Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
A Randomized Double-Blind Placebo-Control Feasibility Trial of Immunoglobulin Treatment for Prevention of Recurrent Acute Exacerbations of COPD
Authors Cowan J , Mulpuru S , Abdallah SJ, Chopra A , Purssell A, McGuinty M, Alvarez GG, Giulivi A, Corrales-Medina V, MacFadden D, Boyle L, Hasimja D, Thavorn K, Mallick R, Aaron SD , Cameron DW
Received 16 September 2021
Accepted for publication 23 November 2021
Published 3 December 2021 Volume 2021:16 Pages 3275—3284
DOI https://doi.org/10.2147/COPD.S338849
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Juthaporn Cowan,1,2 Sunita Mulpuru,1,2 Sara J Abdallah,2 Anchal Chopra,3 Andrew Purssell,1 Michaeline McGuinty,1 Gonzalo G Alvarez,1,2 Antonio Giulivi,2,4 Vicente Corrales-Medina,1,2 Derek MacFadden,1,2 Loree Boyle,1 Delvina Hasimja,1 Kednapa Thavorn,2,5,6 Ranjeeta Mallick,2 Shawn D Aaron,1,2 D William Cameron1,2
1Department of Medicine, University of Ottawa, Ottawa, Ontario, Canada; 2Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; 3Interdisciplinary School of Health Sciences, University of Ottawa, Ottawa, Ontario, Canada; 4Department of Pathology and Laboratory Medicine, The Ottawa Hospital, University of Ottawa, Ottawa, Ontario, Canada; 5School of Epidemiology, Public Health and Preventive Medicine, University of Ottawa, Ottawa, Ontario, Canada; 6Institute for Clinical Evaluative Sciences, Toronto, Ontario, Canada
Correspondence: Juthaporn Cowan Email [email protected]
Background: Observational studies suggest that immunoglobulin treatment may reduce the frequency of acute exacerbations of COPD (AECOPD).
Objective: To inform the design of a future randomised control trial (RCT) of intravenous immunoglobulin (IVIG) treatment efficacy for AECOPD prevention.
Methods: A pilot RCT was conducted. We recruited patients with COPD hospitalized for AECOPD, or from ambulatory clinics with one severe, or two moderate AECOPD in the previous year regardless of their serum IgG level. Patients were allocated in a 1:1 ratio with balanced randomisation to monthly IVIG or normal saline for 1 year. The primary outcome was feasibility defined as pre-specified accrual, adherence, and follow-up rates. Secondary outcomes included safety, tolerance, AECOPD rates, time to first AECOPD, quality of life, and healthcare costs.
Results: Seventy patients were randomized (37 female; mean age 67.7; mean FEV1 35.1%). Recruitment averaged 4.5± 0.9 patients per month (range 0– 8), 34 (49%) adhered to at least 80% of planned treatments, and four (5.7%) were lost to follow-up. There were 35 serious adverse events including seven deaths and one thromboembolism. None was related to IVIG. There were 56 and 48 moderate and severe AECOPD in the IVIG vs control groups. In patients with at least 80% treatment adherence, median time to first moderate or severe AECOPD was 275 vs 114 days, favoring the IVIG group (HR 0.76, 95% CI 0.3– 1.92).
Conclusion: The study met feasibility criteria for recruitment and retention, but adherence was low. A trend toward more robust treatment efficacy in adherent patients supports further study, but future trials must address treatment adherence.
Trial registration number: NCT0290038, registered 24 February 2016, https://clinicaltrials.gov/ct2/show/NCT02690038 and NCT03018652, registered January 12, 2017, https://clinicaltrials.gov/ct2/show/NCT03018652.
Keywords: recurrent AECOPD, pilot RCT, immunoglobulin treatment, IVIG
Plain Language Summary
COPD patients experience acute “flare-ups” as their disease worsens, characterized by periods of increased shortness of breath, cough and phlegm production. Current treatments to prevent COPD flare-ups are only modestly effective. New therapies are needed to improve the quality of life and clinical outcomes for COPD patients.
