Back to Journals » Clinical Ophthalmology » Volume 17
A Retrospective Comparison of Ahmed Glaucoma Valve Implants with or without Ologen Collagen Matrix
Authors Suzuki Junior ER , Maestrini HA, Ruppert ADP, Seixas RCS , Belfort AF , de Miranda Santos HD, Basile Neto A , Balbino M , Lopes da Silva MJ
Received 11 November 2022
Accepted for publication 29 December 2022
Published 4 January 2023 Volume 2023:17 Pages 15—23
DOI https://doi.org/10.2147/OPTH.S396330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Emilio Rintaro Suzuki Junior,1 Heloisa Andrade Maestrini,2 Aline Domingos Pinto Ruppert,3 Regina Cele Silveira Seixas,3 Ana Flávia Belfort,4 Herika Danielle de Miranda Santos,2 Alberto Basile Neto,5 Marcos Balbino,6 Marcelo Jordão Lopes da Silva7
1Department of Ophthalmology, Pontifícia Universidade Católica de Minas Gerais, Belo Horizonte, MG, Brazil; 2Department of Ophthalmology, Hospital Oculare, Belo Horizonte, MG, Brazil; 3Department of Ophthalmology, Grupo OPTY – HCLOE – Oftalmologia Especializada, São Paulo, SP, Brazil; 4Department of Ophthalmology, Clínica de Olhos da Santa Casa de Belo Horizonte, Belo Horizonte, MG, Brazil; 5Department of Ophthalmology, Complexo Hospitalar Padre Bento de Guarulhos, Guarulhos, SP, Brazil; 6Department of Emergency Medicine, Centro Universitário São Camilo, São Paulo, SP, Brazil; 7Department of Ophthalmology, Oftalmocenter Ribeirão Preto, Ribeirão Preto, SP, Brazil
Correspondence: Marcos Balbino, Department of Emergency Medicine, Centro Universitário São Camilo, Cipriano Barata, 1869 – ap. 93. Ipiranga, São Paulo, 04205001, Brazil, Tel +5511-983462354, Email [email protected]
Purpose: To study the effects of Ologen collagen matrix on the outcomes of the Ahmed glaucoma valve implant.
Study Design: Retrospective case-control multicenter study, conducted at four centers, comparing the 6-month outcomes of Ahmed valve implants with or without Ologen.
Participants: The study included 125 eyes in a 4:1 pairing (25 patients with Ologen matched to 100 patients without Ologen).
Intervention: Ologen was placed over the Ahmed plate in the study group. Success was defined as an intraocular pressure (IOP) ≤ 21 mmHg either with no medication (complete success) or regardless of medications (qualified success). Other outcomes included IOP variation, eye drop use, and surgical complications.
Results: Overall, the IOP decreased from 30.72 ± 9.08 to 16.14 ± 4.79 mmHg (p=0.0001). Of the 125 eyes, 26 achieved complete success and 94 achieved qualified success. There was no difference in complete success between the groups (p=0.12); however, there was a difference in qualified success (p=0.01), with better results in the no-Ologen group (80% vs 56%). There were no differences in the decrease in medications (p=0.06), as well as the incidence of complications (p=0.69). Although the need for postoperative surgical reintervention was higher in the no-Ologen group (13% vs 4%), the difference was not significant (p=0.2).
Conclusion: The reductions in IOP and number of medications were similar in both groups after 6 months, with similar complication rates. The qualified success rate was lower in the Ologen group, but further studies are needed to clarify the role of Ologen in Ahmed valve implants.
Keywords: glaucoma, tube shunts, glaucoma incisional surgery, glaucoma anti-fibrotic agents, postoperative glaucoma hypotony
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide.1 In 2020, an estimated 76.02 million people had glaucoma worldwide and that number will keep increasing. Lowering the intraocular pressure (IOP) is currently the only means of reducing progression of the disease.2 Approaches to IOP reduction include topical drops, laser therapy, and surgical therapy.3
The Ahmed glaucoma valve (AGV) provides controlled fluid drainage from the anterior chamber to the subconjunctival space. It contains thin silicone elastomer membranes that open when the intraocular pressure reaches 8 mmHg.4 This valve system avoids hypotony after implantation, and has fewer complications than trabeculectomy or non-valved implants.5–7
One of the causes of surgery failure is encapsulation of the implant plate under the conjunctiva.8–10 Based on animal models of conjunctival wound healing, wound contraction occurs in the early inflammatory stage in the presence of myofibroblasts, which results in a more linear alignment of the collagen fibers.11
Ologen collagen matrix is a degradable, porous, collagen-glycosaminoglycan scaffold designed to reduce conjunctival scarring and wound contraction, resulting in less resistance to the aqueous humor outflow.12 Application of the collagen matrix leads to random reorganization of the regenerating myofibroblasts, fibroblasts, and extracellular matrix. The density and organization of the collagen in Ologen-treated wounds are similar to those of the connective tissue of normal conjunctiva.11–13 Although the use of Ologen matrix with Ahmed valve implantation seems reasonable, there is no consensus about its beneficial effects. Therefore, this study examined the effects of Ologen collagen matrix on the success and safety of the Ahmed glaucoma valve implant.
