Back to Journals » OncoTargets and Therapy » Volume 14
A Radiomic Approach to Access Tumor Immune Status by CD8+TRMs on Surgically Resected Non-Small-Cell Lung Cancer
Authors Min J, Dong F, Wu P, Xu X, Wu Y, Tan Y, Yang F, Chai Y
Received 24 April 2021
Accepted for publication 9 September 2021
Published 27 September 2021 Volume 2021:14 Pages 4921—4931
DOI https://doi.org/10.2147/OTT.S316994
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Jie Min,1 Fei Dong,1 Pin Wu,2 Xiaopei Xu,1 Yimin Wu,2 Yanbin Tan,1 Fan Yang,1 Ying Chai2
1Department of Radiology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, 310009, Zhejiang Province, People’s Republic of China; 2Department of Thoracic Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, 310009, Zhejiang Province, People’s Republic of China
Correspondence: Ying Chai
Department of Thoracic Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, No. 88 Jiefang Road, Hangzhou, 310009, Zhejiang Province, People’s Republic of China
Tel +86-57187783642
Fax +86 571-87068001
Email [email protected]
Purpose: Immunotherapy has made breakthroughs in the treatment of non-small-cell lung cancer (NSCLC); however, only a subset of patients achieved long-term survival, so it is of great importance to find a biomarker of lung cancer thus guide immunotherapy. Studies have shown that the infiltration level of tissue resident memory CD8+ T cells (CD8+ TRMs) is positively correlated with lung cancer prognosis and can be an ideal biomarker for assessing the tumor local immune status. We screened the radiomic features associated with CD8+ TRMs as targets in NSCLC surgical specimens by radiomic approaches, and established a radiomic predictive model to assess the local immune status, which may provide a scientific reference for lung cancer treatment strategies.
Patients and Methods: We retrospectively analyzed the NSCLC surgical specimens immune cell database and extracted CD8+ TRMs cell data, preoperative CT scan data were achieved. A total of 97 patients containing complete preoperative data were included, radiomic features were extracted from the preoperative CT image data. All the patients were divided into two groups, namely high-CD8+ TRMs infiltrated group and low-CD8+ TRMs infiltrated group, based on the proportion of CD8+ TRMs cells subset in the immune cell population. The most valuable radiomic features and semantic features were extracted and selected, and a neural network model was established to predict the level of CD8+ TRMs cell infiltration level to assess the tumor local immune status.
Results: The NSCLC tumor immune status predictive model was built to discriminate high- from low-CD8+ TRMs with an area under the curve (AUC) of 0.788 (95% CI) in the training set and 0.753 (95% CI) in the validation set.
Conclusion: The radiomic models using CT image data showed a good predictive performance for accessing NSCLC immune status thus has great potential for personalized therapeutic decision making.
Keywords: non-small-cell lung cancer, NSCLC, radiomic, tumor immune status, tissue resident memory CD8+ T cells, CD8+ TRMs
Introduction
In recent years, immune checkpoint inhibitors (ICIs), represented by anti-programmed cell death protein (PD)-1 and anti-programmed cell death ligand 1 (PD-L1), have been used in the clinical treatment of cancer patients and have significantly changed the treatment strategy, including non–small-cell lung cancer (NSCLC).1,2 Although some patients (around 20%) have achieved satisfactory treatment results, however, 50% of lung cancer patients do not benefit from the treatment and some even hyper-progressed,3,4 there is therefore a need for development of methods to identify patients who are most likely to respond to immunotherapy. PD-L1 expression status was recommended as a predictive biomarker for immunotherapy.5 However, only a subset of PD-L1-positive patients benefit from anti-PD-1/PD-L1 therapies and some patients with PD-L1-negative tumors can respond favorably to anti-PD-L1 therapy for unclear reasons.6,7 In addition, it has been shown that some PD-L1-negative patients are effective in ICIs and some patients can benefit from immunotherapy regardless of PD-L1 expression status.7,8 This is due to the fact that PD-L1 expression status in the tumor microenvironment is influenced by several factors, which makes PD-L1 expression status still not an ideal predictive biomarker for immunotherapy response.6,9 Since immunotherapy relies on the activation of immune cells to generate an immune response to recognize and clear tumor cells. Therefore, accurate assessment the tumor immune status especially the key immune-mediated responding cell in tumor immune microenvironment is an important guidance to predict the clinical immunotherapy response.10
