Back to Journals » Clinical Ophthalmology » Volume 17
A Randomized, Controlled Trial Comparing Tearcare® and Cyclosporine Ophthalmic Emulsion for the Treatment of Dry Eye Disease (SAHARA)
Authors Ayres BD, Bloomenstein MR, Loh J, Chester T , Saenz B , Echegoyen J, Kannarr SR, Perez VL, Rodriguez TC, Dickerson Jr JE
Received 25 October 2023
Accepted for publication 11 December 2023
Published 18 December 2023 Volume 2023:17 Pages 3925—3940
DOI https://doi.org/10.2147/OPTH.S442971
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Brandon D Ayres,1 Marc R Bloomenstein,2 Jennifer Loh,3 Thomas Chester,4 Bobby Saenz,5,6 Julio Echegoyen,7 Shane R Kannarr,8 Victor L Perez,9 Tomasita C Rodriguez,10 Jaime E Dickerson Jr10,11
1Private Practice, Philadelphia, PA, USA; 2Schwartz Laser Eye Center, Scottsdale, AZ, USA; 3Loh Ophthalmology Associates, Miami, FL, USA; 4Cleveland Eye Clinic, Brecksville, OH, USA; 5Rosenberg School of Optometry, University of the Incarnate Word, San Antonio, TX, USA; 6LASIK San Antonio, Kerrville, TX, USA; 7Gordon Schanzlin New Vision Institute, La Jolla, CA, USA; 8Kannarr Eye Care, Pittsburg, KS, USA; 9Department of Ophthalmology, Duke University Medical Center, Durham, NC, USA; 10Sight Sciences, Menlo Park, CA, USA; 11North Texas Eye Research Institute, University of North Texas Health Science Center, Fort Worth, TX, USA
Correspondence: Jaime E Dickerson Jr, Email [email protected]
Purpose: We compare outcomes in eyes with dry eye disease (DED) treated with TearCare (TC) or topical cyclosporine 0.05% (RESTASIS; CsA).
Setting: Nineteen ophthalmic and optometric practices in 11 US states.
Design: Multicenter, randomized, assessor-masked, controlled IRB-approved trial. Eligible subjects: ≥ 22 years of age, dry eye symptoms within 3– 6 months, Tear Break-up Time (TBUT) ≥ 1 to ≤ 7 s, Meibomian Gland Secretion Score (MGSS) ≤ 12, Ocular Surface Disease Index (OSDI) of 23– 79. Randomized (1:1) to TC or CsA. TC subjects treated at baseline and month 5; CsA was twice daily for 6 months.
Methods: Follow-up visits were scheduled for Day 1, Week 1, Months 1, 3, and 6 with primary inference at Month 6. Primary outcomes: TBUT and OSDI; secondary outcomes: MGSS, conjunctival and corneal staining, eye dryness score (EDS), symptoms assessment in dry eye (SANDE) score, and Schirmer tear score (STS). Safety assessments included adverse events, best corrected visual acuity, intraocular pressure, and slit-lamp findings.
Results: Overall, 345 subjects, 172 TC and 173 CsA. TBUT improved at all time points in both groups (p< 0.0001), with statistically greater improvement for TC versus CsA (p=0.0006). OSDI improved significantly at all time points in both groups (p< 0.0001) with no significant differences between treatments. MGSS and other measures of meibomian gland function improved significantly more with TC eyes versus CsA; other secondary outcomes showed significant improvements in both groups with no difference between groups. Treatment-related adverse events were uncommon (10 total, 8 in the CsA group consistent with prior CsA studies); most (9/10) mild.
Conclusion: TC provides statistically superior and sustained improvement in TBUT and multiple measures of meibomian gland secretion, and non-inferior improvement in OSDI, corneal and conjunctival staining, SANDE, EDS, and STS versus CsA. TC should be a preferred treatment for DED associated with MGD.
Keywords: dry eye disease, meibomian gland dysfunction, restasis, TearCare, cyclosporine
A Letter to the Editor has been published for this article.
A Response to Letter by Mr Chaurasiya has been published for this article.
