Back to Journals » Patient Related Outcome Measures » Volume 8
A questionnaire-based audit to assess overall experience and convenience among patients using vaginal progesterone tablets (Lutigest®) for luteal phase support during IVF treatment
Authors Heine P , Sellar L, Whitten S, Bajaj P
Received 28 April 2017
Accepted for publication 5 September 2017
Published 8 December 2017 Volume 2017:8 Pages 169—179
DOI https://doi.org/10.2147/PROM.S140678
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Liana Bruce
Polly Heine,1 Laura Sellar,1 Sue Whitten,2 Priti Bajaj2
1Fertility Team, The Agora Gyneacology and Fertility Centre, Hove, 2Medical Affairs, Ferring Pharmaceuticals, West Drayton, UK
Purpose: The aim of this audit was to assess the overall experience and patient convenience of vaginal progesterone tablets (Lutigest®, marketed as Endometrin® in the USA) used for luteal phase support (LPS) during in vitro fertilization (IVF) treatment.
Patients and methods: This questionnaire-based audit included responses from 100 patients undergoing IVF treatment at six IVF clinics in the UK from September 2015 to November 2016. Fourteen days after starting progesterone supplementation for LPS during their IVF treatment, patients rated overall experience and perceived convenience of the prescribed progesterone by completing a questionnaire.
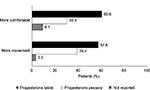
Results: Of the 100 patients included, 96 received vaginal progesterone tablets for LPS. Overall, 53.1% (51/96) indicated that the progesterone tablets were “very easy” to use; 42.7% (41/96) and 44.8% (43/96) found it “very convenient” or “neither convenient or inconvenient” to administer the tablet, respectively. Overall experience with using progesterone tablets was rated as “very comfortable” by 34.4% (33/96) and “neither comfortable or uncomfortable” by 56.3% (54/96) of patients. The applicator was used by 93.8% (90/96) of patients to administer the tablet, and 86.5% (83/96) indicated that the applicator was easy to clean for repeated use. A total of 33 patients had a previous IVF cycle during which they were prescribed vaginal progesterone pessaries for LPS. Compared with progesterone pessaries, the majority found treatment with progesterone tablets to be more comfortable (60.6%; 20/33) and more convenient (57.6%; 19/33) and indicated that the progesterone tablet was their preferred progesterone formulation for LPS (60.6%; 20/33).
Conclusion: These findings offer insights into real-world patient experiences with the progesterone vaginal tablet formulation. The results suggest overall patient convenience, ease, and comfort with using progesterone vaginal tablets for LPS. The majority of patients found progesterone vaginal tablets more convenient and comfortable to use compared with progesterone pessaries.
Keywords: vaginal progesterone tablets, progesterone pessary, progesterone gel, luteal phase support, in vitro fertilization, patient experience, convenience
Introduction
Between ovulation and menstruation – the luteal phase of the menstrual cycle – the corpus luteum produces progesterone which prepares the estrogen-primed endometrium for embryo implantation.1 During in vitro fertilization (IVF), use of gonadotropin-releasing hormone agonists and antagonists for downregulation of luteinizing hormone secretion may disrupt the luteal phase and lower progesterone production;2,3 this could lead to inadequate development of the endometrium and reduce chances of embryo implantation.4 To improve embryo implantation and pregnancy rates, supplementation with exogenous progesterone for luteal phase support (LPS) is an established practice for stimulated IVF cycles.5,6
Progesterone for LPS can be administered vaginally, orally, or intramuscularly.7–9 Benefits of oral progesterone are limited by poor bioavailability (due to liver first-pass metabolism) and sedative side effects.10 Intramuscular administration of progesterone is often associated with complications such as injection site pain, soreness, abscesses, and inflammatory reactions.11 Of the available routes of administration, there is increasing evidence in the literature in favor of vaginal progesterone.11 Progesterone administered vaginally bypasses liver first-pass metabolism and is delivered preferentially to the endometrium, thus resulting in higher progesterone concentrations in the endometrial tissue compared with blood serum.11–15 Pregnancy rates with vaginal progesterone are comparable with those achieved with intramuscular progesterone.16–22 In addition, compared with intramuscular progesterone, vaginal administration of progesterone is painless and does not require special equipment.22 Thus, vaginal progesterone is widely used for LPS and is generally preferred over intramuscular progesterone due to its convenience and ease of administration.20–23