Tissue resident memory CD8+ T cells (CD8+ TRMs) are a subset of CD8+ T cells with high expression of the epithelial adhesion molecule CD103 and/or CD69 molecules as the core feature, which are permanently colonized in mucosal epithelial tissues.11,12 An increasing number of studies suggested the key role for CD8+ TRMs in tumor immunosurveillance and immunotherapy.13 Several clinical studies have shown that the number of tumor-infiltrating CD8+ TRMs cells is positively correlated with the prognosis of lung cancer patients,14,15 and the response level of cytotoxic T cells in patients is positively correlated with the expression level of CD103, a characteristic gene of CD8+ TRMs.14
Radiomics is a novel method for high-throughput extraction of quantitative features from a specified region of interest from images, it can transform digital medical images into mineable high-dimensional data, then by quantitatively analyzing a large amount of imaging data, the relationship between the target content and the tissue structure and pathophysiological changes in the region of interest can be more fully recognized.16,17 Radiomics features include many groups, such as shape-based, first-order and texture features. For shape-based features, radiomics can provides additional information such as degree of sphericity and surface area more than size and volume data in traditional methods. First-order features provide intensity distribution information such as asymmetry (skewness) and flattening (kurtosis), and texture features provide a more in-depth analysis of the relationships between voxels. Analyzing these features might provide a more comprehensive method for exploring a lesion.18 In this study, we used radiomics to establish an assessment model based on the infiltration level of CD8+ TRMs in surgically resected specimens and preoperative CT images, then extracted the radiomic features associated with the infiltration level of CD8+ TRMs in the tumor to assess the local immune status of lung cancer.
Materials and Methods
Study Design and Study Population
Our study was approved by the Institutional research Review Board for human studies (Ethical Committee) of the second affiliated hospital of Zhejiang University School of Medicine and in accordance with principles listed in the Helsinki declaration.
We collected information from patients with a diagnosis of primary NSCLC stage Ib to IIIa, and underwent lung tumor resections with curative intent at the Department of Thoracic Surgery at the second affiliated hospital of Zhejiang University School of Medicine. The patients fulfilled the following inclusion criteria were recruited: a) stage Ib to stage IIIa disease; b) diagnosis of NSCLC with complete pathologic report; c) available preoperative thin-section CT images (Picture Archiving and Communication System, PACS) within one month before surgery and the lesions showed in CT images have sufficient image quality for Radiomics procedure; d) adequacy of tissue samples for immune cell analysis; e) available complete clinical records. Main exclusion criteria were: a) history of drug abuse or medications with potential impact on lung diseases; b) active pneumonitis at the time of CT scan. Eventually, a total number of 97 patients who matched the above criteria were enrolled in the study, including 46 men and 51 women with mean age of 61.8 ± 8.2 years.
Immune Cell Analysis
Immunocellular analysis includes following steps: 1) the lung cancer tissue specimens were collected during thoracoscopic lung cancer resection. After the specimen is isolated, tumor tissue was cut from the aseptic operating table At the same time, the specimen was cleaned with sterile saline, the necrotic and suppurative tissue was removed from the specimen. The pretreated specimen was put into a sterile centrifuge tube containing tissue preservation solution (Miltenyi Biotec). The specimen was put into a 4°C transfer box and transfer to the laboratory as soon as possible. 2) The specimen was processed into a single-cell suspension in the clean station after frozen section pathologic report provided. Isolation of tumor-resident CD8+T cells from human lung tumors was performed using a published protocol and further optimized by our group.19 The specimen was cut to 1–2 mm in a 6-well plate, then collagenase I (1 mg/mL, Thermo Fisher Scientific), collagenase IV (1 mg/mL, Thermo Fisher Scientific), and hyaluronidase (10 ng/mL, STEMCELL) were added, and resuspended to 10 mL with 1640 medium. The specimen was put into a special digestion tube and digested on the Gentle MACS automatic gentle tissue processor (Miltenyi Germany) for 1 hour. When the digestion is completed, the digested suspension was filtered with a 45mm pore size filter to remove the remaining components that have not been degraded. The obtained single-cell suspension was washed with sterile PBS by centrifugation, and the red blood cells were lysed with red blood cell lysate, then washed twice to obtain a stable and uniform single cell suspension. 3) The TRM cell population was labeled with CD45 (Biolegend, HI30), CD3 (Biolegend, UCHT1), CD8 (Biolegend, RPA-T8), CD103 (Biolegend, Ber-ACT8), and CD69 (Biolegend, FN50). After 15 minutes of antibody incubation at room temperature, the unbound antibody was washed away with PBS, and finally resuspended with cell staining buffer. The TRM cells were detected on BD FACSCanto II and sorted by BD FACSAria II.