Material and Methods
Ethics
The Institutional Review Board of HCLOE Clinicas de Oftalmologia Especializada endorsed the study, and all data entered complied with relevant data protection and privacy guidelines. The patients remained anonymous in all steps of the study. The research methods were in accordance with the tenets of the Declaration of Helsinki. The study did not require informed consent due to its retrospective design and non-interventional review of medical records.
Study Design and Population
This retrospective multicenter study compared the 6-month outcomes of eyes that underwent Ahmed valve implant with or without the Ologen collagen matrix from October 2011 to February 2021. All surgeries were performed in one of four ophthalmic surgery centers in Brazil: HCLOE Opty Group Brazil, São Paulo; Hospital Oftalmocenter–Ribeirão Preto, São Paulo; Centro Oftalmológico de Minas Gerais–Belo Horizonte, Minas Gerais; and Oculare Hospital de Oftalmologia–Belo Horizonte, Minas Gerais). All surgeons involved in the study were experienced with this procedure, having implanted the biological matrix in at least 20 surgeries in their careers. The study included only one eye per patient.
Ologen Case–Control Matching
Once the centers identified the Ologen cases, the authors matched them with no-Ologen patients at a 1:4 ratio. Subjects were paired based on their characteristics in the following order: diagnosis, number of previous surgeries, number of drops used preoperatively, preoperative IOP, and age. Each nominal variable was assigned a number. For ordinal variables, the value itself was used. Then this number was used in the formula 10X, where X is the rank of the variable. All participants were assigned a final number and these numbers were ordered; patients were automatically assigned to the most similar value possible in the database. This pairing process reduces selection bias and makes the process much less time-consuming. All participant pairing was revised to ensure that there was no mismatching.
Data Collection and Eligibility
Patients were eligible for inclusion if they were older than 18 years and had indications for anti-glaucoma surgery (per the Brazilian Glaucoma Society guidelines, Appendix 1). Diagnoses included primary open-angle glaucoma (POAG), glaucoma secondary to surgery, neovascular glaucoma, closed-angle glaucoma, glaucoma secondary to uveitis, and other types.
Surgical Procedure
Both groups received a fornix-based flap of the conjunctiva and Tenon’s capsule, performed in the superotemporal quadrant. Then the AGV body was placed 8 to 10 mm from the limbus and sutured to the sclera with a 6–0 or 8–0 silk suture. A 23-gauge needle tract entered the anterior chamber at the scleral spur. The tube tip was cut obliquely, placed through the tract, and a donor sclera patch graft was secured with 10–0 Nylon sutures over the exposed portion of the tube. A 10×10×2 mm rectangle of Ologen (#870051) was placed over the plate in the study group. The conjunctiva was sutured with either 8–0 Vicryl or 10–0 Nylon sutures. Vigamox drops (0.5% moxifloxacin hydrochloride; Alcon Laboratories, Fort Worth, TX, USA) were prescribed four times per day for 14 to 21 days, and Pred Fort (1% prednisolone acetate; Allergan, Irvine, CA, USA) 6 to 8 times per day. The frequency of prednisolone administration was gradually reduced according to the signs of eye inflammation. Appendix 2 Figures 3–9 shows a sequence of seven photos displaying the surgical steps.
Outcomes
The primary outcome was maintaining IOP ≤ 21 mmHg throughout the study. Success was considered “complete” if the patients required no medication in the 6 months of the study and “qualified” both with or without antiglaucoma medications. The data included IOP variation, eye drops, surgical complications, adverse events, and secondary surgeries. Due to the study’s retrospective nature, we decided not to analyze the visual field. The reason is the bias in the data from the patients because the surgeons are prone to measure the visual field only if the patient complains or there is some noticeable concern.
Statistical Analyses
Each eye (right or left) was considered a unit of measurement in the analyses due to the dependence between eyes and to limit the loss of data for particular surgical procedures. A test for skewness and kurtosis verified the normal distribution of the data. We used the t-test (normal distribution) or Wilcoxon rank-sum test (non-normal distribution) to compare means. One-way ANOVA was used to investigate the variance of several means, and the chi-square test was used for categorical variables.
