Back to Journals » Journal of Pain Research » Volume 17
A Randomized Controlled Trial of Clinical Hypnosis as an Opioid-Sparing Adjunct Treatment for Pain Relief in Adults Undergoing Major Oncologic Surgery
Authors Rosenbloom BN, Slepian PM , Azam MA, Aternali A, Birnie KA, Curtis K, Thaker S, Ladak S, Waisman A , Clarke H , Katz J , Weinrib AZ
Received 7 June 2023
Accepted for publication 27 November 2023
Published 4 January 2024 Volume 2024:17 Pages 45—59
DOI https://doi.org/10.2147/JPR.S424639
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Timothy Atkinson
Brittany N Rosenbloom,1– 3 P Maxwell Slepian,1,2,4 Muhammed Abid Azam,1,2 Andrea Aternali,1 Kathryn A Birnie,5,6 Kathryn Curtis,2 Sonal Thaker,2 Salima Ladak,2 Anna Waisman,1 Hance Clarke,2,4 Joel Katz,1,2,4 Aliza Z Weinrib1,2
1Department of Psychology, York University, Toronto, ON, Canada; 2Department of Anesthesia and Pain Management, Toronto General Hospital, University Health Network, Toronto, ON, Canada; 3Toronto Academic Pain Medicine Institute, Women’s College Hospital, Toronto, ON, Canada; 4Department of Anesthesiology and Pain Medicine, University of Toronto, Toronto, ON, Canada; 5Department of Anesthesiology, Perioperative and Pain Medicine, University of Calgary, Calgary, AB, Canada; 6Department of Community Health Sciences, University of Calgary, Calgary, AB, Canada
Correspondence: Joel Katz; Aliza Z Weinrib, Department of Psychology, York University, BSB 232, 4700 Keele Street, Toronto, ON, M3J 1P3, Canada, Email [email protected]; [email protected]
Abstract: Clinical hypnosis is an effective strategy for managing acute pain in the surgical setting. However, the opioid sparing effects of clinical hypnosis are not as well understood. This pre-registered (NCT03730350) randomized, controlled trial (RCT) examined the impact of clinical hypnosis, pre- and post-surgery, on opioid consumption during hospitalization as well as on measures of pain intensity, pain interference, depressed mood, anxiety, sleep, and pain catastrophizing. Participants (M = 57.6 years; SD = 10.9) awaiting oncologic surgery were randomized to treatment-as-usual (n = 47) or hypnosis (n = 45). Intent-to-treat analyses were conducted using linear mixed effects modeling. A significant Group × Time interaction, F(6, 323.34) = 3.32, p = 0.003, indicated an opioid sparing effect of clinical hypnosis during the acute postoperative period. Hypnosis also protected against increases in pain catastrophizing at one-week after surgery, F (1, 75.26) = 4.04, p = 0.048. A perioperative clinical hypnosis intervention had a sparing effect on opioid consumption in-hospital after major oncologic surgery. These findings extend the efficacy of clinical hypnosis as an adjunct tool for perioperative pain management.
Keywords: clinical hypnosis, oncologic surgery, postoperative opioid use, postoperative pain, pain catastrophizing
Introduction
The prevalence of long-term, high-dose opioid use for pain is a key factor in the current opioid public health crisis.1 Opioid use for acute pain after surgery is part of standard care; however, some patients who use opioids for post-surgical pain may ramp up to high opioid doses and subsequently have difficulty weaning back down. Furthermore, patients who are taking opioid medication prior to surgery and also have a history of anxiety and/or depression, are at increased risk of prolonged use and substantial increase in opioid doses after surgery.2–4
Given the current concern over opioid use for pain management, psychological pain management options, such as clinical hypnosis, are being revisited. Hypnosis is established with
an induction procedure to enhance responsiveness and reduce peripheral awareness followed by delivering suggestions within a specific sociocultural context to guide participants to experience cognitive, sensory, motor, or perceptive alteraions.5
Hypnosis is one of the oldest strategies for the management of pain but interest in it as a pain management strategy has ebbed and flowed over time.6 Over the past twenty years, there has been a new wave of scientific investigation into the efficacy of hypnosis for acute and chronic pain.7
A meta-analysis of RCTs investigating the efficacy of clinical hypnosis for pain management in the perioperative context shows small to medium effects in favor of hypnosis on various post-surgical outcomes (eg, pain, emotional distress, medication consumption, and post-surgical recovery) across 2597 patients.8 Further, a more recent double-blind randomized controlled trial evaluating the use of post-surgical hypnosis for patients who underwent coronary artery bypass graft surgery showed that hypnosis resulted in lower post-operative pain intensity, anxiety and depressive symptoms, and opioid use.9 It is possible that the opioid system may also be linked to a person’s hypnotizability. Prescuittini et al10 suggest that a hypnotic assessment can aid in predicting responsiveness to opioids. Overall, there is promising preliminary evidence that clinical hypnosis for pain relief is associated with reduced opioid consumption.11 However, most published studies of clinical hypnosis for surgical patients have been limited to a one-time session of clinical hypnosis prior to surgery.8,9
The primary aim of the current RCT was to examine the effect of clinical hypnosis on opioid consumption during the first week after surgery. We hypothesize that the hypnosis treatment group will use less morphine-equivalent opioid medication daily during the first week after surgery than the standard care group. The secondary aims of this study were to evaluate the effect of hypnosis on post-surgical pain intensity, pain interference, anxiety, depressive symptoms, sleep, pain catastrophizing and the amount of opioids dispensed by pharmacy in the month after surgery. We also evaluated the effects of clinical hypnosis on cardiac vagal activity before and one-month after surgery using a measure of high-frequency heart-rate variability (HRV). Results of the HRV analyses will be reported in a companion manuscript.