Introduction
Dry eye disease (DED) is likely the most common ocular condition affecting adults in the United States and the world. DED has a gender- and age-specific prevalence, affecting 5.7% of women under 50 years of age and rising to 9.8% of women aged 75 years or older,1 and affecting 3.9% of men aged 50–54 years and 7.7% of men aged 80 years or older.2 Causal and risk factors for DED are multiple; however, meibomian gland dysfunction (MGD) may be the leading cause of DED globally,3 accounting for up to 86% of DED cases.4
Treatments for DED and MGD typically begin with lid hygiene. Numerous medications are available to treat DED, including topical cyclosporine A5 and lifitegrast,6,7 and more recently intranasal varenicline.8,9 Likewise, several in-home and in-office procedural-based strategies that include lid warming and massage treatments, as well as intense pulsed light therapy, meibomian gland probing, radio frequency, and microblepharoexfoliation therapies are available to manage MGD; also, various pharmacological therapies—including oral tetracyclines, topical hypochlorous acid, and corticosteroids—can be used to treat MGD.10
TearCare (TC) (Sight Sciences, Menlo Park, CA, USA) is an office-based thermal therapeutic eyelid technology cleared by the US Food and Drug Administration for the treatment of evaporative DED due to MGD.11 The TC procedure involves the debridement of all four eyelid margins at the slit lamp, calibrated thermal liquification of gland obstructions, and the removal of melted meibum from the meibomian glands within all four eyelids. The TC system12,13 consists of two wearable devices (SmartLids) affixed to the upper and lower eyelids of both eyes using medical-grade adhesive such that blinking is unimpeded. These devices are connected to a central controller (SmartHub) that gradually increases the temperature at the eyelid surface to 45°C (above the 41°C threshold necessary to soften and melt meibum).14 The treatment temperature is safely maintained by constant (240x per second) SmartLid sensor/SmartHub communication on an eyelid-by-eyelid basis.15 The thermal procedure is followed by manual gland expression using the provided purpose-designed device (Clearance Assistant Plus) supplied with the disposable SmartLids. In a pair of randomized clinical trials, the TC procedure provided statistically significant improvements in tear break-up time (TBUT), meibomian gland secretion score (MGSS), corneal and conjunctival staining, eye dryness score (EDS), symptoms assessment questionnaire in dry eye (SANDE) score, and ocular surface disease index (OSDI) score from baseline.13,16
Cyclosporine is an immunomodulatory drug derived from the fungi Tolypocladium inflatum.17 Cyclosporine 0.05% ophthalmic emulsion (CsA; Restasis; Allergan, an AbbVie company, Dublin, Ireland) was approved in 2002 by the FDA to increase tear production in patients with deficient tear production thought to be due to inflammation associated with keratoconjunctivitis sicca.18 The mechanism of action of cyclosporine is thought to be two-fold. First, by binding cyclophilin A, calcineurin is inhibited and consequently T-cell activation. Second, by binding to cyclophilin D, cyclosporine inhibits initiation of cellular apoptosis.17 Since its introduction, CsA has become widely prescribed for DED of various etiologies, with multibillion annual sales in the United States.19,20
In this prospective randomized trial, we have compared common and clinically relevant clinical outcomes with TC versus CsA; in eyes with DED associated with MGD.
Methods
Study Design
This was a prospective, randomized, assessor-masked, multicenter, controlled superiority trial conducted at 19 ophthalmic and optometric practices in 11 US states (AZ, CA, CO, FL, IL, KS, MO, OH, PA, RI, TX). Subjects were recruited from April 2021 to September 2022 with the last subject completing the 6-month primary endpoint visit in March 2023. The protocol was approved by all relevant ethics boards (Central IRB was WCG IRB Puyallup WA, USA) and the study was registered at clinicaltrials.gov (NCT04795752) prior to study commencement. The trial was conducted in accordance with the tenets of the Declaration of Helsinki and all subjects provided written informed consent to participate.
Study Population
Eligible subjects were adults aged 22 years or older reporting that they had experienced dry eye symptoms in the preceding 3–6 months requiring the use of artificial tears or other lubricants regularly in the preceding 1 month for symptomatic relief. Criteria specific to ocular surface disease required in both eyes included an anesthetized Schirmer tear score (STS) >5 mm and <15 mm, OSDI score between 23 and 79, TBUT >1 and <7 s, MGSS of <12 in both eyes, at least 15 expressible meibomian glands in each lower lid using a cotton swab at the slit lamp, and best-corrected visual acuity (BCVA) 20/100 or better in both eyes. Key exclusion criteria included use of any of the following medications: cyclosporine or lifitegrast eye drops within 60 days of baseline, antihistamines by any route within 7 days, isotretinoin at any time, oral tetracyclines or azithromycin within 30 days, topical ophthalmic antibiotics, anti-glaucoma medications, steroids, or nonsteroidal anti-inflammatory drugs (NSAIDs) within 30 days, or any systemic medication known to cause ocular dryness if the dose had been changed within 30 days. Use of any of the following dry eye treatments was also exclusionary: office-based treatments (intense pulsed light, TC, thermal pulsation, etc.) within 12 months of baseline, planned or recent (within 90 days) eye or eyelid surgery, meibomian gland expression within 6 months, blepharoexfoliation or debridement within 3 months, punctal occlusion or plugs, tear neurostimulator use within 2 weeks, or any prior meibomian gland probing. Other exclusion criteria included eyelid or ocular surface surgery within the prior 12 months, contact lens use within 2 weeks, history of herpetic eye disease, active or recurrent ocular inflammation, clinically significant anterior blepharitis with or without collarettes or flakes, or other findings of eye or ocular adnexa that could confound study results.