We observed a favorable effect of an antibody (immunoglobulin) treatment on the frequency of flare-ups, doctor visits, medications, and hospitalizations for COPD patients in a non-controlled setting. Here, we conducted a pilot-controlled trial to evaluate immunoglobulin treatment in patients with frequent COPD flare-ups to determine if this treatment is safe, tolerable, and potentially effective in reducing the frequency of flare-ups. We found that the clinical trial was feasible in that we were able to recruit and retain participants throughout the study period. Treatment was safe and tolerable. There was a trend toward extending the period of flare-up free period. This supports further study. However, treatment adherence was an issue affecting data analysis. We must find an alternative study design to improve treatment adherence to definitively study the efficacy of immunoglobulin treatment.
Introduction
Acute exacerbations of COPD (AECOPD) are associated with poor quality of life, worsening lung function, greater healthcare services use, and mortality.1,2 AECOPD severity may be mild (not requiring antibiotics and/or systemic corticosteroids), moderate (requiring outpatient treatment with antibiotics and/or systemic corticosteroids) or severe (requiring hospitalization).2 Patients with COPD identify AECOPD as a very important health outcome.3 Despite optimal medical management, patients experience 1–1.5 AECOPD per person-year on average, highlighting the need for more effective therapies.4–6
In patients with humoral immunodeficiency, immunoglobulin (Ig) therapy effectively prevents recurrent infections.7 In patients with COPD, hypogammaglobulinemia (serum IgG level <7.0 g/L) is associated with increased risks of AECOPD8,9 and hospitalizations.10 While there is no established IgG threshold level that predicts recurrent AECOPD, the relationship between serum IgG level and AECOPD appears to be linear and extends into the “normal” range.9 In a retrospective, longitudinal, within-subject risk interval analysis, Ig treatment was associated with a reduction in AECOPD rates from 4.7±3.1 to 0.6±1.0 per patient-year.11 The overall rate of AECOPD decreased consistently across the severity of COPD or baseline serum IgG level. This observation and others12 suggest that Ig treatment may reduce the frequency of recurrent AECOPD. Well-designed, adequately powered clinical trials are needed to test this hypothesis. To inform the design of a future study of intravenous immunoglobulin (IVIG) treatment efficacy for AECOPD prevention, we conducted a pilot placebo-control RCT to determine feasibility, estimate outcome rates and effect size.
Methods
Study Design
This was a pilot, single centre, double-blind, placebo-control RCT of IVIG vs normal saline (NS) for the prevention of AECOPD conducted at The Ottawa Hospital. The trial was registered (ClinicalTrials.gov Identifier: NCT02690038, and NCT03018652), and its protocol was published.13 The study received regulatory approval from Health Canada and ethics approval (Protocol 20150925–01H, 20160077–01H and 2017005–01H). All patients provided informed written consent prior to study participation in accordance with the Declaration of Helsinki.
Study Population
Eligible patients had a prior diagnosis of COPD supported by a FEV1/FVC ratio <0.70. To be considered for enrollment, patients (a) had to be hospitalized for a diagnosis of AECOPD; or (b) had received care for COPD in an ambulatory clinic and had ≥1 severe AECOPD (requiring hospitalization) or ≥2 moderate AECOPD (requiring outpatient or emergency department treatment) within the previous 12 months (Supporting Table S1). Our target sample size, based on hospital patient-volume, consisted of 48 inpatient subjects, 16 with and 32 without hypogammaglobulinemia, and 22 outpatient subjects. We excluded patients with active cancers, transplantation, and immunotherapy treatment. Patient recruitment commenced in September 2016 for inpatients, March 2018 for outpatients, and concluded in November 2018.
Intervention
Patients were randomized in a 1:1 ratio to receive 10% IVIG or NS monthly. We used 0.8 g/kg for hospitalized patients with hypogammaglobulinemia, and 0.5 g/kg for all others.14 The first treatment was administered during hospitalization for inpatients, or in the Clinical Investigation Unit (CIU) for outpatients, and then every 4±1 weeks in the CIU for 48 weeks (ie, 13 infusions in one year). Patients continued to receive standard treatment for COPD as directed by their treating physician.