We calculated survival estimates using Kaplan–Meier analysis and assessed possible differences with the Log rank test. We set p-value < 0.05 as the threshold for statistical significance. Stata software (release 13; StataCorp, College Station, TX, USA) was used for the analyses.
Results
In the 6 months, 25 Ologen patients were matched with 100 no-Ologen patients, as described above. The data for 125 eyes correspond to 125 patients. Table 1 summarizes the patient demographics. Within-column relative frequencies were used to show the balance between the treatment groups.
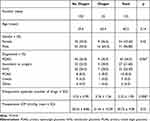 |
Table 1 Patient Demographics. Within-Column Relative Frequencies Were Used to Show the Balance Between the Treatment Groups |
Intraocular Pressure
At the 6-month follow-up, for all eyes included in the study, IOP decreased from 30.72 ± 9.08 (95% confidence interval [CI], 29.11–32.33) to 16.14 ± 4.79 (15.30–17.0) mmHg (p=0.0001). Although the final IOP was lower at 6 months postoperatively in the no-Ologen® group (p=0.009, Table 2), there were no differences between the two treatment groups (p=0.23, Table 2; ANOVA Prob > F = 0.82, Figure 1).
 |
Table 2 Comparison of the Preoperative IOP, Final IOP, and 6-Month Reduction in the IOP in the No-Ologen® and Ologen® Groups |
 |
Figure 1 Six-month IOP variation (mmHg) in the two treatment groups. ANOVA Prob > F = 0.82. The top line shows all the IOP (mmHg) medians at each time point. |
Success Rates
Of the 125 eyes, 26 achieved complete success (IOP ≤ 21 mmHg without medications), and 94 achieved qualified success (IOP ≤ 21 mmHg with or without medications, Table 3). There were no significant differences in complete success between the groups (p=0.12, odds ratio [OR], 0.466; 95% CI, 0.160–1.46); however, there was a significant difference in qualified success between the groups (p=0.01, OR, 3.14; 95% CI, 1.10–8.72), with better results in the no-Ologen group (80% vs 56%, Figure 2).
 |
Table 3 Rates of Complete and Qualified success. The Log Rank Test Was Significant for Qualified Success (p=0.01), but Not for Complete Success (p=0.09) |
Number of Glaucoma Medications
The mean number of medications in the overall cohort decreased from 3.32 ± 1.09 (95% CI, 3.10–3.53) preoperatively to 2.00 ± 1.18 (95% CI, 1.77–2.31) at 6 months (p=0.0001). Table 4 shows the eye drops usage in both treatment groups. Preoperatively, the eyes in the no-Ologen group were on more glaucoma medications than those in the Ologen group. Ologen patients entered the study on a mean of 2.76 ± 1.36 drugs (95% CI, 2.20–3.32), while the no-Ologen patients used 3.35 ± 0.99 drugs (95% CI, 3.17–3.55) (p=0.008). However, both groups had comparable medication burdens at 6 months: 1.67 ± 1.56 drugs (95% CI, 1.13–2.31) for the Ologen group versus 2.02 ± 1.11 drugs (95% CI, 1.79–2.26) for the no-Ologen group (p=0.12). The medication decrease did not differ between groups (Ologen group 0.91 ± 2.00, no-Ologen group 1.42 ± 1.15; p=0.06).
 |
Table 4 Eye Drops Usage in Both Treatment Groups in the Preoperative Period and at Day 180 |
Complications
Postoperative complications are listed in Table 5. Hyperfiltration (in one no-Ologen eye) was the single intraoperative complication. There were no differences in the incidence of complications between the two treatment groups (p=0.69, Table 5). Fourteen eyes required surgical reintervention because of postoperative complications, only one in the Ologen group. Although the need for postoperative surgical reintervention was higher in the no-Ologen group (13% vs 4%), the difference was not significant (p=0.2, Table 5).