Potential participants underwent a comprehensive baseline assessment to determine both eligibility and baseline values for study outcomes of interest. At this visit, participants completed (in the following order) the OSDI, SANDE, visual analog EDS, and underwent assessments of BCVA, slit-lamp examination, TBUT, corneal and conjunctival staining, STS, MGSS, and intraocular pressure (IOP). Qualifying subjects were randomized in a 1:1 ratio to either TC or CsA twice daily. Computer-generated randomization was stratified by investigative site with a block size of 4 and was administered through an electronic data capture system. Treatment commenced following randomization on the same day.
For subjects randomized to TC treatment, eyelid margins underwent debridement at the slit lamp under topical anesthesia. The eyelids were then thoroughly wiped to remove makeup and/or other foreign materials and allowed to dry as described in the product’s Instructions for Use.21 The upper and lower SmartLids were then applied to the subject’s eyelids and the temple housing affixed to the subject’s temple. The SmartLid devices were then connected to the SmartHub. Subjects were then advised that the thermal cycle would begin and to keep their eyes open and blink naturally. The TC procedure then commenced, and temperature settings increased automatically every 30 s to the final meibum melting temperature of 45°C. After 15 min, the SmartHub automatically stopped heating and the SmartLids were removed. Following the thermal portion of the TC procedure, repeat debridement was performed at the slit lamp if needed, followed by four-lid meibomian gland expression using the Clearance Assistant Plus Device as follows: for the nasal, central, and temporal aspect of each lid, the forceps were used to express the glands from fornix to lid margin using a double-pass approach. Additional passes of expression were allowed at the Investigator’s discretion to ensure complete expression.
TC was performed once at study entry and again at month 5; CsA was dosed twice daily for 6 months. Key outcome assessments were performed by masked study personnel periodically after the initiation of treatment. These included BCVA, TBUT, ocular surface staining, STS, MGSS, EDS, OSDI, SANDE, and IOP. Subjects were reassessed 1 day, 1 week, and 1, 3, and 6 months after randomization. In both groups, following the initiation of treatment, subjects were asked to refrain from using artificial tears/lubricants and topical medications, and to record their use if required. Similarly, during the duration of study participation, subjects were not to be treated with other in-office or at-home dry eye treatments including punctal plugs, lid scrubs, or warm compresses. The study design is schematically illustrated in Figure 1.
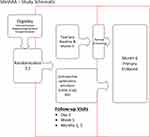 |
Figure 1 Study schematic. Abbreviations: BID, twice-daily; CsA, cyclosporine ophthalmic emulsion 0.05% (Restasis). |
Endpoints
The primary effectiveness endpoints were the changes from baseline in TBUT and OSDI at 6 months. Data from both eyes were analyzed for eye-level outcomes. The change from baseline in TBUT was analyzed using linear mixed effects (LME) models accounting for inter-eye correlations, adjusting for baseline and site, and with a random subject effect. The change from baseline in total OSDI was analyzed using analysis of covariance (ANCOVA) models adjusting for baseline and site. Models at the 6-month primary endpoint for the two primary outcomes accounted for missing data using the last observation carried forward (LOCF) approach. These two endpoints were tested in hierarchical fixed sequence: TBUT was evaluated first, and if superiority of TC over CsA was established, the trial was considered a success and OSDI was then evaluated. Secondary outcomes included MGSS, SANDE score, EDS, number of meibomian glands yielding any liquid secretions (MGYLS), number of meibomian glands yielding clear secretions (MGYCS), corneal staining score, conjunctival staining score, and STS. These were tested using LME models for eye-level outcomes and ANCOVA models for subject-level outcomes using a two-sided alpha of 0.05. Primary and secondary outcome analyses were conducted in a modified intent-to-treat (mITT) population of all randomized subjects who received assigned therapy. Safety outcomes (conducted in the safety population of all subjects on whom TC or CsA therapy was attempted) included the nature and frequency of adverse events and changes in IOP and BCVA. With an estimated 10% drop out rate at 6 months, and assuming a common standard deviation of 2 s for the change from baseline in TBUT at 6 months, a minimum of 175 subjects in each group was determined to yield enough evaluable subjects to provide at least 90% power to detect a mean difference of 1 s between TC and CsA at a two-sided significance level of 0.05.
Endpoint Assessments
To assess TBUT, a saline-moistened fluorescein-impregnated strip was placed against the superior bulbar conjunctiva. Subjects were asked to blink several times and to move their eyes to distribute fluorescein throughout the tear film. TBUT was measured approximately 30 s after fluorescein instillation in the slit lamp under cobalt blue illumination with a Wratten filter, a beam width of 4 mm, and full height. The beam was moved slowly from side to side to cover the entire cornea. Subjects were asked to blink 3 times and hold their eye open. The time from upstroke of the last blink to the first tear film break or dry spot was measured with a stop watch and recorded. Three measurements were taken and averaged.
MGSS was obtained by expression of meibomian glands in the lower eyelids following the method of Korb and Blackie.22 In brief, expression of five central glands in the lateral, central, and temporal thirds of the lower lids was carried out using the Meibomian Gland Evaluator (TearScience, Inc, Morrisville, NC) according to the manufacturer’s instructions. The quality of secretions was graded as described by Lane et al, 0 = nothing, 1 = toothpaste, 2 = cloudy, and 3 = clear.23 The sum of the grade for each of the 15 glands was the MGSS (Maximum = 45). The number of glands yielding any or clear liquid was also recorded.