Vaginally administered progesterone achieves adequate endometrial secretory transformation for LPS, and the pharmacokinetic properties are greatly dependent on the formulation used.11 Several progesterone formulations for vaginal administration are currently available – vaginal progesterone pessaries, gel, capsules, and tablets.2 Although all the different vaginal progesterone formulations contain the same active ingredient, associated side effects, inconvenience, and difficulty of use differ.24–29 Progesterone pessaries (Cyclogest®) contain natural progesterone and are commonly used for LPS during IVF procedures.29–31 However, melting of the pessary at body temperature in the vagina can lead to increased vaginal discharge, vaginal buildup, and vaginal irritation.32,33 Both progesterone gel (Crinone®) and progesterone capsules (Utrogestan®) contain micronized progesterone.34,35 However, progesterone gel is known to cause vaginal irritation,36 while progesterone capsules have been associated with local intolerance (e.g., burning, pruritus, or fatty discharge).35 In addition, progesterone capsules contain soya lecithin as an excipient which may cause hypersensitivity reactions.35
Vaginal progesterone tablets (Lutigest®, marketed as Endometrin® in the USA) contain 100 mg of micronized progesterone in a base of lactose monohydrate, adipic acid, and sodium bicarbonate.37 Decreasing the size of the progesterone particles through micronization increases the bioavailability of the hormone, which is dependent on the size of the progesterone particles in suspension. In addition, the advantage of the progesterone tablet is its unique vaginal delivery system – the effervescent vaginal tablet dissolves upon contact with vaginal secretions and disintegrates into a powder that adheres to the vaginal epithelium, thereby facilitating sustained release of progesterone.33 This allows rapid absorption of progesterone across the vaginal epithelium and achieves high concentrations of progesterone in the endometrial tissue while limiting systemic exposure and providing sustained plasma concentrations suitable for IVF treatment protocols.12,38 In healthy premenopausal women who administered 100 mg of vaginal progesterone tablets thrice daily, serum progesterone concentrations peaked at 19.8 ng/mL on the first day,37 and steady state concentrations were achieved within 24 hours.38 This peak serum level of 19.8 ng/mL was within the effective progesterone range shown to support embryo implantation in hormone replacement-cryopreserved embryo transfer.39 Compared with vaginal progesterone gel, vaginal progesterone tablets reached a higher maximum serum concentration (Cmax), achieved steady state more rapidly, produced greater systemic exposure (area under the curve [AUC]0–24), and had less between-subject variability in pharmacokinetic parameters after 5 days of treatment.38 In addition, higher endometrial progesterone concentrations were achieved with vaginal progesterone tablets compared with intramuscular progesterone.12 A Phase III clinical trial demonstrated that vaginal progesterone tablets administered at 100 mg thrice daily for LPS yielded comparable pregnancy and live birth rates with vaginal progesterone gel and was safe and well tolerated by women undergoing IVF.40
Given that the different vaginal progesterone formulations provide similar efficacy outcomes, the progesterone formulation that provides the most convenience and ease of use would be the formulation of choice for both patients and clinicians. However, studies that have attempted to assess patient convenience and experiences with vaginal progesterone formulations for LPS during IVF cycles are currently limited and the most convenient formulation to meet the highest patient compliance has not yet been elucidated.24–28 The objective of this questionnaire-based audit was to gather real-world evidence regarding patient experience and convenience with using progesterone tablets for LPS as part of their IVF treatment.
Materials and methods
Audit design and participants
This was a questionnaire-based audit conducted at six IVF clinics in the UK from September 2015 to November 2016. Standard IVF treatment protocols for each participating clinic were followed. During the audit period, women who were undergoing fresh IVF cycles at the participating clinics and who agreed to use vaginal progesterone tablets (Lutigest® [marketed as Endometrin® in the USA], Ferring Pharmaceuticals Ltd., UK) for LPS based on the patient information provided were prescribed 100 mg of progesterone tablets administered vaginally three times daily starting at oocyte retrieval.
The questionnaire was provided to women on the day of embryo transfer, and they were requested to complete the questionnaire 14 days after starting vaginal progesterone tablet treatment and to return the questionnaire by post. A total of 100 completed responses were received. The questionnaire was anonymous without any identification of the patient. Institutional review board approval was not required for this audit as it was considered a service evaluation project. Patients were not randomized, and treatment allocation was a joint decision by the clinician and patient.