Methods
Overview
This was a single-center, stratified, parallel-group, randomized-controlled trial conducted at the Toronto General Hospital (TGH) Transitional Pain Service. The study was designed and is reported according to the 2010 CONSORT statement (see Supplementary Table 1).12 This study was conducted in accordance with the Declaration of Helsinki. The study was reviewed and approved by the University Health Network (UHN) Research Ethics Board (certificate #: 17–5441) and the Human Participants Review Committee at York University (certificate #: e2019-031). TGH is one of several hospitals that make up UHN and the UHN REB oversees research at all UHN-affiliated hospitals. The trial was registered with ClinicalTrials.gov (registration #: NCT03730350) prior to recruitment. Not all the measures (ie, pain catastrophizing, one-month opioid use) and data analysis plans were included when the trial was registered at ClinicalTrials.gov, which was an oversight. However, the protocol, all outcome measures, and all plans for data analysis were included in the original and approved REB applications which pre-dated the trial’s registration at ClinicalTrials.gov. All participants provided informed written consent to participate prior to enrollment. Data will not be available for sharing.
Participants
Participants were all adults aged 18 years and older, scheduled for a surgical oncology procedure at the Toronto General Hospital. Exclusion criteria included patients with limited comprehension of English who could not understand the verbal component of clinical hypnosis due to hearing impairment, cognitive deficits, dementia or other causes that would limit comprehension; or a history of serious mental illness (eg, schizophrenia, dissociative identity disorder, or PTSD with dissociation) for which clinical hypnosis is contraindicated.
Sample Size Estimation
A priori sample size estimation for a within-between interaction effect in a repeated measures linear mixed-effects model indicated that a total of 80 participants were needed to identify small-to-medium effect size in post-surgical opioid consumption (in milligrams morphine equivalence; MME) with a type 1 error rate of 0.05, power = 0.80, and 0.5 correlation between repeated measures (G*Power, Dusseldorf, DE). An additional 12 participants (total N = 46 per group) were recruited to account for potential 15% drop out.
Procedure
Recruitment
Patients were recruited from the pre-admission surgery clinic and surgical oncology clinics approximately 1–3 weeks prior to surgery.
Pre-Surgical Study Visit
Immediately after informed written consent was obtained, participants completed an initial set of questionnaires and underwent electrocardiogram and respiration recording for heart rate variability (HRV) analysis. Participants were then randomly assigned to the clinical hypnosis or treatment-as-usual (TAU) group.
Stratified Randomization and Allocation Concealment
Participants were randomly assigned to one of two intervention arms, clinical hypnosis or treatment-as-usual. A computer-generated randomization schedule was created by a study co-investigator (JK) who had no clinical involvement in the trial using the software available at www.randomization.com. Randomization was stratified by patient’s current and/or past use of opioid medications (ie, opioid-experienced versus opioid naïve) with a 1:1 allocation using random block sizes of 10. Random allocation was concealed through the use of sequential, numbered, opaque, sealed envelopes.13 Randomization took place at the conclusion of the pre-surgical study visit after all assessments had taken place at which time a researcher opened the envelope containing the participant’s group allocation and informed the participant of their group assignment.
Inpatient Medical Data Collection
The following information was obtained from participants’ medical records regarding their inpatient stay. 1) Dose of opioid medication converted to mg in morphine equivalents (MME) used daily from time of surgery to time of hospital discharge; 2) pain ratings daily from day of surgery to day of hospital discharge; 3) duration of surgical procedure; 4) length of hospital stay; 5) number of days to transition from patient-controlled analgesia to oral opioid medication. This information was gathered by personnel who were blind to treatment allocation and not otherwise involved in the trial.
Post-Surgical Hypnosis Visit
Participants assigned to receive clinical hypnosis were visited in-hospital 1–3 days after surgery for a 15–20-minute session of clinical hypnosis.
One-Week Post-Surgical Survey
One week post-surgery, participants completed a short questionnaire packet assessing pain intensity, pain interference, mood, sleep, and pain catastrophizing. Questionnaires were administered in hospital to participants still admitted and by phone to participants who had been discharged.
One-Month Post-Surgical Assessment
All participants returned to hospital one month after surgery for HRV assessment and follow-up questionnaires. Questionnaires were the same as those completed at the pre-surgical study visit. If participants were unable to attend an in-person visit, follow-up questionnaires were mailed or completed over the phone. Participants also consented for the study team to contact their pharmacy to collect information on all opioid prescriptions filled since surgery.