Radiomics Procedure
CT Examinations
CT scans were performed by four CT scanners including two 16-slice (sensation 16; Siemens, Germany), one 64-slice (Definition AS; Siemens, Germany) and a second generation dual-source 128-slice (Definition Flash; Siemens, Germany) scanner. All scans were acquired at end-inspiration in craniocaudal direction, capturing the entire lung volume from the apices to the pleural recesses and reconstructed with a slice thickness ranging 1.5–2.5 mm. For the purpose of this study, the Digital Imaging and Communications in Medicine (DICOM) datasets were retrieved from PACS.
Feature Extraction
CT images of thin slice lung window were used for tumor segmentation since lung window had a better performance in identifying the lesion from the surrounding normal tissue. The tumor outline in every slice was drawn as the region of interest (ROI) by a radiologist with 9 years experience in thoracic imaging reporting who had no knowledge of the tumors other than their locations by using a free open-source software package (ITK-SNAP, version 3.6.0; http://itksnap.org) to provide the ROI for computer-based image analysis.
Radiomics feature extraction procedures were proceed using pyradiomics (http://www.radiomics.io/pyradiomics.html). A total of 107 quantitative parameters including 14 shape features, 18 first-order features, 24 gray-level co-occurrence matrix (GLCM) features, 15 gray level dependence matrix (GLDM) features, 16 gray-level run-length matrix (GLRLM) features, 16 gray level size zone matrix (GLSZM) features, 5 neigbouring gray tone difference matrix (NGTDM) features were extracted from the image data. Four semantic features including tumor boundary type, tumor texture type, tumor pathology type and lymph node metastasis type were used in our image feature extraction. Tumor boundary type was defined as the boundary of the tumor is clear or vague from the surrounding normal lung parenchyma. Tumor texture type was defined as the proportion of parenchymal component of the tumor, and those with more than 80% solid component were defined as solid tumor, while those with less than this proportion were defined as mixed density tumor. Pathological type characteristics were classified according to the routine postoperative pathological findings. Since adenocarcinoma patients accounted for 80% of the cases in this group, only two types of pathological types were distinguished: adenocarcinoma and non-adenocarcinoma. Lymph node involvement characteristics were classified as lymph node involvement and non-involvement according to the pathological findings of postoperative lymph node sampling.
To evaluate intra-observer reproducibility, 30 cases were chosen at random for ROI delineation and ROI-based feature extraction by the same radiologist one month later. To select the robust radiomic features, we used intra-class correlation coefficient (ICC) to evaluate the stability and reproducibility in radiomic feature extraction. The intra observer ICC was calculated based on the twice feature extractions by the radiologist. In general, ICC >0.75 was deemed to have a good reliability or reproducibility. To make our results more reliable, we performed feature screening with a more stringent ICC >0.80 criterion.