 |
Table 5 Complications in the Treatment Groups |
Discussion
This retrospective case–control study included 125 eyes in groups with or without Ologen collagen matrix in a 4:1 pairing. The IOP in the overall cohort decreased from 30.72 ± 9.08 to 16.14 ± 4.79 mmHg (p=0.0001). This reduction was similar to the results of other studies.9,10,14–17 There were no differences between the groups in IOP variation (p=0.23) and complete success rate (p=0.12) at the 6-month follow-up, although the final IOP was lower at 180 days postoperatively in the no-Ologen group (p=0.0009). These findings differ from Rho et al, who found a complete success rate of 86.4% in an Ahmed valve implantation group augmented with collagen matrix and 38.1% in a conventional group (p=0.002).17 Other studies have also found that the Ahmed valve implant augmented with Ologen is superior.18,19 By contrast, in a randomized prospective multicenter clinical trial of 58 patients, Sastre-Ibáñez et al found no statistical difference between the two groups in terms of the hypertensive phase or complete or qualified success rates.20
Another recent study reported no differences in the success rates at 6 months, but detected a lower hypertensive phase rate in the Ologen group (61.5% vs 38.5%).21 In our study, defining qualified success as IOP ≤ 21 mmHg with or without medication, the Ologen group had worse results than the no-Ologen group (p=0.01). The OR indicated that the use of Ologen matrix reduced the chance of achieving qualified success more than three times (OR, 3.14; 95% CI, 1.10–8.72). No other study found similar results.
The reduction in medication burden was significant in both groups but did not differ (p=0.06) between them. A between-group difference might have been seen with a larger sample. For example, Rho et al found a significant medication burden reduction at the 6-month follow-up,17 while another study found a significant reduction at 3 months but not at 6 months.18 Song et al19 reported no differences in their Ologen-6 (#862051) group compared to controls but found a significant reduction with Ologen-7 (#870051). Further studies need to investigate the effects of these two collagen matrixes on the reduction of medication burden. Our results are in agreement with other studies that have found no differences between groups.20,21
There were no differences in the number of complications between groups. The most common was pressure peaks, although one patient in the no-Ologen group developed retinal detachment and another had phthisis bulbi. The authors described a TASS case in the study. After revisiting the documents from the patient, they noticed that the patient presented discrete hypopyon on the third postoperative day, which improved with topical antibiotics and corticoids. The local hospital infection control committee identified a failure in one sterilization process at that time, which caused a few other cases of TASS. No vision-threatening complications occurred in the Ologen group. Other studies have also reported no increased complication risk with Ologen.9,14–21
The present study has its limitations. The study’s retrospective design is inferior to the standard prospective randomized strategy but sometimes is reasonable due to tribulations in randomized studies for new surgical techniques. The number of ologen-treated eyes is small (25 eyes), despite the author’s efforts to increase the power of analysis with a 4:1 ratio in the case-control sample. The timeframe for observations could be longer (up to one year); however, similar studies have shown little difference between six months and one-year follow-up,19 and some authors have published papers with an equal time for follow-up.17
In theory, Ologen should help wound healing by reducing or modulating collagen synthesis by fibroblasts over the valve plate and minimizing its resistance to aqueous outflow to the subconjunctival space. Nevertheless, this effect has been observed in some studies and utterly absent in others. Some hidden variables must interfere with the results, even using similar techniques. It is still too soon to discard the positive effects of Ologen combined with valve implants, as all current evidence comes from relatively small studies. More evidence is needed to support its use in larger populations from a public health financing perspective, particularly in developing countries.
Conclusion
In this retrospective, case-control study, the reductions in IOP and number of medications were similar in the Ologen and no-Ologen groups with Ahmed glaucoma valve implants after 6 months. The similar complication rates suggest that Ologen does not add additional risk to the procedure. The qualified success rate was lower in the Ologen group, but further studies with more homogeneous types of glaucoma are needed to clarify the role of Ologen collagen matrix in Ahmed glaucoma valve implants.
Ethical Approval
The Institutional Review Board of Grupo OPTY: HCLOE Clinicas de Oftalmologia Especializada endorsed the retrospective study, and all data entered complied with relevant data protection and privacy guidelines. The patients remained anonymous in all study steps, and only one author manipulated the gathered data, which remained in an encrypted file.
Informed Consent for Medical Photographs
A short video resulted in photos 3–9. The patient in question gave voluntary written consent to publish the entire video or part of it for scientific and academic purposes.
Acknowledgments
The authors would like to thank Regina Cele Silveira Seixas for their leadership and her continuous search for quality and excellence in research, essential to the conclusion of this paper.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors state that this research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
All authors declare that they have no conflicts of interest concerning this publication.
