Back to Journals » Infection and Drug Resistance » Volume 15
A Rare Case of Post-Primary Tuberculosis Which Was Pathologically Diagnosed as Lipoid Pneumonia
Authors Yu M, Zhong J, Bu X, Tan X, Zhan D, Hu X, Gu Y, Xu J, Zhang P , Wang L
Received 21 March 2022
Accepted for publication 21 July 2022
Published 4 August 2022 Volume 2022:15 Pages 4235—4239
DOI https://doi.org/10.2147/IDR.S367312
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Min Yu,1,2,* Jiacheng Zhong,1,2,* Xueyong Bu,3,* Xinjuan Tan,1,2 Danting Zhan,1,2 Xiaoyi Hu,1 Yingying Gu,4 Jing Xu,5 Peize Zhang,6 Lingwei Wang1,2
1Shenzhen People’s Hospital, Shenzhen Institute of Respiratory Diseases, Shenzhen, 518055, China; 2Shenzhen Key Laboratory of Respiratory Diseases, Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University; The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen, 518055, China; 3Department of Radiology, Shenzhen Longgang District Maternity & Child Healthcare Hospital, Shenzhen, 518172, China; 4Department of Pathology, Guangzhou Institute of Respiratory Health, Guangzhou, 510120, China; 5Department of Pathology, Shenzhen People’s Hospital, Shenzhen, 518055, China; 6Department of Pulmonary Medicine & Tuberculosis, The Third People’s Hospital of Shenzhen, Shenzhen, China
*These authors contributed equally to this work
Correspondence: Lingwei Wang, Email [email protected]
Case Presentation: The patient was a middle-aged housewife who had been using the household spray for a long time, and the main symptoms were cough and sputum production. Chest CT showed lobar ground-glass opacities (GGOs) with small patchy consolidation in the right middle lobe (RML), specifically, lung tissue pathology showed a large number of foamy cells and scattered multinucleated giant cells. The patient received empirical anti-infective treatment, but no clinical improvement was observed. Laboratory tests, including smears and cultures of sputum, blood and bronchoalveolar lavage fluid (BALF), did not provide clear evidence for pathogenic microorganisms. Therefore, the presumptive diagnosis was exogenous LP (ExLP). After 28 days of prednisone treatment, her symptoms improved, but 2 months later, she presented with a worsening cough, and the GGOs had progressed into lobar consolidation. Transbronchial lung biopsy (TBLB) culture showed mycobacterium tuberculosis (MTB), and lung tissue pathology showed granulomatous inflammation. After anti-tuberculosis treatment, the consolidation in the right middle lobe was gradually absorbed, along with a considerable symptom improvement. The final diagnosis of the patient was MTB infection with an endogenous lipoid pneumonia (EnLP)-like presentation.
Conclusion: The current case highlights that the MTB infection should be considered when pathology shows LP accompanied by scattered multinucleated giant cells.
Keywords: mycobacterium infection, aetiology, pathological diagnosis
Background
Lipoid pneumonia (LP) is an uncommon pulmonary disease that is manifested by the accumulation of lipid within the lungs, it can be further categorized into exogenous LP (ExLP)/endogenous LP (EnLP), according to the origin of the pathogenic lipid.1 ExLP is related to the aspiration of oil-based substances such as animal fats, mineral oils, petroleum jelly, etc.2 On the other hand, EnLP could be a consequence of various diseases such as obstructive pulmonary disease, pulmonary infection, lung tumour, disorders of lipid metabolism and so on.3 Owing to atypical clinical symptoms and imaging manifestations, LP is commonly misdiagnosed or delayed-diagnosed.4 Currently, LP-like presentation of mycobacterium tuberculosis (MTB) infection is seldom described in the literature. Here, we report the diagnosis and treatment of a rare case of post-primary tuberculosis (PPTB) which was pathologically diagnosed as LP.
Case Presentation
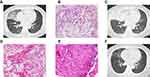
A 59-year-old woman complained of productive cough without fever since April 2018. She is a housewife and has no history of diabetes and smoking. She had undergone routine blood examinations in another hospital, which showed no significant abnormality. The initial diagnosis was a pulmonary infection. Her cough and sputum production, although attenuated, persisted despite receiving 15 days of treatment with ceftriaxone and tazobactam. The patient then received combined administration of amoxicillin and metronidazole for 4 weeks, after which the chest computed tomography (CT) showed that reticulations were superimposed on lobar ground-glass opacities (GGOs), accompanied by small patchy consolidation in the right middle lobe (RML) (Figure 1A). Since then, she had not received any treatment, despite suffering from occasional coughing.