Questionnaire
The questionnaire was designed to assess patients’ experiences with progesterone formulations for LPS during IVF treatment. The questionnaire comprised 11 questions (Figure S1); six questions assessed tolerability, convenience, ease of administration, and overall experience with using progesterone tablets in the current IVF cycle; for patients who had undergone a previous IVF cycle, the remaining five questions assessed their current experience with using progesterone tablets compared with the progesterone formulation prescribed in the previous IVF cycle.
Statistical analyses
Responses obtained were summarized using descriptive statistics. Responses that did not fall within the available multiple choice responses in the audit questionnaire were considered to be invalid or not reported. The chi-square test for independence was used to test for differences in the distribution of responses between naive and non-naive patients; invalid responses were excluded from the analyses. A P-value less than 0.05 was considered to be statistically significant. All analyses were conducted using Microsoft Excel 2013.
Results
Patients’ progesterone formulation prescription history
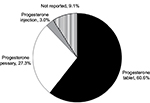
A total of 100 patients completed the questionnaire. Among these patients, 96 received progesterone tablets for LPS during their current IVF cycle in the audit period and were included in the analysis (Table 1). The remaining four patients received either progesterone pessaries (n=3) or progesterone capsules (n=1) for LPS during their current IVF cycle and were excluded from the analysis.
In the current audit, of the 96 patients who were prescribed progesterone tablets in their current IVF cycle, 57 were in their first IVF cycle (naive patients) and 39 had a previous IVF cycle in which they were prescribed a progesterone formulation for LPS (non-naive patients). Table 1 provides the details of the progesterone formulation prescription history of the audit population.
Tolerability, convenience, ease of use, and overall experience with using progesterone tablets
Patients’ assessment of convenience, ease of use, and overall experience with using progesterone tablets is shown in Figure 1. In response to a question about the convenience of administering vaginal progesterone tablets, 42.7% (n=41) of patients indicated that it was “very convenient” while 44.8% (n=43) described it to be “neither convenient or inconvenient” (Figure 1A). Only 10.4% (n=10) of the patients described the convenience of administering progesterone tablets to be “very difficult.” The majority of patients (53.1%; n=51) found the progesterone tablets “very easy” to use and only a few patients (10.4%; n=10) found them “very difficult” to use (Figure 1B). Overall experience with using progesterone tablets was rated as “very comfortable” by 34.4% (n=33) of patients and “neither comfortable or uncomfortable” by 56.3% (n=54) of patients (Figure 1C). Only 8.3% (n=8) of the analysis population described their overall experience with progesterone tablets to be “very uncomfortable”. Overall, 90.6% (n=87) reported experiencing vaginal discharge and 89.6% (n=86) indicated that cleaning was required more often.
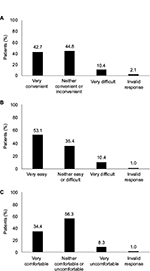  | Figure 1 Patients’ assessment of convenience (A), ease of use (B), and overall experience (C) with using vaginal progesterone tablets (n=96). |
Patient responses regarding convenience, ease of use, overall experience, and extra vaginal discharge with using vaginal progesterone tablets were not significantly different between naive patients and non-naive patients.
Experience with using the progesterone tablet applicator
The majority of the analysis population (93.8%; n=90) used the applicator to administer the progesterone tablets, and most (86.5%; n=83) reported that the applicator was easy to clean for repeated use. There were 24 respondents for the question “if you did not use the applicator how easy did you find it to insert the progesterone preparation?” Of these, 14 (58.3%) reported that insertion of the progesterone tablet was “very easy” without using the applicator; seven (29.2%) indicated that it was “neither easy or difficult” while three (12.5%) found it “very difficult” to insert the progesterone tablets without the applicator.
Patient responses regarding the ease of insertion of progesterone tablets without using the applicator were not significantly different between naive and non-naive patients.
Comparison of overall experience and convenience of progesterone tablets with progesterone pessaries prescribed in a previous IVF cycle
Of the 96 patients who received progesterone tablets for LPS in the current IVF cycle, 57 were in their first IVF cycle. The remaining 39 patients had a previous IVF cycle in which they were prescribed either progesterone tablets (n=2), progesterone pessaries (n=33), progesterone capsules (n=1), or progesterone injection (n=2) for LPS (Table 1). One patient could not recall the progesterone formulation prescribed for LPS in her previous IVF cycle.