Interventions
Clinical Hypnosis
Participants randomized to the clinical hypnosis group received standard perioperative care with the addition of the clinical hypnosis intervention. Participants completed one in-person session of clinical hypnosis 1–2 weeks prior to surgery, and a second session in-hospital 1–3 days after surgery. The scripts for all hypnosis sessions were developed by the TPS pain psychologist and study co-investigator (Dr. Aliza Weinrib), based on the clinical literature,14,15 and were manualized for use in this study. Hypnosis scripts included a combination of principles of Acceptance and Commitment Therapy (ACT) (ie, acceptance of acute pain) as well as Eriksonian pain intensity reduction.15 All clinicians who provided hypnosis during this study (ie, TPS psychologist and psychology trainees) were trained by the TPS pain psychologist and certified in clinical hypnosis.
Pre-Surgical Hypnosis
The pre-surgical hypnosis session was 20–25 minutes in duration and took place immediately after the pre-surgical assessment. The session aimed to prepare the participant for surgery by reducing anxiety and introducing pain coping strategies, including breathing, visualization, and relaxation techniques, that could be used after surgery. At the outset of the session, the clinician provided information on clinical hypnosis, including the use of hypnosis for pain management and an overview of the pre-surgery hypnosis session. The participant was instructed to think of a relaxing “special place” to visualize during the session (eg, a peaceful beach the participant had been to on vacation, a cabin on a lake, a beautiful garden). The clinician then led the pre-surgical hypnosis intervention, including (1) hypnotic induction with slow, deep breathing; (2) hypnotic deepening using progressive relaxation with suggestions of warmth spreading through the body; (3) suggestions of mental imagery and pleasurable sensory experiences in the “special place” of the participant’s choosing; (4) suggestions of positive imagery for the participant’s experiences leading up to, during, and after surgery; (5) suggestions for the participant to engage in self-hypnosis using imagery and breathing techniques before surgery and during surgical recovery; and (6) alerting with counting. Throughout hypnosis, the clinician observed the participant and adapted instructions according to bodily cues of relaxation (eg, facial muscles relaxing, slow breathing rhythm).
Post-Surgical Hypnosis
Participants were visited by a clinician from the TPS pain psychology team on post-operative day one or at the first available time prior to hospital discharge. Participants were guided through a clinical hypnosis session targeted at increasing comfort and pain relief. Participants were given the option to incorporate their chosen “special place” from the pre-surgery hypnosis session or to be guided in a hypnosis session of having “a trip to the beach”. Participants were also given the option to be alerted at the end of the session or to be left to drift off to sleep. The post-surgery hypnosis session lasted 15–20 minutes and included the following components: (1) hypnotic induction with slow, deep, breathing; (2) suggestions for minimizing impact of hospital room noise; (3) hypnotic deepening with progressive relaxations and suggestions of warmth; (4) suggestions of mental imagery and pleasurable sensory experiences in a “special place” or “a trip to the beach”; (5) suggestions for sensory substitution, reduction of pain intensity, and reduction of pain unpleasantness; (6) suggestions for participant to engage in self-hypnosis; and (7) alerting with counting, or leaving patient to drift off to sleep.
Participants were also provided with audio recordings of hypnosis scripts for use during hospitalization and at home after hospital discharge. The audio tracks were accessible on the study’s private YouTube webpage. If participants did not have a device that could access recordings, an MP3 player was provided for the duration of the hospital stay. Participants were encouraged to listen to hypnosis recordings daily.
Treatment-as-Usual
Participants randomized to the TAU study arm received standard perioperative care. HRV was measured during two phases for participants in both conditions: 1) Rest in a seated position with eyes closed (5 minutes) and 2) guided relaxation using features of hypnotic induction, including deep breathing and relaxing suggestions (eg, warmth, heaviness throughout body) (10 minutes). After the completion of all measures at the one-month post-surgical questionnaires and HRV assessment, participants in the TAU group were invited to undergo a session of clinical hypnosis and were provided access to the hypnosis audio recordings.