The patient was admitted to our department in November 2018 because of a worsening cough. She was afebrile with a respiratory rate of 20 breaths per minute, heart rate of 72 beats per minute, blood pressure of 135/84 mmHg, oxygen saturation of 97% on room air, and BMI of 23 kg/m2. The breath sounds were clear, and no rales were heard during the auscultation of both lungs. No heart murmur was heard. Laboratory test results were all normal, including routine blood, urine and stool examinations and serum CRP, fasting blood glucose, PCT, tumour markers and HIV test. The opacities in RML were almost unchanged in comparison to previous imaging. Smears and cultures of sputum, blood and bronchoalveolar lavage fluid (BALF) for bacteria, fungi and acid-fast bacillus were all negative. Histopathology of the specimens obtained by transbronchial lung biopsy (TBLB) showed a large number of foamy cells and cholesterol clefts in alveolar spaces with scattered multinucleated giant cells, and multifocal lymphocyte infiltration with fibrotic proliferation in the interstitial space (Figure 1B). Upon further questioning, the patient admitted that she used household spray and hair spray frequently. Collectively, the patient’s clinical history, imaging features, laboratory test results, histopathology as well as the lack of evidence for pulmonary infection led to the presumptive diagnosis of ExLP. The patient was instructed to stop using household spray and hair spray (withdrawal from lipid exposure) and treated with oral prednisone at a dose of 30 mg/day for 15 days, after which the dose was reduced to 15 mg/day for 7 days, and tapered to discontinuation within the following 6 days. Her symptoms improved progressively, so she was discharged and followed up in our outpatient clinic.
In early April 2019, she presented to our hospital again because of rough coughing accompanied by a lot of yellow sticky sputum. Chest CT showed that GGOs had progressed into lobar consolidation with air bronchogram in RML (Figure 1C). Routine blood, urine and stool examinations, liver and kidney function tests, serum PCT tests and coagulation function studies did not show abnormalities. The interferon-gamma release assay test was negative. Serum high-sensitivity C-reactive protein (hs-CRP) concentration and erythrocyte sedimentation rate (ESR) were both elevated at 12.32mg/L and 71mm/h, respectively. The pathogenic examinations were all negative, including sputum and BALF smears for acid-fast bacilli; sputum, BALF and blood cultures for bacteria and fungi; (1,3)-β-D-glucan (G test) and Galactomannan (GM test) tests of BALF. Through next-generation sequencing of blood and BALF for pathogen discovery, no clinically significant pathogenic microorganisms were found. Histopathology of the specimens obtained by TBLB still revealed a large number of foamy cells filled in alveoli, accompanied by granulomatous lesions scattered in the interstitial space (Figure 1D and E), no caseous necrosis was found. The tissue samples obtained by TBLB were cultured for bacteria, fungi, and acid-fast bacilli. Fortunately, the presence of MTB was confirmed on the 25th day of lung tissue culture.
Then she was transferred to a specialized TB hospital and received quadruple therapy with isoniazid, rifampin, pyrazinamide and ethambutol for 3 months, followed by isoniazid and rifampicin. Her symptoms gradually relieved as treatment progressed. After 4 months of treatment, chest CT showed that the lung opacities had been absorbed almost completely (Figure 1F). The patient returned to her normal lifestyle and no longer suffered from symptoms associated with the use of household spray/hair spray. Satisfactory therapeutic results have been achieved, which confirmed a diagnosis of MTB infection.