Statistical Analysis and Radiomics Model Building
Based on the results of immune cell analysis, the proportion of CD8+ TRMs in immune cells was tested for normality, and patients were divided into high- and low-CD8+ TRMs groups according to the median value of the proportion of CD8+ TRMs. The group’s differences in gender and age were compared using two-sample t or χ2 test in SPSS 19.0 software (IBM). Four semantic features including tumor boundary, tumor texture type, tumor pathology type and lymph node metastasis type were enrolled in the feature selection procedures. Feature selection was performed in the following three steps to reduce redundancy. Firstly, we validated the data according to the ICC > 0.8. Secondly, the difference of every remained radiomic feature in the previous step and 4 semantic features between the high– and low-CD8+ TRMs groups was compared. The differences in features between the two groups were compared using t-test and Wilcoxon Rank-Sum Test. Thirdly, the remained radiomics features and semantic features were selected to build a neural network radiomic model to predict the high– or low-CD8+ TRMs infiltration type.
Predictive Performance of Radiomic Model
The radiomic model performance was evaluated by receiver operating characteristic (ROC) curves. To quantify the discriminatory power of the radiomic model, the parameters including the area under the curve (AUC), sensitivity, specificity, and accuracy were provided. Then, the same parameters on the validation set were obtained from the training set to test the prediction performance of this model. The radiomic workflow is shown in Figure 1.
 |
Figure 1 The workflow of radiomic. Abbreviation: CD8+ TRMs, tissue resident memory CD8+ T cells. |
Result
A total of 97 patients were eligible for enrollment in the lung cancer tissue immune cell database (114 specimens) (Table 1), of which (46 men and 51 women; mean age, 61.8 ± 8.2 years), the proportion of CD8+ TRMs in all enrolled patients was distributed (10.5–76.9%; 44.7 ± 15.4%), and patients were divided into a low CD8+ TRMs infiltration group (≤44.5%; 49 patients) and a high CD8+ TRMs infiltration group (>44.5%; 48 patients) according to the median. (Figure 2) The low CD8+ TRMs infiltration group consisted of 25 men and 24 women (62.7 ± 8.5 years; range 44–73 years), while the high CD8+ TRMs infiltration group contained 21 men and 27 women (61.0 ± 7.9 years; range 38–74 years); There is no statistical differences were found in gender (P = 0.473), age (P = 0.302). Pathological findings showed 80 adenocarcinomas (82.5%) and a total of 17 squamous-cell carcinoma, adenosquamous carcinoma and other types (17.5%), with no statistical difference in the level of CD8+ TRMs infiltration between adenocarcinoma and non-adenocarcinoma specimens (p = 0.1198).
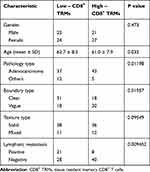 |
Table 1 Results of the Analysis of Basic Demographic and Clinical Data |
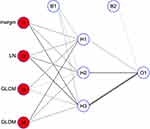
A total of 107 radiomic features were extracted from patients CT image data before tumor resection. The 107 quantitative parameters including 14 shape features, 18 first-order features, 24 gray-level co-occurrence matrix (GLCM) features, 15 gray level dependence matrix (GLDM) features, 16 gray-level run-length matrix (GLRLM) features, 16 gray level size zone matrix (GLSZM) features, 5 neigbouring gray tone difference matrix (NGTDM) features were extracted from the image data. In the feature selection step, the feature with ICC >0.8 was deemed to have a good reliability or reproducibility in intra-observer analyses. As a result, a total of 46 features were robust and then applied for subsequent feature selection. The difference of radiomic features and 4 semantic features between the high– and low-CD8+ TRMs groups was compared using t-test and Wilcoxon Rank-Sum Test. Two radiomic features and two semantic features remained showed correlation with CD8+ TRMs Infiltrating phenotypes. The 2 radiomic features were original_glcm_MCC (p=0.0383) and original_gldm_Large Dependence High Gray Level Emphasis (p=0.0377) showed significant difference between high- versus low-CD8+ TRMs. The ability of the 2 radiomic signature to classify high- versus low-CD8+ TRMs infiltration was shown to have an AUC of 0.622 and 0.621, respectively. (Figure 3) The 2 semantic features Boundary type (p=0.01957) and Lymphatic metastasis (p=0.009452) showed significant difference between high- versus low-CD8+ TRMs. The selected features were provided in Table 2. Finally, the remaining four features were chosen to build a neural network radiomic model (Figure 4).