Responses from the 33 patients who were prescribed progesterone pessaries in a previous IVF cycle were analyzed for comparison of patient experiences with progesterone tablets and progesterone pessaries. When asked about their previous experience with progesterone pessaries, some patients reported experiencing vaginal discharge/leakage (36.4%; n=12), vaginal buildup (15.1%; n=5), and vaginal irritation (6.1%; n=2). Compared with progesterone pessaries, the majority found treatment with progesterone tablets to be more comfortable (60.6% [progesterone tablets] vs. 30.3% [progesterone pessaries]) and more convenient (57.6% [progesterone tablets] vs. 39.4% [progesterone pessaries]; Figure 2). Among the 33 patients who were prescribed progesterone pessaries in a previous IVF cycle and progesterone tablets in the current IVF cycle, 30 patients reported experiencing vaginal discharge with progesterone tablets, six of whom reported less vaginal discharge compared with when they used progesterone pessaries in a previous IVF cycle. More patients preferred using a treatment that was administered vaginally (66.6%; n=22) rather than rectally (24.2%; n=8) while a few did not indicate a preference (9.1%; n=3). When asked about their preferred progesterone formulation if given a choice, the majority (60.6%) expressed a preference for progesterone tablets while 27.3% indicated progesterone pessaries as their preferred progesterone formulation (Figure 3).
Discussion
Progesterone supplementation is standard practice for LPS during IVF treatment and has been shown to improve ongoing pregnancy and live birth rates.5,6 Various progesterone formulations for LPS in IVF treatment are available (vaginal tablets, pessaries, capsules, gel, and intramuscular injections), and several comparison studies have reported no differences in pregnancy rates between the various formulations.6,16–18,22,28,40 However, besides pregnancy rates, patient experiences and preferences are also important aspects of fertility care.41 Insights into patient experiences and perceptions of the various progesterone formulations can help clinicians make informed decisions when prescribing progesterone for LPS during IVF treatment and may also improve patient experience and compliance with treatment. This questionnaire-based audit of 100 patients provides a comprehensive assessment of patient-reported convenience, ease of use, and tolerability with vaginal progesterone tablets, as well as patient-reported experience with progesterone tablets in comparison with progesterone pessaries.
Overall, the majority of patients found progesterone tablets “very easy” to use and the applicator easy to clean for repeated use. Most patients (90.7%) described their overall experience with the vaginal progesterone tablets as “very comfortable” or “neither comfortable or uncomfortable,” and 87.5% found them to be “very convenient” or “neither convenient or inconvenient” to administer. Only 10.4% described the convenience of administering progesterone tablets to be “very difficult” to use and only 8.3% described their overall experience with progesterone tablets to be “very uncomfortable.” Compared with progesterone pessaries prescribed in a previous IVF cycle, most patients found treatment with progesterone tablets to be more convenient and more comfortable, and 60.6% indicated that the progesterone tablet was their preferred progesterone formulation.
IVF treatment success rates are influenced by patient compliance with treatment.42 Treatment compliance may depend on a multitude of factors including dosing complexity, dosing frequency, patient convenience, and overall experience with treatment. In the current audit, the majority of patients found vaginal progesterone tablets easy to use and comfortable to administer. In addition, despite a three-times daily dosing regimen, a significant proportion of patients found dosing of progesterone tablets to be “very convenient.” One possible reason for the perceived convenience of progesterone tablets may be its vaginal route of administration, which offers a painless and comfortable way to administer progesterone compared with intramuscular injection.11,17 A previous study by Beltsos et al22 reported better patient satisfaction, ease of use, and convenience with vaginal progesterone tablets compared with intramuscular progesterone. In addition, ease of cleaning of the progesterone tablet applicator for repeated use, as reported by 86.5% of patients in the current audit, may also be a contributory factor to the perceived convenience of progesterone tablet administration. Even when the progesterone tablet applicator was not used, the majority of patients still found progesterone tablet administration to be “very easy.” Considering that patient convenience and overall experience with treatment prescribed are associated with treatment compliance, the perceived convenience, comfort, and ease of administering progesterone tablets for LPS may be clinically important in influencing IVF treatment compliance and outcomes.