Outcomes
Primary Outcome
Daily opioid consumption (in milligrams morphine equivalence; MME) during hospitalization was the primary outcome of the study. Opioid use on each day was abstracted from the medical record and converted to MME.16
Secondary Outcomes
Present Pain Intensity
The “pain now” scale of the Brief Pain Inventory17 was administered to participants who were asked to rate their current pain on an 11-point NRS from 0, no pain, to 10, pain as bad as you can imagine. Numerical rating scales have been extensively validated (eg, see18), and the “pain now” pain intensity rating scale has been recommended as a clinical trial outcome.19
Pain Interference
The pain interference subscale of the Brief Pain Inventory17 was used to measure the extent to which pain interferes in everyday activities. Respondents are asked to rate how pain interferes with seven daily activities including general activity, mood, sleep, walking ability, work, relationships with other people, and enjoyment in life, on an 11-point NRS, from 0, does not interfere, to 10, completely interferes (maximum score of 70). The BPI has been used extensively in a wide range of pain conditions and has excellent psychometric properties.20,21
Sleep
An 8-item self-report measure from the Patient Report Outcome Measurement Information System (PROMIS) was used to assess sleep disturbance and sleep impairment.22 Sleep disturbance items assess perceptions of sleep quality, sleep depth, and restoration, whereas sleep impairment items focus on alertness, sleepiness, tiredness and associated functional impairment. Responses are recorded on a scale from 1, not at all, to 5, very much. Items are summed to create a total score (maximum of 40). The PROMIS short-form sleep measures are validated and have been found to outperform other commonly used self-report sleep measures.23
Depressed Mood
The Center for Epidemiological Studies – Depression scale (CES-D) was used to measure depressive symptoms.24 The CES-D is a 20-item measure that asks respondents to rate symptoms of depression (eg, restless sleep, feeling lonely, poor appetite) on a scale from 0, rarely or none of the time, to 3, most or almost all of the time. The CES-D has previously been validated for use with patients with pain.25 Cut-off scores for mild, moderate, and severe depression are 10, 16, and 25, respectively.24
Anxiety
The Generalized Anxiety Disorder – 7 (GAD-7) was used as a measure of anxiety symptoms.26 This 7-item scale assesses signs of generalized anxiety (eg, “feeling afraid as if something awful might happen”) with response options ranging from 1, not at all, to 4, nearly every day. Scores of 5, 10, and 15 represent cut-offs for mild, moderate, and severe levels of anxiety, respectively.26 The GAD-7 is frequently used in primary and outpatient care populations and has been found to have adequate validity and good reliability.26
Pain Catastrophizing
Catastrophic thinking about pain was assessed using the Pain Catastrophizing Scale (PCS), which measures an individual’s tendency to ruminate, exaggerate, and feel helpless about their pain.27 Participants indicate the degree to which they agree, from 0, not at all, to 4, all the time, with thirteen statements. The PCS has high internal consistency (Cronbach’s α = 0.87) and has been extensively validated.27–30 The 13 items of the PCS are summed to create a total score (maximum score of 52). A cut-off score of 24 indicates clinically significant catastrophic thinking about pain.31
One-Month Opioids Dispensed
Data on opioid use after hospital discharge was collected by contacting participant’s pharmacists at one month after surgery and collecting information on all opioid prescriptions filled since the date of surgery. The amount of opioids dispensed was converted to MME.16
Data Analysis
Linear mixed effects (LME) models were used to conduct intent-to-treat analyses of the impact of hypnosis on primary and secondary outcomes. F-test results from LME models can be interpreted as if mixed design ANOVA was conducted with fixed effect factor (treatment) and repeated measures factor (time). However, LME modeling increases degrees of freedom, and thus power, for examination of within-subject effects.32 Also, LME is robust against missing data due to the use of maximum likelihood parameter estimation, and has been recommended as a strategy for handling missing data in intent-to-treat analyses.33,34 All participants contributing at least one data point were included in intent-to-treat analyses. Dependent responses were entered at Level 1 of the model, while Subject ID was used to group Level 2 units, accounting for non-independence of repeated observations. In each model, time was entered as a repeated factor and a first-order autoregressive covariance structure was specified.
The primary aim of the current study was to examine the effect of hypnosis on opioid consumption (daily MME) during the first week after surgery. A 2 (Group: hypnosis, TAU) by 7 (Time: POD 0 through POD 6) LME was conducted. Secondary outcomes included pain intensity, pain interference, anxiety, depressive symptoms, one-month opioid use, sleep, and pain catastrophizing. Each secondary outcome was examined using 2 (Group: hypnosis, TAU) by 2 (Time: pre-surgery, one-week post-surgery) LME models. Length of hospital stay was included as a covariate in each model.
Results
Recruitment and Participant Flow
Recruitment was conducted from November 6, 2018 to November 1, 2019 and concluded after the target number of participants (N = 92) had been randomized. Figure 1 shows the CONSORT flow diagram for the present study. Participants were randomized to receive clinical hypnosis (n = 45) or TAU (n = 47). Nine participants were excluded prior to randomization, and one participant in the TAU group had a cancelled surgery after randomization. All patients in the clinical hypnosis group received the pre-surgery hypnosis session, and 38 participants completed the post-surgery hypnosis session. Twenty-nine participants in the clinical hypnosis group and 30 participants in the TAU group completed the one-month post-surgery assessment. Participant attrition rates were 35.6% for the clinical hypnosis group and 36.2% for the TAU group. Reasons for attrition in the clinical hypnosis group included personal reasons (n = 2), death (n = 1), unable to schedule follow-up visit (n = 5), lost to follow-up (n = 8), and development of cardiac complications (n = 1). Reasons for attrition in the TAU group included cancelled surgery (n = 1), personal reasons (n = 1), unable to schedule follow-up visit (n = 1), and lost to follow-up (n = 13). Table 1 shows the number of days between relevant study time points for the two groups, including surgery and inpatient visit; surgery and one-month follow-up; and inpatient visit and one-month follow-up.