Allocation of Study Treatment and Blinding
We used computer-generated randomization in blocks of four. Randomised allocation was balanced by stratification for IgG level above or below 7 g/L in inpatients. The CIU nurses were unblinded to patient allocation. The study infusion treatments were concealed with a cardboard box to patients, investigators, and other research staff.
Study Visits and Procedures
At each treatment visit, medical history, physical examination, intercurrent and concomitant adverse events (AEs) and medications were reviewed, and the COPD Assessment Test (CAT) was administered.15 At weeks 0, 4, 12, 24, 36 and 48, the St. George’s Respiratory Questionnaire (SGRQ),16 5-level EQ-5D (EQ-5D-5L),17 and the COST questionnaire were administered, and spirometry was performed.
Outcomes
The primary outcome of feasibility was defined as the recruitment of an average of four patients per month; with ≥80% of patients adhering to 80% of allocated treatment; and ≥80% of individuals retained at twelve-month follow-up regardless of treatment adherence. Secondary outcomes included safety, tolerance, AECOPD annual rate, time to first AECOPD, quality of life (QoL), and healthcare costs. AECOPD was defined as deterioration in at least two of three cardinal symptoms (breathlessness, cough, and/or sputum production) that exceeded normal variation, led to a new prescription (antibiotics and/or prednisone), use of healthcare services,18 and occurred >30 days after previous AECOPD. Interval AECOPD and other AEs were identified at regular visits, and confirmed via health records, primary care providers and pharmacies. AECOPD outcomes and AEs were reviewed and adjudicated by DH and LB who were not involved in data collection or analysis.
Statistical Analyses
For descriptive data, we used mean (standard deviation), median (interquartile range), or frequency (%), as appropriate. The intention-to-treat (ITT) analysis included all patients randomized to receive IVIG or placebo, while a per-protocol (PP) analysis, performed for secondary outcomes, included only “adherent” patients who received 80% of allocated treatments. Study treatment adherence was defined as the percentage of planned study treatments received. The adherence rate was the proportion of patients adhering to ≥80% of planned study treatments. The study retention rate was the proportion of patients available for follow-up regardless of adherence until death or 12 months from randomization.
AEs and tolerability were summarized using point estimates with 95% CI. The time to first moderate or severe AECOPD was calculated by survivorship analysis and presented using a Kaplan–Meier curve. The hazard ratio (HR) was calculated using Cox-proportional hazards regression, adjusting for pre-defined variables (age, sex, baseline IgG, % predicted FEV1 value, and baseline AECOPD rate). Changes in CAT, SGRQ, 5D-5Q-5L19 values were assessed using a repeated measures linear regression analysis with an adjustment for the baseline values. Statistical analysis was performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA). Figures were generated using GraphPad Prism 7.0d.
The Data Safety Monitoring Board (DSMB) consisted of three members experienced in clinical trials appointed to ensure that all patients were not exposed to unnecessary or unreasonable risks and that the study was conducted with the highest scientific and ethical standards. DSMB meeting occurred five times during the study period.
Results
Study Population
Seventy of 444 screened patients (15.91%) were randomised to either IVIG (n = 35) or control (n = 35) (Figure 1). Baseline clinical characteristics are presented in Table 1 and Supporting Table S2. Pulmonary function, baseline IgG and rate of AECOPD in the year before randomization were clinically comparable between groups.
 |
Table 1 Baseline Characteristics |
Recruitment
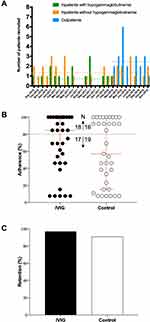
Combined recruitment rate from all recruitment settings was 4.5±0.9 patients per month (range 0–8, Figure 2A). The highest rate was from the outpatient setting (2.4±0.6). Treatment visit frequency was the most common reason for participation refusal (n = 32; 27.8%).
Study Treatment Adherence
Overall mean study treatment adherence was 68.8±5.7% (median: 85%) for IVIG, and 59.4±6.3% (median: 57%) for control (Figure 2B). Eighteen patients (51.4%) randomized to IVIG adhered to 80% of their allocated treatment, compared to 16 (45.7%) in the control group.
