Back to Journals » Journal of Pain Research » Volume 16
A Protocol for a Single-Centered, Pragmatic, Randomized, Controlled, Parallel Trial Comparing Comprehensive Nonsurgical Therapy Options for Individuals with Lumbar Spinal Stenosis
Authors Sun Y, An Y, Fan X, Liu C, Li D, Lei Y, Weng Z, Gong Y, Wang X, Yu C
Received 3 December 2022
Accepted for publication 20 February 2023
Published 9 March 2023 Volume 2023:16 Pages 773—784
DOI https://doi.org/10.2147/JPR.S398897
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Alaa Abd-Elsayed
Ya’nan Sun,1,* Yi An,2,* Xiran Fan,2 Changxin Liu,3 Duoduo Li,3 Yuan Lei,3 Zhiwen Weng,3 Yuanyuan Gong,3 Xiyou Wang,3 Changhe Yu3
1Traditional Chinese Medicine Department, Xuanwu Hospital Capital Medical University, Beijing, People’s Republic of China; 2First Clinical College, Beijing University of Chinese Medicine Affiliated Dongzhimen Hospital, Beijing, People’s Republic of China; 3Tuina and Pain Management Department, Beijing University of Chinese Medicine Affiliated Dongzhimen Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiyou Wang; Changhe Yu, Email [email protected]; [email protected]
Aim: Lumbar spinal stenosis (LSS) is a long-term degenerative disease. Considering the risks and advantages of the patient’s age range and the characteristics of the condition, non-surgical treatment is recommended. To determine the best first-line non-surgical therapy for LSS, few studies have examined different non-surgical therapies. Therefore, the main objective of this study is to determine whether the selection of comprehensive Chinese medicine (CM) treatment for LSS is more successful than non-surgical conservative treatment.
Patients and Methods: In this two-armed, parallel, single-centered, pragmatic randomized controlled study, 94 LSS participants will be randomized to receive 24 sessions of comprehensive CM therapy or conservative treatment for 3 months, with follow-up assessments at 6, 9, 12, and 15 months. The primary outcome will be based on the success rate of the Zurich Claudication Questionnaire (ZCQ) for the most clinical important difference (MCID) at 3 and 15 months. Secondary outcomes include Numerical Rating Scale (NRS) scores for back and leg pain, ZCQ scores, Oswestry Disability Index scores for lumbar dysfunction, and Short-Form 12 scores for health-related quality of life at 3, 6, 9, 12, and 15 months. Adverse events and incidences of surgery will be reported anytime during the trial and follow-up.
Conclusion: This protocol examines the comparative efficacy of comprehensive CM therapy compared with conventional care through a pragmatic randomized controlled trial to present data to facilitate clinical or policy decision-making. The outcomes will make it easier to decide which patient-centered treatments to prioritize for LSS.
Keywords: lumbar spinal stenosis, Chinese medicine, comprehensive treatment, conservative treatment, pragmatic randomized controlled trial, patient centered
Introduction
The condition, known as lumbar spinal stenosis (LSS), is primarily acquired degenerative stenosis, characterized by reduced space in the neural foramen or lumbar spinal canal, resulting in compression of the nerves or blood vessels. More than 100 million patients are affected.1,2 Neurogenic claudication (NC) and other neurological symptoms in the lower back and lower limbs, with or without pain, may be brought on by degenerative LSS. Walking makes LSS symptoms worse while lying down makes them go away. When treating LSS, non-surgical procedures are often advised as first-line therapy, with surgical procedures being reserved for individuals who do not improve with conservative care (CC).3
Improved pain management, increased walking distance, and relief of neurological symptoms are the three main treatment objectives of the non-surgical CC of LSS. Physiotherapy, including massage, acupuncture, thermotherapy, and kinesiotherapy, are presently regarded as the most effective therapies in rehabilitation.4 Other therapies, such as steroid epidural injection (ESI) and pharmaceutical treatments, often center on pharmacology.5,6 Non-surgical CC is advised for the clinical improvement of LSS, but no particular therapy or combined therapy has been established as a superior intervention.