Enzymes, microflora, and vaginal secretions in the vagina affect the spreading and retention of vaginal pharmaceutical formulations, as well as absorption and drug release in the vagina.43 In the presence of vaginal moisture, solid dosage formulations should disperse in the vaginal canal immediately after insertion – this will avoid inconvenience to the users due to the progesterone vaginal formulation lying in situ in the vagina for prolonged periods and also allow absorption through the vaginal epithelium. It has also been suggested that the use of excipients and vaginal drug delivery systems that offer advantages over conventional systems would be desirable. In addition, there is a need for desirable product dispersion throughout the vagina, retention for intended intervals, and adequate release of drug.43 The advantage of the effervescent vaginal progesterone tablet is that it absorbs the vaginal secretions and disintegrates into an adhesive powder that adheres to the vaginal epithelium, thus facilitating sustained release.33
Vaginal progesterone preparations are known to be associated with vulvovaginal side effects,24–29 with vaginal discharge being the most common and reported in over 80% of women using vaginally administered progesterone.24 As expected, most patients in the current audit reported experiencing extra vaginal discharge while using progesterone tablets for LPS. As the progesterone tablets used in this audit were effervescent vaginal tablets that dissolve upon contact with vaginal secretions,33 it is likely that patients experienced this effervescent action as vaginal discharge. It is perhaps also noteworthy that the presence of extra vaginal discharge was specifically enquired in the audit questionnaire, unlike other studies which may have taken a more passive approach.40 Nevertheless, most instances of vaginal discharge are normal and physiological unless accompanied by odor, discomfort, or pruritus.44 Misperceptions about the vaginal discharge associated with vaginal progesterone administration for LPS can lead to reluctance on the part of some health care professionals to recommend various vaginally administered progesterones.45 In the current audit, despite reporting vaginal discharge, most patients stated that they perceived the progesterone vaginal tablet to be comfortable, convenient, and easy to use. Detailed patient information provided by health care professionals regarding the expected vaginal discharge, reason for discharge, and how to manage it may help to prime patient expectations and further improve patient tolerance of the side effects of vaginal discharge and irritation related to vaginal progesterone preparations.
Depending on the vaginal progesterone formulation used and the excipients it contains, the incidence24–29 and consistency of vaginal discharge may differ (e.g., cloddy discharge with progesterone gel,25 fatty discharge with progesterone capsules,35 and pessaries46). Incidences of vaginal burning and pruritus have been reported with the progesterone capsule,35 while vaginal irritation has been associated with the progesterone gel, pessary, and tablet.28,32,33,36 Notably, a trend toward less perineal irritation due to vaginal discharge was observed in patients using progesterone tablets compared with those using progesterone pessaries.28 As allergens such as soya lecithin and peanut oil (found in other vaginal progesterone preparations) are not used as excipients in the pharmaceutical formulation of the vaginal progesterone tablet, it may also be suitable for hypersensitive patients.37
Few studies have attempted to assess and compare side effects and patient convenience between the various vaginal progesterone formulations.24–28 A recent study found no significant differences in side effects and patient convenience between vaginal progesterone pessaries and vaginal progesterone tablets administered twice a day.28 Our findings add to current literature comparing patient convenience between progesterone pessaries and progesterone tablets. Among patients who were prescribed progesterone pessaries in a previous IVF cycle and progesterone tablets in the current IVF cycle, most found treatment with progesterone tablets to be more convenient and more comfortable compared with progesterone pessaries. Furthermore, the majority indicated that the vaginal progesterone tablet was their preferred progesterone formulation. In the current audit, two-thirds of patients preferred the vaginal route of administration over the rectal route. Notably, rectal administration of progesterone is commonly associated with side effects such as constipation and flatulence.29,31 As route of administration may influence treatment compliance and ultimately IVF outcomes, patient preferences should be taken into consideration when prescribing a progesterone formulation for LPS.
Importantly, our findings need to be interpreted within the limitations of the audit. One limitation of the current audit was its open-label design, where randomization or blinding of participants was not performed. In addition, by nature of the audit design, patients were prescribed vaginal progesterone tablets for LPS, and direct comparison with another progesterone formulation was not possible. Nevertheless, patients who have had more than one IVF cycle provided a comparison of their experience with using vaginal progesterone tablets in their current IVF cycle with the progesterone formulation prescribed in their previous IVF cycle. It should be noted, however, that this comparison was based on retrospective recall and patient opinion, which may be limited by recall bias or partially influenced by pregnancy outcomes of the previous IVF cycle. A follow-up improved study design which examines patient perceptions in relation to pregnancy outcomes of the previous IVF cycle may help to address these potential biases. In addition, local IVF protocols were followed at each participating clinic; however, this was not expected to influence patients’ assessment of ease of use and dosing convenience with the progesterone tablets. Finally, as the recommended duration for progesterone supplementation for LPS varies widely between IVF clinics (ranges from 2 to 12 weeks after oocyte retrieval),47 patient experience with vaginal progesterone tablets for LPS beyond 14 days was not evaluated in the current audit.