Study Retention
One patient randomized to IVIG and two to NS withdrew consent. One patient in the IVIG group was lost to follow-up. Therefore, 66 patients were retained. Retention rate was 97.1% (n = 34) and 91.4% (n = 32) in IVIG and control groups, respectively (Figure 2C). Seven died during the study period (IVIG: n = 3, control: n = 4).
Safety and Tolerability
ITT analysis. There were 137 AEs in 33 patients and 126 AEs in 27 patients in the IVIG and control, respectively (p = 0.55). Median number (interquartile range) of AEs per patient was 2 (1–5) for IVIG and 3 (1–5) for control. The five most commonly reported AEs in the IVIG and control groups were upper respiratory tract symptoms (17 vs 11), musculoskeletal pain (8 vs 11), pneumonia (7 vs 7), increased shortness of breath (4 vs 9) and chest discomfort (7 vs 5). There were 18 and 10 all-cause hospitalizations in the IVIG and control groups, respectively. PP analysis. There were 70 AEs in 18 patients and 78 AEs in 13 patients in the IVIG and control, respectively (p = 0.61). There were no significant differences in the number of patients that experienced serious AEs or AE ≥ grade 3. One patient developed an infusion reaction (cold sensation at the infusion site, hand swelling, throat itchiness, throat thickness without airway compromise) that led to treatment discontinuation. Symptoms were resolved within hours of infusion discontinuation and IV diphenhydramine. Subsegmental pulmonary embolism occurred in one patient in the IVIG group, which was not determined to be related to IVIG. A full list of AEs can be found in the Supporting Tables S3–S4.
A higher number of patients continued to receive study treatment at week 48 in the IVIG group (58%) vs the control group (46%), indicating tolerability to IVIG.
AECOPD Outcomes
Overall, there were 56 (40 moderate, 16 severe) AECOPD events in the IVIG vs 48 (28 moderate, 20 severe) in the placebo groups. Among “adherent” patients (n = 18 vs n = 16), there were 24 AECOPD events (20 moderate, 4 severe) in IVIG and 24 (20 moderate, 4 severe) in placebo. The severe AECOPD rates appeared to be reduced in the IVIG group in the ITT (RR 0.78, 95% CI 0.37 to 1.65) and PP analyses (RR 0.43, 95% CI 0.07–2.49) (Table 2). The median time to first moderate or severe AECOPD was not different between groups in ITT (HR 0.91, 95% CI 0.59–1.41) and PP (HR 0.98, 95% CI 0.50–1.93). However, in the PP analysis, there was a trend toward longer median time to first AECOPD by 161 days in the IVIG group (Figure 3B and Supporting Table S5). In addition, the median time to first moderate or severe AECOPD appeared to be greater in the adherent than non-adherent IVIG groups. This readout was not different between adherent and non-adherent controls.
 |
Table 2 Rates of Acute Exacerbation of COPD (AECOPD) by Treatment Allocation |
 |
Figure 3 Kaplan–Meier curve of cases remaining without moderate or severe AECOPD, (A) regardless of non-adherence (ITT), (B) in cases with >80% adherence (PP) to study treatments. |
Pulmonary Function and HRQoL Outcomes
Pulmonary function (FEV1) and measures of HRQoL (CAT, SGRQ and EQ-5D-L) were not significantly different between groups at baseline, or during the study period in PP analyses (Supporting Figure S1 and Table S6). Notably, in contrast to controls, the mean change in the CAT and SGRQ scores of the IVIG group between weeks 0 and 4 exceeded their respective MCIDs by 2 points and 4 units.
Healthcare Costs Associated with AECOPD
The mean total healthcare costs related to AECOPD-emergency department visits and hospitalizations over the 12-month study period were not significantly different between IVIG (Can$4,226.58 ± $11,428.53) and placebo (Can$4,263.47 ± $8,927.10).
Discussion
Our study determined the feasibility of a randomized, double-blind, placebo-control, hospital-based trial of monthly IVIG treatment to reduce the frequency of AECOPD. We demonstrated that two of the three pre-specified study feasibility goals were met: recruitment and retention, although treatment adherence was low. There was a trend toward reduced severe AECOPD rates and time to first moderate or severe AECOPD in the adherent IVIG group.