 |
Table 2 Selected Features with Descriptions of the High– and Low-CD8+TRMs Infiltrating Phenotypes |
All patients were randomly divided into training and validation sets according to the ratio of 8:2, with 79 patients in the training set and 18 patients in the validation set. There was no significant difference in age and gender between the two groups of patients. In the training set, the radiomic model had good discrimination capacity (AUC =0.788, 95% confidence interval [CI]; sensitivity= 0.5385; specificity =0.7500; and accuracy = 0.6456) for discriminating the high– from the low-CD8+ TRMs groups. In the validation set, this radiomic model had a similar discrimination capacity (AUC = 0.753, 95% CI; sensitivity = 0.4444; specificity = 0.7778; and accuracy = 0.6111) (Figure 5).
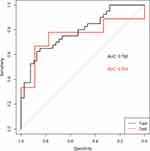 |
Figure 5 ROC curves of the radiomic model in the training set (black curve) and the validation set (red curve). |
Discussion
With increasing use of immunotherapy in cancer, knowledge of an individual’s immune status could help to identify those who holds good response to treatment.20,21 Although ICI has revolutionized the management of specific cancer types, the majority of patients are intrinsically resistant and fail to respond. Overall, only approximately 20% to 30% of patients showed treatment response, although this varies depending on the tumor type.3,4 Furthermore, administration of immunotherapy can result in severe side effects, especially immune mediated reactions against healthy organs.3 In addition, immunotherapy is particularly expensive, therefore, for the most cost-effective use of ICI, it is important to have validated biomarkers that upfront predict whether a patient is likely to respond to these therapies.4
Since immunotherapy relies on the activation of immune cells to generate an immune response to recognize and clear tumor cells, the key immune-mediated responding cell in tumor immune microenvironment is an important guidance to predict the clinical immunotherapy response.10 In 1999, Federica Sallusto et al established the doctrine of immunosurveillance centered on circulating CD8+ T cells by studying the phenotype and function of CD8+ T cells in tissues, such as blood and lymph nodes.22 However, this doctrine does not explain well the rationale for local immunosurveillance within epithelial tissues that occupy the largest body surface area of the body, such as skin and mucosal epithelium.23 CD8+ TRMs are a part of CD8+ T cells with high expression of the epithelial adhesion molecule CD103 and/or CD69 molecules as the core feature. An increasing number of studies suggested the key role for CD8+ TRMs in tumor immunosurveillance and immunotherapy.13 Study on lung cancer molecular features associated with the robustness of anti-tumor immune responses showed that a greater density of TRM cells in tumors was predictive of a better survival outcome in lung cancer, and this effect was independent of that conferred by cytotoxic T lymphocytes density.14 Another study directly confirmed the association of CD8+ TRMs with the major cell subpopulation secreting antitumor-associated cytokines in human early-stage lung cancer infiltrating CD8+ TRMs.24 However, detection of CD8+ TRMs in tumor tissues can currently only rely on lung puncture biopsy, which is often difficult to accurately reflect the number of CD8+ TRMs in tissues due to the limited amount of tissue collected and tumor heterogeneity compared to surgical specimens, which can more accurately reflect the number of CD8+ TRMs in lung cancer tissues. Considering there are a large number of inoperable patients with lung cancer, thus it is important to establish a non-invasive method to accurately assess the number of CD8+ TRMs in lung cancer tissues.
In this study, we developed and validated an effectively preoperative neural network radiomic model using CT image data to differentiate the high– from the low– CD8+ TRMs infiltration phenotype and further to predict NSCLC tumor local immune status. It may be a potential imaging method for assessing lung cancer prognosis and guiding clinical treatment decision making in a noninvasive way.
Previous study use radiomics approach to assess tumor-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy showed that imaging biomarker could be useful in estimating CD8 cell count and predicting clinical outcomes of patients treated with immunotherapy, when validated by further prospective randomised trials.25 However, the study included different tumor types at various sites, and the number of lung cancer cases only accounted for 20% of the total, and no specific study was conducted for lung cancer, so the application value in lung cancer is still not sufficient.