Conclusion
Overall, the vaginal progesterone tablet was reported to be convenient, easy, and comfortable to use. Compared with progesterone pessaries, most patients found treatment with progesterone tablets to be more convenient and more comfortable, and the majority indicated that the progesterone tablet was their preferred progesterone formulation. Notably, the vaginal route was preferred over the rectal route for the administration of progesterone for LPS. The findings of this questionnaire-based audit provide important information on real-world patient experiences that may aid clinical decisions when selecting a progesterone formulation for LPS as part of IVF treatment.
Acknowledgments
The authors thank the following clinics and nurses who provided patient information to the participating patients and coordinated distribution and collection of the questionnaires: Caroline Lewis (Nuffield Health Woking Hospital, Woking, UK), Frances Stevens (Care Fertility Tunbridge Wells, Tunbridge Wells, UK), Alice Denge (Assisted Conception Unit, Guy’s Hospital, Great Maze Pond, London, UK), Lister Fertility Clinic (Chelsea Bridge Road, London, UK), and Care Fertility Nottingham (Nottingham, UK). This audit was sponsored by Ferring Pharmaceuticals, UK. Medical writing and editorial support were funded by Ferring Pharmaceuticals and provided by Bao Hui Lee and Naina Sharma from Tech Observer Pvt. Ltd.
Author contributions
Polly Heine had full access and control to all the data in the audit. Polly Heine and Laura Sellar were involved in data collection. Sue Whitten and Priti Bajaj took responsibility for the integrity of the data and the accuracy of the data analysis. All the authors participated in the audit design, data collection process, data analyses, data interpretation, manuscript writing, revision, and approval of the manuscript for submission.
Disclosure
Sue Whitten and Priti Bajaj are employees of Ferring Pharmaceuticals, UK. The other authors report no conflicts of interest in this work.
References
Reed BG, Carr BR. The normal menstrual cycle and the control of ovulation [Updated May 22, 2015]. In: De Groot LJ, Chrousos G, Dungan K, et al., editors. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc.; 2000. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279054/. Accessed October 03, 2016. | ||
Check JH. Luteal phase support in assisted reproductive technology treatment: focus on Endometrin(R) (progesterone) vaginal insert. Ther Clin Risk Manag. 2009;5(4):403–407. | ||
Rosenberg SM, Luciano AA, Riddick DH. The luteal phase defect: the relative frequency of, and encouraging response to, treatment with vaginal progesterone. Fertil Steril. 1980;34(1):17–20. | ||
Shah D, Nagarajan N. Luteal insufficiency in first trimester. Indian J Endocrinol Metab. 2013;17(1):44–49. | ||
National Collaborating Centre for Ws, Children’s H. National Institute for Health and Clinical Excellence: Guidance. Fertility: Assessment and Treatment for People with Fertility Problems. London, UK: RCOG Press National Collaborating Centre for Women’s and Children’s Health; 2004. | ||
van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2015;(7):Cd009154. | ||
Practice Committee of American Society for Reproductive Medicine in collaboration with Society for Reproductive E, Infertility. Progesterone supplementation during the luteal phase and in early pregnancy in the treatment of infertility: an educational bulletin. Fertil Steril. 2008;90(5 suppl):S150–S153. | ||
Fatemi HM, Popovic-Todorovic B, Papanikolaou E, Donoso P, Devroey P. An update of luteal phase support in stimulated IVF cycles. Hum Reprod Update. 2007;13(6):581–590. | ||
Vaisbuch E, Leong M, Shoham Z. Progesterone support in IVF: is evidence-based medicine translated to clinical practice? A worldwide web-based survey. Reprod Biomed Online. 2012;25(2):139–145. | ||
Pritts EA, Atwood AK. Luteal phase support in infertility treatment: a meta-analysis of the randomized trials. Hum Reprod. 2002;17(9):2287–2299. | ||
Tavaniotou A, Smitz J, Bourgain C, Devroey P. Comparison between different routes of progesterone administration as luteal phase support in infertility treatments. Hum Reprod Update. 2000;6(2):139–148. | ||