While the combined recruitment rate was 4.5±0.9 patients per month, we observed higher recruitment in the ambulatory care setting. Treatment adherence presents a significant barrier to feasibility. Low adherence was likely due to frailty in our targeted patient population. The frequency of monthly visits was another barrier to both recruitment and adherence. Despite the equivalent number of “treatment adherent” individuals between the two groups, the median adherence rate was higher in the IVIG group (85% vs 57%). The reason for this is unknown. It is notable that health status at week 4 was better in the IVIG group; therefore, patient perception of a benefit could be contributing to better adherence in this group. To improve treatment adherence, home-based IVIG infusions, or self-administered subcutaneous Ig should be considered. These alternative infusion strategies are more cost-effective than hospital-based infusions,20 and have proven benefit in chronic disease.21 However, a suitable sham therapy or placebo must be identified.
IVIG was safe and well tolerated as more participants allocated to IVIG stayed on study treatment (58% vs 48%) at week 48. This is expected as IVIG has been used with acceptable safety profiles in many diseases for decades. Our study did not demonstrate any significant change in FEV1, but a better HRQoL at week 4 was observed in the IVIG group. A rapid improvement of HRQoL is surprising since Ig has a half-life of 25–35 days and reaches a steady state after 3–5 doses. We speculate that there may be an immediate effect of Ig, such as decreased inflammatory state or reduced autoantibodies in the acute phase of AECOPD. A prospective measurement of inflammatory markers following Ig administration, such as erythrocyte sedimentation rate and C-reactive protein or sputum autoantibodies, would be informative.22,23
The study was not powered to determine the efficacy of IVIG on AECOPD outcomes. Although there were no differences in rate or time to the first event, there was a trend toward lower AECOPD rate and a longer time to the first event.4 Based on the calculated HR obtained from this study, if one were to conduct a definitive study with the same inclusion/exclusion criteria, a 1-year accrual and 1-year follow-up time, 1500 participants per arm will be required. This sample size is large and may not be feasible. Hence, patients who may be most responsive to Ig treatment should be identified prior to conducting a large definitive trial.
Some recurrent AECOPDs may be precipitated by recurrent respiratory tract infections, which are common in individuals with functional antibody deficiency. Ig treatment prevents recurrent infections and improves quality of life in immunodeficient patients. Hence, it is possible that a subset of patients with COPD could have functional antibody deficiency and would receive the most benefit from Ig treatment. This subgroup is more difficult to identify because low serum IgG alone is not a direct indication of functional antibody deficiency. Dynamic testing of a specific immune response to a polysaccharide vaccine antigen, which induces an antibody response without the help of T-cells, can be used to identify functional antibody deficiency.24
The study has several limitations. First, we recruited both hospitalized and non-hospitalized COPD patients. This resulted in heterogeneity in our sample, including lower FEV1 values and higher burden of respiratory disease in the hospitalized patients. Future trials should focus more on non-hospitalized patients for recruitment feasibility. Second, a different IVIG dose was administered to the inpatients with hypogammaglobulinemia vs the inpatients without hypogammaglobulinemia and outpatients. However, the reported effect size in the study was analyzed for all adherent patients regardless of their recruitment setting, or baseline IgG. This should be considered if the effect size of this study is used for a future trial. Third, the low adherence rate limits the interpretation of the secondary outcomes. Lastly, AECOPD outcome may not be appropriate measures of treatment efficacy because exacerbations are heterogenous with different phenotypes. Many patients independently sought medical treatments or started antibiotics and/or prednisone for respiratory symptoms that may not have reflected true AECOPDs, such as anxiety-related breathlessness. Recently, an international multidisciplinary clinical research network (DECODE-NET) identified challenges in the design and conduct of clinical trials on AECOPD.25,26 The group proposed that a core outcome set should be developed to homogenise outcome measures in COPD trials.
Conclusion
In conclusion, a trial of IVIG treatment for patients with recurrent exacerbations of COPD is not feasible with the current study design. Monthly treatment visits were a barrier to adherence. Study design modifications, including home-based Ig administration, and recruitment of non-hospitalized patients who are less frail may improve both recruitment and adherence. Further, identifying a targeted subgroup of COPD patients for Ig treatment, such as those with evident functional antibody deficiency, is warranted.