 |
Table 1 Timing of Participant Assessment Visits Relative to Surgery |
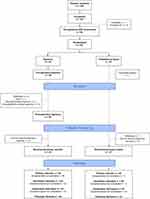 |
Figure 1 Consolidated Standards of Reporting Trials 2010 flow diagram showing participant flow for enrolment, group classification, randomization to intervention, and analyses. |
Participant Characteristics
Participant demographic, medical, and surgical histories are summarized in Table 2. The mean age of participants was 57.6 years (SD = 10.9), the mean duration of surgery was 260 minutes (SD = 150.2), and the average length of hospital stay was 4.9 days (SD = 5.3). The majority of participants were female (53.6%) and had pain prior to surgery (56%). Only two participants (one patient per group) were taking opioids prior to surgery. Groups did not differ on any medical or surgical variable, all p > 0.05. Descriptive statistics for opioid outcomes are listed in Table 3 and descriptive statistics for pain and psychological outcomes are listed in Table 4.
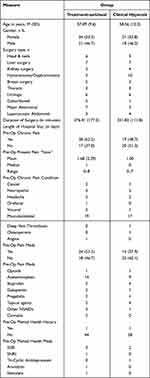 |
Table 2 Demographic and Clinical Characteristics of Study Groups |
 |
Table 3 Means and Standard Deviations for Daily Opioid Consumption (in MME) |
 |
Table 4 Means and Standard Deviations for Secondary and Exploratory Outcome Variables |
Opioid Use During the First Week of Hospitalization
Opioid use on each day during the first week after surgery was obtained from the medical record and analyzed using a 2 (Group: hypnosis, TAU) by 7 (Time: POD 0 through POD 6) LME model. Length of hospital stay was entered into the model as a covariate. On average, opioid consumption decreased for all individuals over the first week after surgery, F (6, 323.79) = 2.43, p = 0.03. However, this was qualified by a significant interaction between Group and Time, F (6, 323.34) = 3.32, p = 0.003. Post-hoc tests of least significant differences were conducted to examine group differences on each day during the first week after surgery. Individuals who received hypnosis consumed significantly less opioids on the first day after surgery (POD 1), mean difference = 15.6 MME, d = 0.39, p = 0.01, and on the fourth day after surgery (POD 3), mean difference = 13.9 MME, d = 0.36, p = 0.04. A test of simple slopes indicated that clinical hypnosis protected against an increase in opioid consumption from day of surgery to the first day after surgery, clinical hypnosis mean change = 9.9 MME, TAU mean change = 23.9 MME, p = 0.03 d = 0.46. Differences in opioid consumption during the first week after surgery are depicted in Figure 2.
 |
Figure 2 Daily opioid consumption in MME during the first week after major oncologic surgery. Observed means and standard errors are plotted. *p < 0.05. |
Secondary Outcomes
Self-report measures of pain severity, pain interference, sleep impairment, depressed mood, and anxiety were completed at pre-surgical assessment, and one week after surgery. Separate 2 (Group: hypnosis, TAU) by 2 (Time: pre-surgery, one-week post-surgery) LME models were examined for each outcome.
Pain Intensity
On average, pain intensity increased from baseline to one-week after surgery, F (1, 75.98) = 18.2, p < 0.001. However, this change was not impacted by treatment group as evidenced by a non-significant Group × Time interaction effect, F (1, 83.66) = 0.01, p = 0.93.
Pain Interference
On average, pain interference increased from baseline to one-week after surgery, F (1, 77.82) = 28.88, p < 0.001. This change did not differ between the two treatment groups, F (1, 78.89) = 0.00, p = 0.99. Across time points, the main effect of treatment group was not significant, F (1, 83.81)= 3.48, p = 0.066; however, of note, individuals who received hypnosis reported marginally less pain interference both before and after surgery.
Sleep Impairment, Depressed Mood, and Anxiety
On average, sleep impairment, depressed mood, and anxiety did not change from the pre-surgical assessment to one-week after surgery, all p’s > 0.05. Likewise, there were no interactions between treatment group and time for these outcomes, all p’s > 0.05.
Pain Catastrophizing
Pain catastrophizing was assessed using 2 (Group: hypnosis, TAU) by 2 (Time: pre-surgery, one-week post-surgery) LME models. Changes in pain catastrophizing after surgery differed between treatment groups, F (1, 75.26) = 4.04, p = 0.048. Follow-up tests of simple effects indicated that PCS scores increased in the TAU group from the pre-surgical assessment to one-week after surgery, mean increase = 2.83, F (1, 74.92) = 4.29, p = 0.042, whereas pain catastrophizing did not change for individuals who received hypnosis, mean increase = −1.23, F (1, 75.41) = 0.69, p = 0.41. However, the simple effect of Group was not significant at one-week after surgery, p = 0.19. This treatment effect is depicted in Figure 3.
 |
Figure 3 Catastrophic thinking about pain from pre-surgical assessment to one week after surgery. Observed means and standard errors are plotted. |
One-Month Opioids Dispensed
The effect of clinical hypnosis on one-month opioid prescribing totals was examined using a non-parametric Mann–Whitney U-test to account for non-normality of data. MME dispensed to participants in the month following surgery did not differ significantly between the hypnosis group (median = 43.75 MME, IQR = 100.0) and the treatment-as-usual group (median = 0.0 MME, IQR = 165.0), U = 629.0, p = 1.0.