Corneal and conjunctival staining were assessed exactly as described in Gupta et al using fluorescein dye (cornea) and lissamine green (conjunctiva).13
Results
Demographics, Disposition, and Exposure
A total of 348 subjects were enrolled with 3 exited pre-randomization (one each was lost to follow-up, did not meet eligibility criteria, and unspecified reason); 345 were randomized. The modified ITT population consisted of 341 subjects (168 randomized to TC, 173 to CsA; 4 subjects assigned to TC) did not meet eligibility criteria. Demographic data for study participants are given in Table 1. Overall, subjects were on average (SD) 56.2 (14.0) years of age, female (71.6%), and Caucasian (88.6%), with no significant differences in any parameters between groups. In the TC group, 13 (7.7%) subjects discontinued the study prior to the month 6 primary endpoints (5 by subject choice, 2 by investigator choice, 3 lost to follow-up, and 3 other); in the CsA group, 11 subjects discontinued the study prior to the month 6 primary endpoints (8 by subject choice, 2 lost to follow-up, and 1 other). TC was performed twice in 165 eyes (95.9%) and only once in 7 eyes (4.1%) initially assigned to TC treatment. Subjects randomized to CsA therapy used a mean of 5.7 (1.2) 5.5 mL bottles of study medication during the 6-month treatment period; each is intended to provide a 1-month supply (85% of subjects used ≥6, 96% ≥5).
 |
Table 1 Baseline Demographic and Baseline Ocular Characteristic Data of the Modified Intent-to-Treat Population (N=341) by Treatment Group |
Tear Break-Up Time
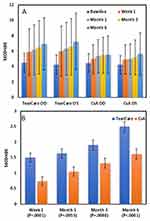
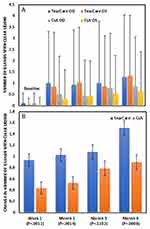
Both TC and CsA produced statistically significant improvements (all p<0.0001) in mean TBUT in both eyes at all study time points (Figure 2A). By 6 months in the TC group, mean TBUT had improved by a mean of 2.30 (3.24) seconds in right eyes and 2.88 (3.42) seconds in left eyes (p<0.0001 for each). Similarly, in the CsA group, by 6 months mean TBUT had improved by 1.08 (2.36) and 1.41 (2.57) seconds in right and left eyes, respectively (p<0.0001 for each).
Comparison of the changes from baseline in mean TBUT between treatment groups revealed statistically significantly greater improvements (all p<0.0080) in the TC group versus the CsA group at every time point (Table 2, Figure 2B). At the month 6 primary endpoint, the observed mean difference between groups in TBUT improvement was 1.49 s (LS mean difference of 0.76 s, 95% CI 0.32, 1.19; p=0.0006) demonstrating superiority of TC over CsA.
 |
Table 2 Mixed-Effects Model Analyses of Tear Break-Up Time and Ocular Surface Disease Index Primary Outcomes in the Modified Intent-to-Treat Population |
Ocular Surface Disease Index
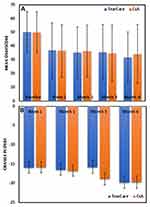
Both TC and CsA produced statistically significant improvements (all <0.0001) in mean OSDI at all time points (Figure 3A). Improvements were evident within 1 week of initiating treatment. By month 6, mean OSDI in TC eyes had improved by −17.84 (17.95) points. In CsA eyes, mean OSDI had improved by −15.49 (19.90) points. Results of the mixed-effects ANCOVA analysis are presented in Table 2.
Comparison of the changes from baseline in mean OSDI between treatment groups revealed no statistically significant differences at any time point (Figure 3B). At the month 6 primary endpoint, the observed mean difference between groups in OSDI improvement was 2.35 (LS mean difference 0.16, 95% CI −4.295, 4.617) demonstrating non-inferiority (p=0.9843).
Secondary Endpoints
Meibomian Gland Secretion Score
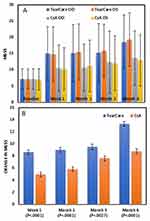
The mean MGSS improved significantly in both eyes (p<0.0001) in both treatment groups at all time points (Figure 4A). These changes from baseline were statistically significantly greater favoring TC at each time point (p<0.0027) including the month 6 primary endpoint (p<0.0001) (Figure 4B).
Meibomian Glands Yielding Any Liquid Secretions
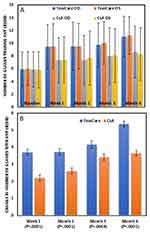
The mean number of MGYLS increased significantly (p<0.0001) in both eyes of both treatment groups at all time points (Figure 5A). Changes from baseline were statistically significantly different, favoring TC at all time points (p<0.0064) including the month 6 primary endpoints (p<0.0001) (Figure 5B).