Other studies used radiomic approach to evaluate the tumor immune microenvironment (TIME) of lung cancer surgical specimens with high and low CD8-to-CD3high/PD-1- to-CD8low), and concluded that higher order CT extracted features associated with specific TIME profiles may reveal a radio-immune signature with prognostic impact on resected NSCLC.26 Meanwhile, a studies suggesting that total CD8+ T cells do not correlate with survival in NSCLC patients unless CD8+ T cells are present in the tumor tissue.15
Several clinical studies have shown that tumor-infiltrating CD8+ TRMs are positively associated with lung cancer patient prognosis,14,15 and the evidence suggests that CD8+ TRMs are the key cell subpopulation within the lung cancer microenvironment that is specifically activated and exerts a long-term anti-tumor immune response. Therefore, by directly detecting the infiltration level of CD8+ TRMs within the tumor tissue can more accurately reflect the local immune status of the tumor and maybe a good biomarker for predict the immunotherapy response.
In our study, we used lung cancer surgical specimens to detect the proportion of CD8+ TRMs among immune cells in lung cancer surgical specimens to reflect the local immune status of the tumor, and then applied radiomic approaches to extract the imaging features associated with the proportion of CD8+ TRMs, and the results showed that radiomic features could screen the infiltration proportion of CD8+ TRMs in lung cancer tissues. The AUC values were 0.788 and 0.753 in the training set and validation set, respectively. These were achieved by using the selected radiomic features and semantic features to build a neural network model to predict the infiltration level of CD8+ TRMs within lung cancer tissues, indicating that radiomic can be used to predict the infiltration level of CD8+ TRMs within lung cancer tissues and reflect the local immune status of the tumor, which provides a new pathway for us to non-invasively assess the immune status of lung cancer tissues, and the model can be used to predict the local immune status and immunotherapy prognosis of patients before treatment, providing a scientific reference basis for clinical treatment decisions.
In addition, our results showed that the degree of lymph node involvement in NSCLC was correlated with the proportion of CD8+ TRMs, with 8 cases of lymph node involvement and 40 cases of no lymph node involvement in the high-CD8+ TRMs infiltration group, while 21 cases of lymph node involvement and 28 cases of no lymph node involvement in the low-CD8+ TRMs infiltration group, with a significant difference between the two groups (P = 0.009452), which indirectly reflected that the presence of CD8+ TRMs could inhibit the lymph node metastasis of tumor cells and influence the clinical stage of patients, and could also explain the association of CD8+ TRMs with the prognosis of lung cancer patients. In our study, the lymph node metastasis factor was based on the surgery result, however, this factor can also be assessed by imaging techniques such as CT or PET-CT, and previous study showed that PET-CT is useful in predicting not only lymph node metastasis but also other malignant features of early-stage lung adenocarcinoma.27 Therefore, the lymph node metastasis factor in our radiomics model can be replaced by imaging result in practice. Meanwhile, whether the boundary of the lesion is vague or clear is often used as an indirect evidence to judge the benignity and malignancy of the lesion in clinical imaging diagnosis. Our results showed that the boundary characteristics of the lesion were also related to the infiltration level of CD8+ TRMs, according to which we can speculate that CD8+ TRMs may have a role in limiting the infiltration and invasion of the tumor into the surrounding tissues.
Our study also has some limitations. Firstly, our study is a retrospective and single-center study, and the number of cases is limited. With only limited number of cases trained and validated, more data from multiple centers are needed to validate the results. Secondly, although we have established a predictive model for local immune status of lung cancer, there is not enough data to validate the predictive ability of immunotherapy patients in clinical practice, which makes the clinical application limited. We will continue to collect clinical data from immunotherapy patients to further validate the model.