Discussion and Conclusion
LP is a rare chronic reactive inflammation that results from the accumulation of lipids within the alveolar spaces.1 As for ExLP, when lipids are sucked into the alveolar cavities, they are emulsified and subsequently swallowed by alveolar macrophages.5 Since these cells cannot metabolize fatty substances, their deaths could result in the repeated intra-alveolar release of lipids,6 which may culminate in giant cell granulomatous response, chronic inflammatory response, as well as alveolar and interstitial fibrosis.7 The pathophysiological mechanisms of EnLP are more complicated and might involve continuous epithelial cell secretion, cell disruption, vascular leakage, and chronic hypoxia, among others.7
According to the duration of exposure, ExLP can be categorized into acute and chronic forms: the acute form is very rare, it may have severe clinical manifestations, such as fever and hemoptysis; the symptoms of the chronic form are typically insidious, and some patients are even asymptomatic.8 In contrast, EnLP might provoke non-specific symptoms that vary in severity, such as cough, expectoration, and dyspnea.9
The patient in our case displayed imaging manifestations of LP including GGOs, pulmonary consolidation and “spun glass” appearance, which lack specificity and resembled many other pulmonary diseases.7 For instance, GGOs were preliminary used to describe pulmonary alveolar proteinosis,10 however, it is also observed in diffuse lung diseases that affect alveoli or interstitium, such as pneumonia, pulmonary oedema and diffuse pulmonary haemorrhage.11 In light of this, pathology was necessary for a definite diagnosis. Microscopically, the patient’s TBLB specimens displayed an accumulation of lipids and foamy cells (lipid-laden macrophages) in alveoli, which were typical characteristics of LP.7
After confirmation of LP, it is imperative to further determine whether it is ExLP or EnLP. The diagnosis of ExLP requires a clear history of lipid inhalation.8 To diagnose EnLP, it is important to find the etiological factors. Our patient was presumptively diagnosed as having ExLP, given the prolonged usage of household spray/hair spray and the absence of evidence of infection. Although her symptoms were relieved after corticosteroid treatment, an infective respiratory exacerbation was found after 2 months. Upon further examinations, a diagnosis of MTB infection was proposed, considering the following clinical features:
- In addition to previously observed typical features of LP, histopathology first showed giant cell granulomatous inflammation. Granulomatous lesions can be caused by LP itself,12 but it is also common in a variety of infectious diseases.13 Such histopathological alteration, when combined with a large number of foamy cells, might suggest infection with EnLP-like presentation, especially when conventional anti-infective therapy fails.
- The MTB infection was finally confirmed based on the patient’s TBLB specimens. Previously, she underwent repeated pathogenic examinations, however, none of the results was positive for MTB, which might be ascribed to the relatively low MTB loads when the patient was immunocompetent. Although she achieved transient symptom relief after corticosteroid treatment, the resulting reduction in overall immunity, which facilitates the reproduction of microorganisms, might render MTB more detectable. This could account for the presence of MTB at her last pathogenic examination. Therefore, despite the confirmation of LP, corticosteroids should be used with great caution when the aetiology was unclear.
- We found that the GGOs of the patient had progressed into lobar consolidation. In the context of EnLP, infectious lesions are surrounded by lung tissues that have been consolidated by non-infectious inflammation, which prevents the microorganisms, to some extent, from spreading.14 This might explain that, in the current case, multiple CT scans were performed over a prolonged length of time, but almost no progression or deterioration of the GGOs was observed until the corticosteroid was applied.
As expected, the patient achieved a satisfactory clinical outcome after anti-tuberculosis treatment. The preceding evidence indicates that we encountered a rare case of post-primary TB (PPTB) which was pathologically diagnosed as LP. In primary infection, although the majority of MTBs were eliminated by an ongoing immune response, a few survivors might fall into dormancy, waiting for the opportunity to start multiplying rapidly again, which caused PPTB after several decades.15 In immunocompetent individuals, PPTB begins with the accumulation of foamy macrophages in alveoli, such phenomenon could be attributed to the host immune response against MTB, during which the release of gamma interferon could facilitate the generation of foamy macrophages.16 These suggest a very close association between LP and the early stage of PPTB.
Although 90% of PPTB can heal spontaneously, the remaining 10% will eventually develop into caseous pneumonia.15 Hence, once the diagnosis of MTB infection is established, anti-tuberculosis treatment should be performed in time.
Our patient has a clinical course similar to a previously described 37-year-old woman with progressive pleural effusion and semi-turbid exudative lymph dominant fluid,17 although that case was further complicated by comorbidities such as rheumatoid arthritis and cytomegalovirus infection: the patient was initially tested negative for MTB infection; corticosteroid treatment only achieved a short-term remission, before the subsequent symptom aggravation.
There are some limitations in our study. Firstly, GeneXpert (cartridge based nucleic acid amplification test), a technique recommended for patients suspected of having either pulmonary or extrapulmonary MTB infection, was not used in this study. Second, although nutrition status has an essential role in combat against TB,18 we did not perform a nutritional evaluation for our patient. Third, this study did not provide strong evidence suggesting a causal relationship between MTB infection and EnLP.
In summary, this case underscored that when empirical anti-infective treatments fail in patients who display LP-like clinical manifestations accompanied by scattered multinucleated giant cells, the possibility of PPTB should be considered.