Meibomian Glands Yielding Clear Liquid Secretions
The mean number of MGYCS increased significantly in both eyes at every time point in the TC group (p<0.0001) and in the CsA group (p<0.0075) (Figure 6A). These changes from baseline were statistically significantly greater, favoring TC over CsA at week 1 (p=0.0011), month 1 (p=0.0014), and the primary endpoint at month 6 (p=0.0040), but not at month 3 (p=0.1102) (Figure 6B).
Conjunctival Staining Score
The mean conjunctival staining score improved significantly in both eyes at all time points in the TC group (p<0.0019) (Supplemental Figure 1A) Conversely, in the CsA group, significant improvement was only seen at month 3 in right eyes (p=0.0326) and month 6 in both eyes (p<0.0056), while scores in both eyes at week 1 and month 1 worsened insignificantly. Changes from baseline between groups were significantly greater favoring TC at week 1 (p=0.0020), month 1 (p=0.0160), and month 3 (p=0.0394), but not at month 6 (p=0.2680) (Supplemental Figure 1B).
Corneal Staining Score
The mean corneal staining score improved significantly in both eyes at all time points in the TC group (p<0.0001) and in the CsA group at all time points (p<0.0167) except week 1 in left eyes (p=0.0830) (Supplemental Figure 2A). Changes from baseline were not significantly different between treatment groups at any time points (p>0.1532) (Supplemental Figure 2B).
SANDE Score
The mean SANDE score improved significantly (p<0.0001) in both treatment groups at all time points (Supplemental Figure 3A). Changes in baseline between groups were statistically significantly different, favoring TC at 1 week (p=0.0240) and 1 month (p=0.0285) but not at month 3 (p=0.8190) or month 6 (p=0.3090) (Supplemental Figure 3B).
Eye Dryness Score
The mean EDS improved significantly (baseline 64.7 TC, 64.3 CsA; Month 6 39.3 and 46.5, respectively) in both treatment groups at all time points (p<0.0001) (Supplemental Figure 4A). The between-group difference was only significantly favoring TC at week 1 (p=0.0418) and insignificant at other time points (p>0.1018) (Supplemental Figure 4B).
Schirmer Tear Score
The mean STS improved significantly from baseline in both eyes at all time points in both the TC group (p<0.0001 for all) and the CsA group (p<0.0006) (Supplemental Figure 5A). Changes from baseline were not significantly different between treatment groups at any time point (p>0.0983) (Supplemental Figure 5B).
Artificial Tear Use
The use of artificial tears (rescue therapy) was permitted if needed for ocular comfort. Artificial tears were used by 85 subjects (49.4%) in the TC group and by 66 subjects (38.2%) in the CsA group.
Safety
Both treatments were generally safe and well tolerated. Of 19 treatment-emergent adverse events in each treatment group (11.0%), only 2 (1.2) in the TC group and 8 (4.6) in the CsA group were graded as definitely/probably/possibly related to study treatment and all of these were graded as mild (n=9) or moderate (n=1) in severity (Table 3). The only ocular TEAEs occurring in two or more eyes were two cases of loss of BCVA>10 letters (both in the CsA group), two cases of medication instillation discomfort (both in the CsA group), and two cases of conjunctival hyperemia (one in each group). Two serious adverse events were recorded; both were non-ocular and unrelated to study treatment (cholelithiasis and pulmonary edema). No statistically or clinically significant changes or differences between treatment groups were seen in BCVA or IOP.
 |
Table 3 Nature and Incidence (n [%]) of Treatment-Emergent Adverse Events (TEAEs) in the Safety Population (N=345). Multiple Occurrences of the Same TEAE in the Same Eye are Counted Once |
Discussion
In this prospective, multicenter, randomized, assessor-masked, active-controlled trial, TC provided superior improvement in TBUT compared to CsA in eyes with DED. Additionally, TC provided significantly greater improvements in meibomian gland function—assessed by MGSS, MGYLS, and MGYCS—compared to CsA, and comparable improvements in OSDI, conjunctival and corneal staining, SANDE, EDS, and Schirmer tear scores compared to CsA at month 6. TC was also better tolerated than CsA, with treatment-related adverse events being 4 times more common in CsA eyes versus TC eyes.
