. Vascular targeting to increase the efficiency of immune checkpoint blockade in cancer. Front Immunol. 2018;9:3081. PMID: 30627131; PMCID: PMC6309238. doi:10.3389/fimmu.2018.03081
27. Xu Q, Wang J, Sun Y, et al. Efficacy and safety of sintilimab plus anlotinib for PD-L1-positive recurrent or metastatic cervical cancer: a multicenter, single-arm, prospective phase II trial. J Clin Oncol. 2022;40(16):1795–1805. PMID: 35192397; PMCID: PMC9148684. doi:10.1200/JCO.21.02091
28. Chung HC, Ros W, Delord JP, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37(17):1470–1478. PMID: 30943124. doi:10.1200/JCO.18.01265
29. Hao YY, Qiao YP, Cheng JD. Clinical activity and safety of anlotinib combined with PD-1 blockades for patients with previously treated small cell lung cancer. Int J Gen Med. 2021;14:10483–10493. PMID: 35002304; PMCID: PMC8722563. doi:10.2147/IJGM.S337316
30. Chen Q, Li Y, Zhang W, Wang C, Yang S, Guo Q. Safety and efficacy of ICI plus anlotinib vs. anlotinib alone as third-line treatment in extensive-stage small cell lung cancer: a retrospective study. J Cancer Res Clin Oncol. 2022;148(2):401–408. PMID: 34797416; PMCID: PMC8800903. doi:10.1007/s00432-021-03858-2
31. Yu L, Xu J, Qiao R, Han B, Zhong H, Zhong R. Efficacy and safety of anlotinib combined with PD-1/PD-L1 inhibitors as second-line and subsequent therapy in advanced small-cell lung cancer. Cancer Med. 2023;12(5):5372–5383. PMID: 36250532; PMCID: PMC10028028. doi:10.1002/cam4.5360
32. Yuan M, Zhu Z, Mao W, et al. Anlotinib combined with anti-PD-1 antibodies therapy in patients with advanced refractory solid tumors: a single-center, observational, prospective study. Front Oncol. 2021;7;11:683502. PMID: 34692475; PMCID: PMC8529018. doi:10.3389/fonc.2021.683502
33. Wallis CJD, Lawson K, Butaney M, et al. Association between PD-L1 status and immune checkpoint inhibitor response in advanced malignancies: a systematic review and meta-analysis of overall survival data. Jpn J Clin Oncol. 2020;50(7):800–809. PMID: 32083295. doi:10.1093/jjco/hyaa021
34. Liu X, Guo CY, Tou FF, et al. Association of PD-L1 expression status with the efficacy of PD-1/PD-L1 inhibitors and overall survival in solid tumours: a systematic review and meta-analysis. Int J Cancer. 2020;147(1):116–127. PMID: 31633798. doi:10.1002/ijc.32744
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.