Discussion
This randomized, controlled trial evaluated the effects of a brief clinical hypnosis intervention, delivered pre- and post-operatively (less than 50 minutes of clinical hypnosis in total), on post-operative opioid consumption among adults undergoing major oncologic surgery. Across groups, participants increased opioid consumption from the day of surgery to the next day and then slowly reduced opioid use over subsequent days. However, the changes over time were significantly different for the two groups (ie, Group × Time interaction). Specifically, individuals who underwent clinical hypnosis had a less dramatic increase in opioid usage, and consumed less opioids on the first and third days after surgery. These results indicate that the delivery of clinical hypnosis for patients undergoing cancer-related surgeries significantly decreased MME consumed in the acute post-operative phase. Importantly, the clinical hypnosis provided in this study did not explicitly direct participants to use less opioids, but rather promoted pain relief post-operatively, suggesting that future studies should evaluate the use of clinical hypnosis synergistically with pain medication to promote optimal outcomes (ie, lower acute pain intensity).
The results of the present study extend previous findings that show clinical hypnosis is beneficial in surgical settings. Two past meta-analyses have confirmed that hypnosis in the perioperative period is beneficial for a range of outcomes, including medication use.11,35 However, these analyses did not specifically address opioid sparing effects. The present results are consistent with those of Nowak et al36 who found that intraoperative hypnotic suggestion, administered via MP3, reduced opioid consumption in the 24 hours after surgery. The present results also show that hypnosis can be beneficial for patients undergoing longer, more complex surgeries. The average duration of surgery in the current study was >4 hours, whereas the study by Nowak et al focused exclusively on elective surgeries with a maximum length of three hours. The current study also showed that the opioid sparing effect of clinical hypnosis persisted up to day 3 of the longer hospital stay associated with these more complex surgeries.
The opioid sparing effect of clinical hypnosis identified in the current study was particularly notable on the first day after surgery. There was a small-moderate effect size difference between groups in opioid consumption on the first day after surgery (d = 0.39). Moreover, there was a moderate effect size difference favoring hypnosis in the degree of increase in opioid consumption from the day of surgery to the first day after surgery (d = 0.46). This broadly aligns with the delivery of the post-operative hypnosis session. The majority (62%) of participants had their post-operative session on the first day after surgery, with an additional 25% having session one on the following day. It is likely that active participation in clinical hypnosis provided the greatest benefit and further research is needed on the most beneficial dosing and administration of perioperative hypnosis.
To the authors’ knowledge, this is the first study to examine the effects of clinical hypnosis on pharmacy dispensed opioids in the month after surgery. On average, individuals who received clinical hypnosis had fewer opioids dispensed, although this was not statistically significant. Indeed, a large proportion of participants in each group (54% in the TAU group and 47% in the clinical hypnosis group) did not have any opioid prescriptions filled, and only two participants, both in the TAU group, were prescribed opioids in excess of 50 MME/day. As such, any effects of clinical hypnosis on opioid use in the month after surgery may be obscured by a floor effect. Future studies should evaluate a larger number of patients to determine whether clinical hypnosis is associated with a longer-term reduction in opioid use after surgery.
Contrary to previous meta-analytic evaluations of perioperative clinical hypnosis,11,35 we did not identify any effect of clinical hypnosis on measures of pain intensity, pain interference, sleep impairment, depressed mood, or anxiety at one-week after surgery. Of these secondary outcomes, only pain intensity and pain interference changed from the pre-operative assessment to one-week after surgery as would be expected following major surgery. Notably, however, patients in the clinical hypnosis group reported no difference in pain intensity while using less opioids. Moreover, average scores on measures of sleep impairment, depressed mood, and anxiety were not clinically significant prior to surgery. Thus, the impact of hypnosis on secondary outcomes may have been obscured by floor effects. It is possible that this intervention may have greater benefit in a more distressed population, who would be at higher risk of long-term, high-dose opioid use after surgery.
Exploratory analyses suggest that clinical hypnosis protected individuals against increases in catastrophic thinking about pain after surgery. That is, participants in the TAU group had an increase in catastrophic thinking from pre-operative assessment to one-week post-surgery, but those who received clinical hypnosis did not. The beneficial effect of hypnosis on catastrophic thinking is in line with previous experimental and clinical research that have identified catastrophic thinking about pain as a target for clinical hypnosis interventions.37–39 It is noteworthy, however, that individuals in the current study did not, on average, endorse a clinically significant degree of catastrophic thinking about pain.31 Indeed, some catastrophic thinking about pain after surgery is normative and situational. Future research should examine the impact of perioperative clinical hypnosis for individuals with high levels of catastrophic thinking about pain.