These results confirm and expand on the results of prior TC trials. In a prospective randomized trial comparing TC to daily warm eyelid compresses in eyes with DED, mean TBUT improved by almost 12 s in the TC group versus no improvement in the compresses group at 4 weeks (p<0.001); this improvement lasted through 6 months with some loss of effect between 3 and 6 months.24 In a subsequent extension study, eyes were retreated with TC at month 7 and followed through month 13; TBUT improved to the same level after repeat TC as after the initial TC treatment, with diminution of effect by month 13.12 In another randomized trial, first-generation TC was compared to the LipiFlow thermal pulsation therapy system in eyes with DED associated with MGD; significant improvements from baseline in TBUT, MGSS, EDS, SANDE, and OSDI were seen in both treatment groups, with improvements being arithmetically but not significantly greater in TC eyes.13 Subsequent subgroup analysis of eyes with more severe MGD at baseline showed that improvements from baseline in OSDI and SANDE were significantly greater for TC versus LipiFlow (p=0.01 for each) and borderline greater (p=0.08) for EDS at 1 month.16 A prospective multicenter case series demonstrated significant improvement from baseline at 1 month in TBUT (p<0.0001), OSDI (p<0.001), and MGSS (p<0.0001) after TC treatment.25 Also, a single-center retrospective case series demonstrated significant improvements from baseline in MGSS (p<0.001) and Standardized Patient Evaluation of Eye Dryness [SPEED] questionnaire score (p<0.001) 2–3 months after treatment.26
Previous studies—summarized above—have compared TC to other lid-based treatments (warm compresses or LipiFlow thermal pulsation therapy). To our knowledge, this is the first randomized trial comparing an interventional eyelid procedure or any other thermal therapy to the leading prescription drug therapy for DED, CsA. Several elements of the study design were carefully considered to ensure the fairest possible comparison between these two different treatment modalities. First, eligibility criteria for study entry were developed to accommodate issues related to CsA efficacy and safety. Eligibility required not only impaired TBUT but also a low Schirmer’s tear score. This ensured inclusion of patients with evaporative DED as well as DED arising from impaired tear production, which is the target population in which CsA would be expected to perform most effectively by increasing tear production.18 CsA was approved by the US Food and Drug Administration on the basis of STS improvement, despite only meager outcomes: across four clinical studies, an improvement from a baseline of >10 mm in STS was seen in ~15% of CsA-treated eyes versus ~5% of vehicle-treated eyes,18 a difference that was statistically significant but of marginal clinical significance. SAHARA utilized Schirmer’s testing with anesthesia to minimize reflex tearing and better estimate tear production; in the CsA registry trials, both anesthetized and unanesthetized STS were assessed, and CsA did not outperform its vehicle in terms of change in unanesthetized STS.5 In terms of exclusion criteria, the current study excluded patients using a wide variety of topical and systemic medications (including but not limited to immunomodulatory agents, antihistamines, cholinergic agents, antimuscarinics, beta-blockers, tricyclic antidepressants, and estrogen-progesterone) not because these are contraindications to TC therapy but because use of these drugs could interfere with or confound response to CsA5 Topical anti-inflammatory medications and current use of punctal plugs were also exclusion criteria as CsA does not effectively increase tear production in eyes using these therapies.18,27
The second consideration given to the branded CsA comparator in the study design was with regard to the time of the primary endpoint. Typically, DED therapies achieve maximum therapeutic efficacy in a short period of time, permitting primary endpoint assessments in as little as 1 month (varenicline nasal spray8,9) or 3 months (lifitegrast6,7). As has been seen in this and previous studies, the benefits of TC manifest within 1 month of treatment. The two Phase 3 studies of CsA, however, utilized a 6-month primary endpoint because, as noted in a recent review of the CsA literature, several weeks may be required for CsA to have a therapeutic effect and as much as 6 months for maximum improvement.20 A 6-month endpoint was therefore selected for the current study to ensure that cyclosporine was at maximal efficacy and to maintain fair balance, TC was repeated at month 5 to ensure that it, too was at peak efficacy 1 month after retreatment. Thus, SAHARA was a comparison of TC versus CsA at the time of each treatment’s peak efficacy.
Artificial tear use was more common in the TC group compared to the CsA group. However, the CsA vehicle itself is an effective lubricating solution, and therefore CsA dosing provided twice-daily topical lubrication to 100% of eyes in the CsA group. The CsA vehicle contains glycerin, castor oil, polysorbate 80, carbomer copolymer type A, purified water, and sodium hydroxide as a buffer.18 The authors of the phase 3 study report for CsA stated that the CsA vehicle itself provided substantial palliative benefits, with significant improvements from baseline in several outcome measures. They postulated that sustained residence time of the oil component on the ocular surface could have reduced the evaporation of natural tears.5 This may explain in part why, in the current study, improvements in the CsA group were apparent as early as week 1 despite historical evidence that CsA therapeutic effects typically do not manifest until after longer dosing periods.20
In terms of safety, 80% of the treatment-related treatment-emergent adverse events (8/10) occurred in the CsA group. The nature of adverse events in the CsA group was entirely consistent with the adverse event profile of CsA in its two phase 3 trials.5 Both of the related AEs in the TC group, eyelid irritation and conjunctival hyperemia, were mild and resolved without treatment.