Paulson RJ, Collins MG, Yankov VI. Progesterone pharmacokinetics and pharmacodynamics with 3 dosages and 2 regimens of an effervescent micronized progesterone vaginal insert. J Clin Endocrinol Metab. 2014;99(11):4241–4249. | ||
Bulletti C, de Ziegler D, Flamigni C, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12(5):1073–1079. | ||
Cicinelli E, de Ziegler D, Bulletti C, Matteo MG, Schonauer LM, Galantino P. Direct transport of progesterone from vagina to uterus. Obstet Gynecol. 2000;95(3):403–406. | ||
Miles RA, Paulson RJ, Lobo RA, Press MF, Dahmoush L, Sauer MV. Pharmacokinetics and endometrial tissue levels of progesterone after administration by intramuscular and vaginal routes: a comparative study. Fertil Steril. 1994;62(3):485–490. | ||
Miller CE, Zbella E, Webster BW, Doody KJ, Bush MR, Collins MG. Clinical comparison of ovarian stimulation and luteal support agents in patients undergoing GnRH antagonist IVF cycles. J Reprod Med. 2013;58(3–4):153–160. | ||
Mitwally MF, Diamond MP, Abuzeid M. Vaginal micronized progesterone versus intramuscular progesterone for luteal support in women undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2010;93(2):554–569. | ||
Khan N, Richter KS, Newsome TL, Blake EJ, Yankov VI. Matched-samples comparison of intramuscular versus vaginal progesterone for luteal phase support after in vitro fertilization and embryo transfer. Fertil Steril. 2009;91(6):2445–2450. | ||
Zarutskie PW, Phillips JA. A meta-analysis of the route of administration of luteal phase support in assisted reproductive technology: vaginal versus intramuscular progesterone. Fertil Steril. 2009;92(1):163–169. | ||
Penzias AS, Alper MM. Luteal support with vaginal micronized progesterone gel in assisted reproduction. Reprod Biomed Online. 2003;6(3):287–295. | ||
Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein M. Crinone vaginal gel is equally effective and better tolerated than intramuscular progesterone for luteal phase support in in vitro fertilization-embryo transfer cycles: a prospective randomized study. Fertil Steril. 2010;94(7):2596–2599. | ||
Beltsos AN, Sanchez MD, Doody KJ, Bush MR, Domar AD, Collins MG. Patients’ administration preferences: progesterone vaginal insert (Endometrin(R)) compared to intramuscular progesterone for Luteal phase support. Reprod Health. 2014;11:78. | ||
Schoolcraft WB, Hesla JS, Gee MJ. Experience with progesterone gel for luteal support in a highly successful IVF programme. Hum Reprod. 2000;15(6):1284–1288. | ||
Germond M, Capelli P, Bruno G, et al. Comparison of the efficacy and safety of two formulations of micronized progesterone (Ellios and Utrogestan) used as luteal phase support after in vitro fertilization. Fertil Steril. 2002;77(2):313–317. | ||
Kleinstein J; Luteal Phase Study Group. Efficacy and tolerability of vaginal progesterone capsules (Utrogest 200) compared with progesterone gel (Crinone 8%) for luteal phase support during assisted reproduction. Fertil Steril. 2005;83(6):1641–1649. | ||
Ludwig M, Schwartz P, Babahan B, et al. Luteal phase support using either Crinone 8% or Utrogest: results of a prospective, randomized study. Eur J Obstet Gynecol Reprod Biol. 2002;103(1):48–52. | ||
Ng EH, Miao B, Cheung W, Ho PC. A randomised comparison of side effects and patient inconvenience of two vaginal progesterone formulations used for luteal support in in vitro fertilisation cycles. Eur J Obstet Gynecol Reprod Biol. 2003;111(1):50–54. | ||
Ng EH, Chan CC, Tang OS, Ho PC. A randomized comparison of side effects and patient convenience between Cyclogest suppositories and Endometrin tablets used for luteal phase support in IVF treatment. Eur J Obstet Gynecol Reprod Biol. 2007;131(2):182–188. | ||
Khrouf M, Slimani S, Khrouf MR, et al. Progesterone for luteal phase support in in vitro fertilization: comparison of vaginal and rectal pessaries to vaginal capsules: a randomized controlled study. Clin Med Insights Womens Health. 2016;9:43–47. | ||