This study has several limitations. First, the vast majority of participants were opioid naïve prior to surgery. As such, it is not possible to determine the generalizability of the results to patients who are on large doses of opioids pre-operatively. Future work should focus on pragmatic trials and clinical effectiveness across a broad range of patient populations and delivered by a range of different clinicians trained in clinical hypnosis. Second, participants were not blinded to group assignment. There are limited options for sham clinical hypnosis,40 and such options (eg, white noise) may be an unethical use of patient’s time in the peri-surgical period. Third, fidelity checks were not performed over the course of this RCT. All clinicians conducting hypnosis were trained by the TPS pain psychologist and closely followed the hypnosis scripts. Fourth, clinical hypnosis recordings were provided to patients prior to surgery, but the use of these recordings was not systematically tracked. Therefore, we are unable to evaluate whether the reduction in opioid consumption and pain catastrophizing was associated with the amount of time participants spent listening to recordings. Evaluating dose-response will be an important component of future research on perioperative clinical hypnosis. Given the time-intensive nature of clinical hypnosis, it will be important to determine if audio recorded clinical hypnosis sessions are as effective as in-person clinical hypnosis sessions. Further differentiating which patients/ medical procedures benefit most from clinical hypnosis will aid in determining which populations would benefit most from adjunct clinical hypnosis. Fifth, we did not ask participants who used opioids at home how they were using their opioid prescription (eg, frequency, dose), but rather we examined the dispensing pattern. Future studies should consider closely following patients’ patterns of opioid use. Sixth, we acknowledge that hypnotizability varies in the general population and this suggests that future studies screen for hypnotizability using, for example, a hypnosis assessment. Lastly, it is worth noting that the TAU group received a brief relaxation procedure prior to surgery, as part of the pre-surgery HRV assessment, which is more than what a typical control group receives in perioperative studies.
In sum, individuals who received a brief session of clinical hypnosis before and after surgery consumed less opioids during their hospital stay and reported less catastrophic thinking about their pain compared to those undergoing treatment-as-usual. These results support the clinical efficacy of clinical hypnosis as a tool to reduce opioid requirement in the perioperative period. Clinical hypnosis can reduce opioid use, while keeping patients comfortable after surgery, an important consideration in the age of the opioid crisis.
Trial Registration
ClinicalTrials.gov registration #: NCT03730350.
Disclosure
Brittany Rosenbloom was supported by a Canadian Institutes of Health Research (CIHR) CGS Doctoral Award and a CIHR Banting Fellowship. Muhammad Abid Azam was supported by a CIHR CGS Doctoral Award. Hance Clarke is supported by a Merit Award from the Department of Anesthesiology and Pain Medicine at the University of Toronto. Joel Katz is supported by a CIHR Canada Research Chair in Health Psychology at York University. The authors report no other conflicts of interest in this work.
References
1. Kolodny A, Courtwright DT, Hwang CS., et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36(1):559–574. doi:10.1146/annurev-publhealth-031914-122957
2. Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ. 2014;3:348.
3. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695. doi:10.2147/JPR.S91924
4. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286–1293. doi:10.1001/jamainternmed.2016.3298
5. Geagea D, Griffin B, Kimble R, Polito V, Terhune DB, Tyack Z. Hypnotherapy for procedural pain, itch, and state anxiety in children with acute burns: a feasibility and acceptability study protocol. Pilot Feasibility Stud. 2022;8(1):58. doi:10.1186/s40814-022-01017-z
6. Jensen MP. Hypnosis for chronic pain management: a new hope. Pain. 2009;146(3):235–237. doi:10.1016/j.pain.2009.06.027
7. Thompson T, Terhune DB, Oram C, et al. The effectiveness of hypnosis for pain relief: a systematic review and meta-analysis of 85 controlled experimental trials. Neurosci Biobehav Rev. 2019;99:298–310. doi:10.1016/j.neubiorev.2019.02.013
8. Tefikow S, Barth J, Maichrowitz S, Beelmann A, Strauss B, Rosendahl J. Efficacy of hypnosis in adults undergoing surgery or medical procedures: a meta-analysis of randomized controlled trials. Clin Psychol Rev. 2013;33(5):623–636. doi:10.1016/j.cpr.2013.03.005
9. Akgul A, Guner B, Çırak M, Çelik D, Hergünsel O, Bedirhan S. The beneficial effect of hypnosis in elective cardiac surgery: a preliminary study. Thorac Cardiovasc Surg. 2016;64(07):581–588. doi:10.1055/s-0036-1580623
10. Presciuttini S, Curcio M, Sciarrino R, Scatena F, Jensen MP, Santarcangelo EL. Polymorphism of opioid receptors μ1 in highly hypnotizable subjects. Int J Clin Exp Hypn. 2018;66(1):106–118. doi:10.1080/00207144.2018.1396128
11. Montgomery GH, David D, Winkel G, Silverstein JH, Bovbjerg DH. The effectiveness of adjunctive hypnosis with surgical patients: a meta-analysis. Anesth Analg. 2002;94(6):1639–1645. doi:10.1213/00000539-200206000-00052
12. Schulz KF, Altman DG, Moher D, CONSORT Group*. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732. doi:10.7326/0003-4819-152-11-201006010-00232
13. Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. Crit Care Med. 2005;20(2):187–191. doi:10.1016/j.jcrc.2005.04.005