This study is strengthened by several aspects of its design. Prospective randomized trials represent the most robust primary study design in the hierarchy of evidence. Assessments in this study were performed by study personnel masked to treatment assignment to eliminate bias. Selecting CsA as the active comparator adds significant clinical relevance to the findings, as CsA is the most commonly used prescription therapy for DED worldwide. As discussed above, several aspects of the study design—including eligibility criteria and the selection and timing of endpoints—were tailored to ensure the fairness of comparisons to CsA. Moreover, subject compliance with treatment is known to be better in a clinical trial setting.28,29 This should enhance the effectiveness of a treatment reliant on patient adherence (like CsA) but would not influence outcomes for a treatment like TC, which does not rely on patient behavior. Stated another way, the durable effectiveness observed with TC was not dependent on subject adherence. Having thus set the bar as high as possible for TC, the demonstration of superiority of TC over CsA for TBUT improvement at month 6—the primary study outcome—was thus rendered more robust and clinically significant. A weakness is that subjects were not masked, which could have introduced bias in patient-reported outcomes that included EDS, SANDE, and OSDI. Subject masking was considered but not implemented because the inclusion of CsA vehicle in the TC group (sham CsA) to maintain masking would have a disproportionate effect on outcomes compared to the sham TC treatment that would have been required in the CsA group.
This study is ongoing. Following the month 6 assessment, TC eyes will be followed through month 24, with repeat TC administered at or after month 9 only if prespecified retreatment criteria are met. These include TBUT decreasing to within 2 s of baseline (or below) AND OSDI worsening by >15 points from the previous visit. CsA eyes will be crossed over to a single TC treatment at month 6 and followed through month 12. Results of longer-term follow-up of the study sample will be presented separately in the future.
Due to the significant impact of DED in various areas such as quality of life, office worker productivity, contact lens use, and emotional well-being,30–32 providing patients with an effective and reliable therapy is of great interest. TC demonstrated objective and subjective improvements in DED signs and symptoms. Since traditional therapy for DED has typically relied on eyedrops and because patients with DED do not typically adhere to their eyedrops at a specified frequency, successful treatment is not a common outcome.33 However, because TC does not rely on patient adherence and because of its durability,34 it appears to be an attractive intervention for the treatment of DED, a globally impactful disease.
In summary, TC technology and procedure provided durable and statistically greater improvement in TBUT and multiple measures of meibomian gland secretion and non-inferior improvement in OSDI, corneal and conjunctival staining, SANDE score, EDS, and STS compared to CsA in eyes with evaporative and/or aqueous deficiency DED. TC should be a preferred treatment for patients with DED associated with MGD.
Data Sharing Statement
The authors do not intend to share participant-level data. Other queries or requests should be directed to the corresponding author (JD).
Acknowledgments
We are grateful to Dr. David Badawi for review and advice on this paper. Dr. Tony Realini (Hypotony LLC) provided medical writing services for a preliminary draft of the manuscript. Dr. Yuxin Zhang (Xtiers Consulting) provided statistical analysis.
Disclosure
BA, MB, JL, TC, BS, JE, and SK were Investigators and received research support for the study. BA is a consultant for Allergan and Sight Sciences. MB is a consultant for and reports honoraria from Allergan and Sight Sciences. JL is a consultant for Allergan and Sight Sciences and reports personal fees from Sun Ophthalmics, Alcon, and Bausch and Lomb, outside the submitted work. TC reports grant support, consulting, and speaking for Sight Sciences. BS is a consultant and speaker for Sight Sciences and Allergan. SK is a consultant and speaker for Allergan and a consultant for Sight Sciences. VP reports consulting fees from Sight Sciences, grants from Alcon; stock options from Claris; grants, personal fees from Novartis; personal fees from Dompe, Oyster Point, Oculis, Novaliq, Sun Pharma, Quidel, BrightStar and Kala; stock options from Trefoil; personal fees, stock options from Kuria, outside the submitted work. JD and TR are employees of Sight Sciences. There are no other relevant conflicts.
References
1. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318–326. doi:10.1016/S0002-9394(03)00218-6
2. Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the physicians’ health studies. Arch Ophthalmol. 2009;127:763–768. doi:10.1001/archophthalmol.2009.103
3. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52:1922–1929. doi:10.1167/iovs.10-6997a
4. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31:472–478. doi:10.1097/ICO.0b013e318225415a
5. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–639. doi:10.1016/S0161-6420(99)00176-1
6. Tauber J, Karpecki P, Latkany R, et al. Lifitegrast ophthalmic solution 5.0% versus placebo for treatment of dry eye disease: results of the randomized Phase III OPUS-2 study. Ophthalmology. 2015;122:2423–2431. doi:10.1016/j.ophtha.2015.08.001
7. Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology. 2014;121(2):475–483. doi:10.1016/j.ophtha.2013.09.015
8. Wirta D, Vollmer P, Paauw J, et al. Efficacy and safety of OC-01 (varenicline solution) nasal spray on signs and symptoms of dry eye disease: the ONSET-2 phase 3 randomized trial. Ophthalmology. 2022;129(4):379–387. doi:10.1016/j.ophtha.2021.11.004
9. Wirta D, Torkildsen GL, Boehmer B, et al. ONSET-1 phase 2b randomized trial to evaluate the safety and efficacy of OC-01 (varenicline solution) nasal spray on signs and symptoms of dry eye disease. Cornea. 2022;41(10):1207–1216. doi:10.1097/ICO.0000000000002941
10. Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050–2064. doi:10.1167/iovs.10-6997g
11. US Food and Drug Administration. TearCare system indications for use. Available from: https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K213045;.