Aali BS, Ebrahimipour S, Medhdizadeh S. The effectiveness of luteal phase support with cyclogest in ovarian stimulated intra uterine insemination cycles: a randomized controlled trial. Iran J Reprod Med. 2013;11(4):309–314. | ||
Aghsa MM, Rahmanpour H, Bagheri M, Davari-Tanha F, Nasr R. A randomized comparison of the efficacy, side effects and patient convenience between vaginal and rectal administration of Cyclogest((R)) when used for luteal phase support in ICSI treatment. Arch Gynecol Obstet. 2012;286(4):1049–1054. | ||
EMC [webpage on the Internet]. Cyclogest 200mg (SPC); 2016. Available from: https://www.medicines.org.uk/emc/medicine/15056. Accessed December 15, 2016. | ||
Levy T, Gurevitch S, Bar-Hava I, et al. Pharmacokinetics of natural progesterone administered in the form of a vaginal tablet. Hum Reprod. 1999;14(3):606–610. | ||
U.S. National Library of Medicine [webpage on the Internet]. Crinone® 4% and Crinone® 8% (Progesterone Gel), Physician Information; 2010. Available from: https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=42041. Accessed March 1, 2017. | ||
EMC [webpage on the Internet]. Utrogestan Vaginal 200mg Capsules (SPC); 2016. Available from: https://www.medicines.org.uk/emc/medicine/28065. Accessed December 15, 2016. | ||
EMC [webpage on the Internet]. Crinone 8% Progesterone Vaginal Gel (SPC); 2015. Available from: https://www.medicines.org.uk/emc/medicine/4135. Accessed December 15, 2016. | ||
EMC [webpage on the Internet]. Lutigest 100 mg Vaginal Tablets (SPC); 2015. Available from: https://www.medicines.org.uk/emc/medicine/29749#POSOLOGY. Accessed December 10, 2016. | ||
Blake EJ, Norris PM, Dorfman SF, Longstreth J, Yankov VI. Single and multidose pharmacokinetic study of a vaginal micronized progesterone insert (Endometrin) compared with vaginal gel in healthy reproductive-aged female subjects. Fertil Steril. 2010;94(4):1296–1301. | ||
Yovich JL, Conceicao JL, Stanger JD, Hinchliffe PM, Keane KN. Mid-luteal serum progesterone concentrations govern implantation rates for cryopreserved embryo transfers conducted under hormone replacement. Reprod Biomed Online. 2015;31(2):180–191. | ||
Doody KJ, Schnell VL, Foulk RA, et al. Endometrin for luteal phase support in a randomized, controlled, open-label, prospective in-vitro fertilization trial using a combination of Menopur and Bravelle for controlled ovarian hyperstimulation. Fertil Steril. 2009;91(4):1012–1017. | ||
Gonen LD. Satisfaction with in vitro fertilization treatment: patients’ experiences and professionals’ perceptions. Fertil Res Prac. 2016; 2(1):6. | ||
Gameiro S, Boivin J, Peronace L, Verhaak CM. Why do patients discontinue fertility treatment? A systematic review of reasons and predictors of discontinuation in fertility treatment. Hum Reprod Update. 2012;18(6):652–669. | ||
Dobaria N, Mashru R, Vadia N. Vaginal drug delivery systems: a review of current status. East Central Afr J Pharm Sci. 2007;10(1):3–13. | ||
Powell AM, Nyirjesy P. New perspectives on the normal vagina and noninfectious causes of discharge. Clin Obstet Gynecol. 2015;58(3):453–463. | ||
Alexander NJ, Baker E, Kaptein M, Karck U, Miller L, Zampaglione E. Why consider vaginal drug administration? Fertil Steril. 2004;82(1):1–12. | ||
Netdoctor [webpage on the Internet]. Cyclogest (Progesterone). Find Out How to Use These Pessaries and What Side Effects Are Possible; 2016. Available from: http://www.netdoctor.co.uk/medicines/a8718/cyclogest-progesterone/. Accessed April 02, 2017. | ||
Russell R, Kingsland C, Alfirevic Z, Gazvani R. Duration of luteal support after IVF is important, so why is there no consistency in practice? The results of a dynamic survey of practice in the United Kingdom. Hum Fertil. 2015;18(1):43–47. |
Supplementary material
This questionnaire is designed to find out about your treatment experience when you received progesterone during your treatment cycle for luteal phase support.
Please mark x in the box for your answer
Thank you for completing this questionnaire.
  | Figure S1 Questionnaire for women who have been prescribed progesterone for luteal support as part of fertility treatment. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.