14. Jensen MP. Hypnosis for Chronic Pain Management: Therapist Guide. Oxford University Press; 2011.
15. Patterson DR. Clinical Hypnosis for Pain Control. American Psychological Association; 2010.
16. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic noncancer pain. Can Med Assoc J. 2017;189(18):E659–E666. doi:10.1503/cmaj.170363
17. Cleeland CS, Ryan K. The brief pain inventory. Pain Res Group. 1991;20:143–147.
18. Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399–2404. doi:10.1016/j.pain.2011.07.005
19. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi:10.1016/j.pain.2004.09.012
20. Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–318. doi:10.1097/00002508-200409000-00005
21. Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi:10.1016/j.jpain.2003.12.005
22. Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMISTM sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2012;10(1):6–24. doi:10.1080/15402002.2012.636266
23. Chimenti RL, Rakel BA, Dailey DL, et al. Test–retest reliability and responsiveness of PROMIS sleep short forms within an RCT in women with fibromyalgia. Front Pain Res. 2021;2:19. doi:10.3389/fpain.2021.682072
24. Radloff LS. The CES-D Scale: a self-reported depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi:10.1177/014662167700100306
25. Geisser ME, Roth RS, Robinson ME. Assessing depression among persons with chronic pain using the center for epidemiological studies-depression scale and the beck depression inventory: a comparative analysis. Clin J Pain. 1997;13(2):163–170. doi:10.1097/00002508-199706000-00011
26. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of Internal Medicine. 2006;166(10):1092–1097. doi:10.1001/archinte.166.10.1092
27. Sullivan MJL, Bishop S, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–532. doi:10.1037/1040-3590.7.4.524
28. Lamé IE, Peters ML, Kessels AG, Van Kleef M, Patijn J. Test--retest stability of the pain catastrophizing scale and the Tampa scale for Kinesiophobia in chronic pain over a longer period of time. J Health Psychol. 2008;13(6):820–826. doi:10.1177/1359105308093866
29. Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The pain catastrophizing scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23(4):351–365. doi:10.1023/A:1005548801037
30. Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O’Neill E. Factor structure, reliability, and validity of the pain catastrophizing scale. J Behav Med. 1997;20(6):589–605. doi:10.1023/A:1025570508954
31. Scott W, Wideman TH, Sullivan MJ. Clinically meaningful scores on pain catastrophizing before and after multidisciplinary rehabilitation: a prospective study of individuals with subacute pain after whiplash injury. Clin J Pain. 2013. doi:10.1097/AJP.0b013e31828eee6c
32. Hox JJ, Moerbeek M, van de Schoot R. Multilevel Analysis: Techniques and Applications. Routledge; 2010.
33. Chakraborty H, Gu H. A Mixed Model Approach for Intent-to-Treat Analysis in Longitudinal Clinical Trials with Missing Values. RTI Press; 2009. Available from: http://www.ncbi.nlm.nih.gov/books/NBK538904/.
34. Peters SAE, Bots ML, den Ruijter HM, et al. Multiple imputation of missing repeated outcome measurements did not add to linear mixed-effects models. J Clin Epidemiol. 2012;65(6):686–695. doi:10.1016/j.jclinepi.2011.11.012
35. Holler M, Koranyi S, Strauss B, Rosendahl J. Efficacy of hypnosis in adults undergoing surgical procedures: a meta-analytic update. Clin Psychol Rev. 2021;85:102001. doi:10.1016/j.cpr.2021.102001
36. Nowak H, Zech N, Asmussen S, et al. Effect of therapeutic suggestions during general anaesthesia on postoperative pain and opioid use: multicentre randomised controlled trial. BMJ. 2020;371:m4284.
37. Jensen MP, Ehde DM, Gertz KJ, et al. Effects of self-hypnosis training and cognitive restructuring on daily pain intensity and catastrophizing in individuals with multiple sclerosis and chronic pain. Int J Clin Exp Hypn. 2010;59(1):45–63. doi:10.1080/00207144.2011.522892
38. Kjøgx H, Kasch H, Zachariae R, Svensson P, Jensen TS, Vase L. Experimental manipulations of pain catastrophizing influence pain levels in patients with chronic pain and healthy volunteers. Pain. 2016;157(6):1287–1296. doi:10.1097/j.pain.0000000000000519
39. Lee JK, Zubaidah J, Fadhilah ISI, Normala I, Jensen MP. Prerecorded hypnotic peri-surgical intervention to alleviate risk of chronic postsurgical pain in total knee replacement: a randomized controlled pilot study. Int J Clin Exp Hypn. 2019;67(2):217–245. doi:10.1080/00207144.2019.1580975
40. Kendrick C, Koep L, Johnson A, Fisher W, Elkins G. Feasibility of a sham hypnosis: empirical data and implications for randomized trials of hypnosis. Contemp Hypn Integr Ther. 2012;29(4):317–331.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