Conclusion
In conclusion, our preliminary study showed that the radiomic model had a good performance for preoperatively predicting both local immune status of NSCLC tissue and lymph node metastasis in a noninvasive way.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81572800 and 82073141 to PW); The Fundamental Research Funds for the Central Universities (2019QNA7025 to PW) and the Natural Science Foundation of Zhejiang Province (LY15H160041 to PW).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi:10.1038/nature21349
2. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774
3. Postow MA, Sidlow R, Hellmann MD, Longo DL. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi:10.1056/NEJMra1703481
4. Duffy MJ, Crown J. Biomarkers for predicting response to immunotherapy with immune checkpoint inhibitors in cancer patients. Clin Chem. 2019;65(10):1228–1238. doi:10.1373/clinchem.2019.303644
5. Keenan TE, Burke KP, Van Allen EM. Genomic correlates of response to immune checkpoint blockade. Nat Med. 2019;25(3):389–402. doi:10.1038/s41591-019-0382-x
6. Yi M, Niu M, Xu L, Luo S, Wu K. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol. 2021;14(1):10. doi:10.1186/s13045-020-01027-5
7. Dong W, Wu X, Ma S, et al. The mechanism of anti-PD-L1 antibody efficacy against PD-L1-negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Discov. 2019;9(10):1422–1437. doi:10.1158/2159-8290.CD-18-1259
8. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi:10.1056/NEJMoa1504627
9. Cyriac G, Gandhi L. Emerging biomarkers for immune checkpoint inhibition in lung cancer. Semin Cancer Biol. 2018;52(Pt 2):269–277. doi:10.1016/j.semcancer.2018.05.006
10. Corgnac S, Boutet M, Kfoury M, Naltet C, Mami-Chouaib F. The emerging role of CD8(+) Tissue Resident Memory T (TRM) cells in antitumor immunity: a unique functional contribution of the CD103 integrin. Front Immunol. 2018;9:1904. doi:10.3389/fimmu.2018.01904
11. Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. doi:10.1038/ni.1718
12. Masopust D, Choo D, Vezys V, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207(3):553–564. doi:10.1084/jem.20090858
13. Park SL, Gebhardt T, Mackay LK. Tissue-resident memory T cells in cancer immunosurveillance. Trends Immunol. 2019;40(8):735–747. doi:10.1016/j.it.2019.06.002
14. Ganesan AP, Clarke J, Wood O, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol. 2017;18(8):940–950. doi:10.1038/ni.3775
15. Djenidi F, Adam J, Goubar A, et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194(7):3475–3486. doi:10.4049/jimmunol.1402711
16. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–577. doi:10.1148/radiol.2015151169
17. Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–762. doi:10.1038/nrclinonc.2017.141
18. Gardin I, Gregoire V, Gibon D, et al. Radiomics: principles and radiotherapy applications. Crit Rev Oncol Hematol. 2019;138:44–50. doi:10.1016/j.critrevonc.2019.03.015
19. Wu P, Wu D, Ni C, et al. GammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40(5):785–800. doi:10.1016/j.immuni.2014.03.013
20. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi:10.1038/nature14011
21. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi:10.1038/nature13954
22. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi:10.1038/44385
23. Masopust D, Soerens AG. Tissue-resident T cells and other resident leukocytes. Annu Rev Immunol. 2019;37(1):521–546. doi:10.1146/annurev-immunol-042617-053214
24. O’Brien SM, Klampatsa A, Thompson JC, et al. Function of human tumor-infiltrating lymphocytes in early-stage non-small cell lung cancer. Cancer Immunol Res. 2019;7(6):896–909. doi:10.1158/2326-6066.CIR-18-0713
25. Sun R, Limkin EJ, Vakalopoulou M, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018;19(9):1180–1191. doi:10.1016/S1470-2045(18)30413-3
26. Mazzaschi G, Milanese G, Pagano P, et al. Integrated CT imaging and tissue immune features disclose a radio-immune signature with high prognostic impact on surgically resected NSCLC. Lung Cancer. 2020;144:30–39. doi:10.1016/j.lungcan.2020.04.006
27. Kagimoto A, Tsutani Y, Izaki Y, et al. Prediction of lymph node metastasis using semiquantitative evaluation of PET for lung adenocarcinoma. Ann Thorac Surg. 2020;110(3):1036–1042. doi:10.1016/j.athoracsur.2020.03.032
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.