References
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi:10.1136/bjo.2005.081224
2. Sihota R, Angmo D, Ramaswamy D, Dada T. Simplifying “target” intraocular pressure for different stages of primary open-angle glaucoma and primary angle-closure glaucoma. Indian J Ophthalmol. 2018;66(4):495–505. doi:10.4103/ijo.IJO_1130_17
3. Crawley L, Zamir SM, Cordeiro MF, Guo L. Clinical options for the reduction of elevated intraocular pressure. Ophthalmol Eye Dis. 2012;4:43–64. doi:10.4137/OED.S4909
4. Coleman AL, Hill R, Wilson MR, et al. Initial clinical experience with the Ahmed glaucoma valve implant. Am J Ophthalmol. 1995;120(1):23–31. doi:10.1016/S0002-9394(14)73755-9
5. Christakis PG, Kalenak JW, Zurakowski D, et al. The Ahmed versus Baerveldt study: one-year treatment outcomes. Ophthalmology. 2011;118(11):2180–2189. doi:10.1016/j.ophtha.2011.05.004
6. Budenz DL, Feuer WJ, Barton K, et al.; Ahmed Baerveldt Comparison Study Group. Postoperative complications in the Ahmed Baerveldt Comparison Study during five years of follow-up. Am J Ophthalmol. 2016;163:75–82.e3. doi:10.1016/j.ajo.2015.11.023
7. Ayyala RS, Zurakowski D, Monshizadeh R, et al. Comparison of double-plate Molteno and Ahmed glaucoma valve in patients with advanced uncontrolled glaucoma. Ophthalmic Surg Lasers. 2002;33(2):94–101. doi:10.3928/1542-8877-20020301-04
8. Tsai JC, Johnson CC, Dietrich MS. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma: a single-surgeon comparison of outcome. Ophthalmology. 2003;110(9):1814–1821. doi:10.1016/S0161-6420(03)00574-8
9. Nouri-Mahdavi K, Caprioli J. Evaluation of the hypertensive phase after insertion of the Ahmed Glaucoma Valve. Am J Ophthalmol. 2003;136:1001–1008. doi:10.1016/S0002-9394(03)00630-5
10. Ayyala RS, Zurakowski D, Smith JA, et al. A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology. 1998;105:1968–1976. doi:10.1016/S0161-6420(98)91049-1
11. Hsu WC, Spilker MH, Yannas IV, Rubin PAD. Inhibition of conjunctival scarring and contraction by a porous collagen-glycosaminoglycan implant. Invest Ophthalmol Vis Sci. 2000;41(9):2404–2411.
12. Yuan F, Li L, Chen X, Yan X, Wang L. Biodegradable 3D-porous collagen matrix (Ologen) compared with mitomycin C for treatment of primary open-angle glaucoma: results at 5 years. J Ophthalmol. 2015;2015:637537. doi:10.1155/2015/637537
13. Chen HS, Ritch R, Krupin T, Hsu WC. Control of filtering bleb structure through tissue bioengineering: an animal model. Invest Ophthalmol Vis Sci. 2007;48(2):485.
14. Huang MC, Netland PA, Coleman AL, et al. Intermediate-term clinical experience with the Ahmed glaucoma valve implant. Am J Ophthalmol. 1999;127(1):27–33. doi:10.1016/S0002-9394(98)00394-8
15. Lai JS, Poon AS, Chua JK, et al. Efficacy and safety of the Ahmed glaucoma valve implant in Chinese eyes with complicated glaucoma. Br J Ophthalmol. 2000;84(7):718–721. doi:10.1136/bjo.84.7.718
16. Coleman AL, Smyth RJ, Wilson MR, Tam M. Initial clinical experience with the Ahmed Glaucoma Valve implant in pediatric patients. Arch Ophthalmol. 1997;115(2):186–191. doi:10.1001/archopht.1997.01100150188007
17. Rho S, Sung Y, Ma KT, Rho SH, Kim CY. Bleb analysis and short-term results of biodegradable collagen matrix-augmented Ahmed glaucoma valve implantation: 6-month follow-up. Invest Ophthalmol Vis Sci. 2015;56(10):5896–5903. doi:10.1167/iovs.15-17480
18. Kim TJ, Kang S, Jeoung JW, Kim YK, Park KH. Comparison of 1-year outcomes after Ahmed glaucoma valve implantation with and without Ologen adjuvant. BMC Ophthalmol. 2018;18(1):45. doi:10.1186/s12886-018-0709-2
19. Song M, Lee S, Choe D, Kim S, Roh YH, Rho S. Clinical and biological evaluations of biodegradable collagen matrices for glaucoma drainage device implantation. Invest Ophthalmol Vis Sci. 2017;58(12):5329–5335. doi:10.1167/iovs.17-22579
20. Sastre-Ibáñez M, Cabarga C, Canut MI, et al. Efficacy of Ologen matrix implant in Ahmed glaucoma valve implantation. Sci Rep. 2019;9:3178. doi:10.1038/s41598-019-38656-x
21. Harizman N, Du J, Tai TY. A prospective randomized trial of Ahmed glaucoma drainage device implantation with or without Ologen collagen matrix. Ophthalmol Glaucoma. 2021;4(4):421–426. doi:10.1016/j.ogla.2020.12.005
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