Abbreviations
AECOPD, acute exacerbation of chronic obstructive pulmonary disease; CAT, COPD assessment test; CI, confidence interval; CIU, clinical investigation unit; COPD, chronic obstructive pulmonary disease; EQ-5D-5L, EuroQol-5-dimension-5-level; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HR, hazard ratio; HRQoL, health-related quality of life; Ig, immunoglobulin; IgG, immunoglobulin G; ITT, intention to treat; IVIG, intravenous immunoglobulin; PP, per protocol; SGRQ, St. George’s respiratory questionnaire.
Data Sharing Statement
Individual participant data that underlie the results reported in this article, after deidentification and study protocol, will be available for individual participant data meta-analysis. Data will be available to investigators whose proposed use of the data has been approved by an independent review committee. Proposals should be directed to [email protected] immediately or for up to 36 months following publication. To gain access, data requestors will need to sign a data access agreement.
Ethics Approval and Consent to Participate
The study protocol was approved by the OHRI Research Ethics Board (protocol number 20150925-01H, 20160077-01H and 20170005-01H). All patients provided informed written consent prior to study participation in accordance with the Declaration of Helsinki.
Acknowledgments
We thank the administrative staff at the OHRI for ensuring that the study was being conducted according to Good Clinical Practices. We thank Drs. Tim Ramsay, Alan Tinmouth, and Doug McKim for serving as Data Safety Monitoring committee members. We thank Mrs. Isabelle Seguin, Mrs. Danielle Tardiff, Mrs. Laurie Bretzlaff and Mrs. Wendy Fusee for study coordination and nursing care to all our participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported in part by The Ottawa Hospital Academic Medical Organization Innovative Fund, CSL Behring, and Grifols. Funders were not involved in the study design, study conduct, result analysis or interpretation. DWC is supported in salary with a grant from the University of Ottawa and Department of Medicine.
Disclosure
JC received grants and/or personal fees from CSL Behring, Grifols, The Ottawa Hospital Academic Medical Organization, OctaPharma, Takeda, GSK, Sanofi Genzyme, EMD Serono, and Alexion outside the submitted work. MM reports personal fees from Merck, outside the submitted work. DWC reports grants from CSL Behring Inc., Grifols Inc., during the conduct of the study; personal fees and/or non-financial support from CSL Behring, Grifols, Takeda, Celltrion, AstraZeneca Canada, Gilead Sciences outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi:10.1136/thoraxjnl-2011-201518
2. GOLD. Global strategy for the diagnosis, management and prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease - GOLD; 2017. Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/.
3. Zhang Y, Morgan RL, Alonso-Coello P, et al. A systematic review of how patients value COPD outcomes. Eur Respir J. 2018;52(1):1800222. doi:10.1183/13993003.00222-2018
4. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698. doi:10.1056/NEJMoa1104623
5. Martinez FJ, Calverley PMA, Goehring U-M, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385(9971):857–866. doi:10.1016/S0140-6736(14)62410-7
6. Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26. doi:10.1164/rccm.200707-973OC
7. Hartung H-P, Mouthon L, Ahmed R, Jordan S, Laupland KB, Jolles S. Clinical applications of intravenous immunoglobulins (IVIg) - beyond immunodeficiencies and neurology. Clin Exp Immunol. 2009;158:23–33. doi:10.1111/j.1365-2249.2009.04024.x
8. Leitao Filho FS, Ra SW, Mattman A, et al. Serum IgG subclass levels and risk of exacerbations and hospitalizations in patients with COPD. Respir Res. 2018;19(1):30. doi:10.1186/s12931-018-0733-z