12. Badawi D. TearCare® system extension study: evaluation of the safety, effectiveness, and durability through 12 months of a second TearCare® treatment on subjects with dry eye disease. Clin Ophthalmol. 2019;13:189–198. doi:10.2147/OPTH.S191588
13. Gupta PK, Holland EJ, Hovanesian J, et al. TearCare for the treatment of meibomian gland dysfunction in adult patients with dry eye disease: a masked randomized controlled trial. Cornea. 2022;41:417–426. doi:10.1097/ICO.0000000000002837
14. Blackie CA, Solomon JD, Greiner JV, Holmes M, Korb DR. Inner eyelid surface temperature as a function of warm compress methodology. Optom Vis Sci. 2008;85(8):675–683. doi:10.1097/OPX.0b013e318181adef
15. Dhamdhere K, Badawi D. A blink-assisted, cornea-sparing wearable eyelid device for the effective penetration of therapeutic thermal energy into the meibomian glands for the treatment of dry eye disease. J Clin Exp Ophthalmol. 2021;S12:003.
16. Holland EJ, Loh J, Bloomenstein M, Thompson V, Wirta D, Dhamdhere K. A comparison of TearCare and lipiflow systems in reducing dry eye disease symptoms associated with meibomian gland disease. Clin Ophthalmol. 2022;16:2861–2871. doi:10.2147/OPTH.S368319
17. Foulks GN. Topical cyclosporine for treatment of ocular surface disease. Int Ophthalmol Clin. 2006;46:105–122. doi:10.1097/01.iio.0000212135.77675.6a
18. US Food and Drug Administration. Restasis prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050790s020lbl.pdf;.
19. Schwartz LM, Woloshin S. A clear-eyed view of restasis and chronic dry eye disease. JAMA Internal Med. 2018;178:181–182. doi:10.1001/jamainternmed.2017.7904
20. Utine CA, Stern M, Akpek EK. Clinical review: topical ophthalmic use of cyclosporin A. Ocul Immunol Inflamm. 2010;18(5):352–361. doi:10.3109/09273948.2010.498657
21. Sight Sciences Inc. TearCare System Instructions for Use. Sight Sciences Inc; 2022.
22. Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27(10):1142–1147. doi:10.1097/ICO.0b013e3181814cff
23. Lane SS, DuBiner HB, Epstein RJ, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31(4):396–404. doi:10.1097/ICO.0b013e318239aaea
24. Badawi D. A novel system, TearCare®, for the treatment of the signs and symptoms of dry eye disease. Clin Ophthalmol. 2018;12:683–694. doi:10.2147/OPTH.S160403
25. Karpecki P, Wirta D, Osmanovic S, Dhamdhere K. A prospective, post-market, multicenter trial (CHEETAH) suggested TearCare system as a safe and effective blink-assisted eyelid device for the treatment of dry eye disease. Clin Ophthalmol. 2020;14:4551–4559. doi:10.2147/OPTH.S285953
26. Chester T. A single-center retrospective trial of a blink-assisted eyelid device in treating the signs and symptoms of dry eye. Optom Vis Sci. 2021;98:605–612. doi:10.1097/OPX.0000000000001711
27. Roberts CW, Carniglia PE, Brazzo BG. Comparison of topical cyclosporine, punctal occlusion, and a combination for the treatment of dry eye. Cornea. 2007;26:805–809. doi:10.1097/ICO.0b013e318074e460
28. van Onzenoort HA, Menger FE, Neef C, et al. Participation in a clinical trial enhances adherence and persistence to treatment: a retrospective cohort study. Hypertension. 2011;58:573–578. doi:10.1161/HYPERTENSIONAHA.111.171074
29. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi:10.1056/NEJMra050100
30. Kawashima M, Sano K, Takechi S, Tsubota K. Impact of lifestyle intervention on dry eye disease in office workers: a randomized controlled trial. J Occup Health. 2018;60:281–288. doi:10.1539/joh.2017-0191-OA
31. Mertzanis P, Venkataraman K, Begley C, Chalmers R, Abetz L. The impact of dry eye on daily life: results from a qualitative study. Invest Ophthalmol Vis Sci. 2002;43:74.
32. Nichols KK, Bacharach J, Holland E, et al. Impact of dry eye disease on work productivity, and patients’ satisfaction with over-The-counter dry eye treatments. Invest Ophthalmol Vis Sci. 2016;57:2975–2982. doi:10.1167/iovs.16-19419
33. Uchino M, Yokoi N, Shimazaki J, et al. Adherence to eye drops usage in dry eye patients and reasons for non-compliance: a web-based survey. J Clin Med. 2022;11(2):367. doi:10.3390/jcm11020367
34. Chester T, Ferguson T, Chester E. Localized heat treatment for meibomian gland dysfunction: a single-center retrospective analysis of efficacy over time. Optom Vis Sci. 2023;100(9):625–630. doi:10.1097/OPX.0000000000002053
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.