Abbreviations
PPTB, post-primary tuberculosis; LP, lipoid pneumonia; GGOs, lobar ground-glass opacities; RML, right middle lobe; BALF, bronchoalveolar lavage fluid; ExLP, exogenous lipoid pneumonia; TBLB, transbronchial lung biopsy; MTB, mycobacterium tuberculosis; EnLP, endogenous lipoid pneumonia.
Ethics Approval and Consent to Participate
The study was approved by the research ethics committee of the Third People’s Hospital of Shenzhen (reference number: 2021-015-02). Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Consent for Publication
Written informed consent for publication was obtained from the participant.
Funding
This work was supported by the Shenzhen Science and Technology Program (KCXFZ202002011008256). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
Yu, Zhong and Bu are co-first authors. The authors declare that they have no competing interests.
References
1. Harris K, Chalhoub M, Maroun R, et al. Lipoid pneumonia: a challenging diagnosis. Heart Lung. 2011;40(6):580–584. doi:10.1016/j.hrtlng.2010.12.003
2. Rajagopala S, Selvam N. An unusual cause of respiratory distress. J Postgrad Med. 2019;65(1):38–40. doi:10.4103/jpgm.JPGM_496_17
3. Betancourt SL, Martinez-Jimenez S, Rossi SE, et al. Lipoid pneumonia: spectrum of clinical and radiologic manifestations. AJR Am J Roentgenol. 2010;194(1):103–109. doi:10.2214/AJR.09.3040
4. Hadda V, Khilnani GC, Bhalla AS, et al. Lipoid pneumonia presenting as non resolving community acquired pneumonia: a case report. Cases J. 2009;2(1):9332. doi:10.1186/1757-1626-2-9332
5. Pielaszkiewicz-Wydra M, Homola-Piekarska B, Szcześniak E, et al. Exogenous lipoid pneumonia - a case report of a fire-eater. Pol J Radiol. 2012;77(4):60–64. doi:10.12659/PJR.883631
6. Russo R, Chiumello D, Cassani G, et al. Case of exogenous lipoid pneumonia: steroid therapy and lung lavage with an emulsifier. Anesthesiology. 2006;104(1):197–198. doi:10.1097/00000542-200601000-00027
7. Hadda V, Khilnani GC. Lipoid pneumonia: an overview. Expert Rev Respir Med. 2010;4(6):799–807. doi:10.1586/ers.10.74
8. Marchiori E, Zanetti G, Mano CM, et al. Exogenous lipoid pneumonia. Clinical and radiological manifestations. Respir Med. 2011;105(5):659–666. doi:10.1016/j.rmed.2010.12.001
9. Raya AI, Fernández-de Marco M, Núñez A, et al. Endogenous lipid pneumonia in a dog. J Comp Pathol. 2006;135(2–3):153–155. doi:10.1016/j.jcpa.2006.06.002
10. Borie R, Danel C, Debray M-P, et al. Pulmonary alveolar proteinosis. Eur Respir Rev. 2011;20(120):98–107. doi:10.1183/09059180.00001311
11. Nishino M, Itoh H, Hatabu H. A practical approach to high-resolution CT of diffuse lung disease. Eur J Radiol. 2014;83(1):6–19. doi:10.1016/j.ejrad.2012.12.028
12. Papla B, Urbańczyk K, Gil T, et al. Exogenous lipoid pneumonia (oil granulomas of the lung). Pol J Pathol. 2011;62(4):269–273.
13. Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1–12. doi:10.1016/j.jctube.2017.02.001
14. Burke M, Fraser R. Obstructive pneumonitis: a pathologic and pathogenetic reappraisal. Radiology. 1988;166(3):699–704. doi:10.1148/radiology.166.3.3340764
15. Hunter RL. Pathology of post primary tuberculosis of the lung: an illustrated critical review. Tuberculosis. 2011;91(6):497–509. doi:10.1016/j.tube.2011.03.007
16. Wong KW, Jacobs WR. Postprimary tuberculosis and macrophage necrosis: is there a Big ConNECtion? mBio. 2016;7(1):e01589–e01615. doi:10.1128/mBio.01589-15
17. Jamaati H, Kahkouee S, Farrokhpour M, Mir-Aboutalebi F. Endogenous lipoid pneumonia associated with rheumatoid arthritis, Tuberculosis and cytomegalovirus infection. Paripex Indian J Res. 2017;6(7):1–15.
18. Degefa MG, Bezabih AM, Kahsay ZH, et al. Barriers and facilitators of nutrition assessment, counseling, and support for tuberculosis patients: a qualitative study. BMC Nutr. 2021;7(1):58. doi:10.1186/s40795-021-00463-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.