9. Leitao Filho FS, Won RS, Mattman A, et al. Serum IgG and risk of exacerbations and hospitalizations in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2017;140(4):1164–1167.e6. doi:10.1016/j.jaci.2017.01.046
10. Leitao Filho FS, Mattman A, Schellenberg R, et al. Serum IgG levels and risk of COPD hospitalization: a pooled meta-analysis. Chest. 2020;158(4):1420–1430. doi:10.1016/j.chest.2020.04.058
11. Cowan J, Gaudet L, Mulpuru S, et al. A retrospective longitudinal within-subject risk interval analysis of immunoglobulin treatment for recurrent acute exacerbation of chronic obstructive pulmonary disease. PLoS One. 2015;10(11):e0142205. doi:10.1371/journal.pone.0142205
12. McCullagh BN, Comellas AP, Ballas ZK, Newell JD, Zimmerman MB, Azar AE. Antibody deficiency in patients with frequent exacerbations of Chronic Obstructive Pulmonary Disease (COPD). PLoS One. 2017;12(2):e0172437. doi:10.1371/journal.pone.0172437
13. Cowan J, Mulpuru S, Aaron S, et al. Study protocol: a randomized, double-blind, parallel, two-arm, placebo control trial investigating the feasibility and safety of immunoglobulin treatment in COPD patients for prevention of frequent recurrent exacerbations. Pilot Feasibility Stud. 2018;4(1):135. doi:10.1186/s40814-018-0327-z
14. Bonilla FA. Pharmacokinetic s of Immunoglobulin administered via Intravenous or subcutaneous routes. Immunol Allergy Clin North Am. 2008;28(4):803–819. doi:10.1016/j.iac.2008.06.006
15. Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203. doi:10.1016/S2213-2600(14)70001-3
16. Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(Suppl B):25–27. doi:10.1016/S0954-6111(06)80166-6
17. Nolan CM, Longworth L, Lord J, et al. The EQ-5D-5L health status questionnaire in COPD: validity, responsiveness and minimum important difference. Thorax. 2016;71(6):493–500. doi:10.1136/thoraxjnl-2015-207782
18. Niewoehner DE, Rice K, Cote C, et al. Prevention of exacerbations of Chronic Obstructive Pulmonary Disease with Tiotropium, a once-daily inhaled Anticholinergic Bronchodilator. Ann Intern Med. 2005;143(5):317. doi:10.7326/0003-4819-143-5-200509060-00007
19. Xie F, Pullenayegum E, Gaebel K, et al. A time trade-off-derived value set of the EQ-5D-5L for Canada. Med Care. 2016;54(1):98–105. doi:10.1097/MLR.0000000000000447
20. Luthra R, Quimbo R, Iyer R, Luo M. An analysis of intravenous immunoglobin site of care: home versus outpatient hospital. Am J Pharm Benefits. 2014;6(2):e41–e49.
21. Le Masson G, Solé G, Desnuelle C, et al. Home versus hospital immunoglobulin treatment for autoimmune neuropathies: a cost minimization analysis. Brain Behav. 2018;8(2):e00923. doi:10.1002/brb3.923
22. Wen L, Krauss-Etschmann S, Petersen F, Yu X. Autoantibodies in chronic obstructive pulmonary disease. Front Immunol. 2018;9:66. doi:10.3389/fimmu.2018.00066
23. Liang Z, Wang F, Zhang D, et al. Sputum and serum autoantibody profiles and their clinical correlation patterns in COPD patients with and without eosinophilic airway inflammation. J Thorac Dis. 2020;12(6):3085–3100. doi:10.21037/jtd-20-545
24. Orange JS, Ballow M, Stiehm ER, et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the basic and clinical immunology interest section of the American Academy of allergy, asthma & immunology. J Allergy Clin Immunol. 2012;130(3):S1–S24. doi:10.1016/j.jaci.2012.07.002
25. Mathioudakis AG, Sivapalan P, Papi A, Vestbo J. The disEntangling Chronic Obstructive pulmonary Disease exacerbations clinical trials NETwork (DECODE-NET): rationale and vision. Eur Respir J. 2020;56(1):1. doi:10.1183/13993003.00627-2020
26. Mathioudakis AG, Moberg M, Janner J, Alonso-Coello P, Vestbo J. Outcomes reported on the management of COPD exacerbations: a systematic survey of randomised controlled trials. ERJ Open Res. 2019;5(2):00072–02019. doi:10.1183/23120541.00072-2019
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.