Chinese medicine (CM) plays a vital role in treating LSS. Acupuncture and acupotomy can benefit patients with LSS regarding pain, symptoms, and functional outcomes up to 6 months after treatment.7,8 A network meta-analysis shows that Chinese Herbal Medication (CHM) alone or combined with other treatments might improve pain and functional outcomes better than other treatments.9 Compared to self-directed or group exercise, manual therapy combined with supervised activities increases short-term walking capacity and moderately reduces pain and symptom severity.10 For LSS patients with NC, multimodal or comprehensive treatments, such as education, lifestyle modifications, and behavioral change strategies, combined with home exercises, manual therapy, and rehabilitation, maybe first considered.6,11
As to comprehensive CM therapies, compared to conventional treatment, the integrative treatment of Tuina, acupuncture, with or without herbal medication, showed a substantial reduction in pain intensity and walking impairment at 3 and 6 months.12 Our systematic review (the article will be published separately) showed that a combination of 2 or 3 CM therapies (ie, cupping, acupuncture, Tuina, CHM, and bloodletting therapy) showed positive results on pain intensity and walking disability in short- or intermediate- term. CM therapies are safe interventions with few adverse effects. It is advised for reducing pain and fostering functional rehabilitation in individuals with degenerative LSS by the Clinical Practice Guidelines of CM.13 Therefore, this study will assess the effectiveness of comprehensive CM therapy versus first-line conservative therapy treatment for LSS.
Patients and Methods
Study Design and Ethics
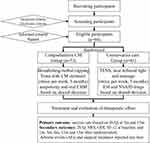
This investigation is a pragmatic, single-centered, randomized, controlled clinical trial. The comprehensive CM group (CMG) or the comprehensive CC group (CCG) will be randomly allocated to the ninety-four LSS patients who voluntarily agree to participate in a 4:3 ratio (Figure 1).
Before patient recruitment, the Institutional Review Committee of Dongzhimen Hospital, connected to the Beijing University of Chinese Medicine (2021DZMEC-123-02), authorized the study. The trial will be conducted in accordance with the Declaration of Helsinki. The research protocol (version 2, 2021.11) has been submitted to ClinicalTrials.gov (NCT05273346) and will be updated. The research protocol complies with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) standards and checklist. It also partly adheres to the CONSORT extension for pragmatic clinical trials (Supplementary Files). Although group allocation and patient information will remain a secret from researchers and participants throughout the trial, assessors and statisticians will be blinded.
Randomization and Allocation
The CMG or the CCG is randomly allocated to the eligible participants who complete the informed consent form in a ratio of 4:3. An impartial statistician created a random sequence before the initial enrolment. A complete randomization approach is used to create a “Proc plan” program, which used SAS 9.3 software to create random sequences. The randomly generated line is enclosed in a covert envelope. The whole randomization sequence is hidden from everyone but saved by the statistician. One research assistant opens a randomization envelope for each patient and assigns the patient to the proper group.
Eligibility and Setting
Inclusion criteria are included: 1) diagnostic of LSS (L1-S1),1,2 using of magnetic resonance imaging, 2) back or leg pain with neurogenic claudication lasting at least 3 months, 3) attempting conservative treatment (oral medications, physiotherapy, and epidural steroid injections) in recent 3 months, 4) indications for surgery2 but refuses surgery, and 4) age 40–85 years. The research protocol must be understood and approved by each participant. There will be written informed consent.
The following conditions or circumstances will excluded: vascular claudication; pathologies of non-spinal origin; spinal scoliosis > 15° or disc instability (rotation > 10° or translation > 4 mm); soft tissue pathology or other systemic illnesses that may result in radiating leg discomfort or lumbar back pain, such as a spinal tumor, rheumatism, or fractures; any other chronic condition that would impede receiving treatment or understanding the outcomes, such as anemia, control in diabetes, poor glycemic dementia, tumors or stroke; prescription medication that might inhibit the interpretation of the findings, such as immunosuppressants, corticosteroids, or psychotropic drugs; epidural steroid injections within 6 months and painkillers or physical therapy within 7 days; allergy history and constitution of CHM; subcutaneous lump, red skin or skin lesions in the treatment sites; pregnant, during the time of the research, you were or were intending to be nursing; spinal surgery; preparing to participate in other research studies while the study is ongoing; and is determined by the researchers to be unfit for participation, such as poor compliance; severe cauda equina syndrome.
Interventions
We systematically reviewed CM or CM-centered therapy trials versus western medical care to develop LSS CM clinical practice Guidelines (No. IPGRP-2022CN3332) for the registry (http://www.guidelines-registry.cn/guid/1710). We discussed the evidence-based CM interventions through Delphi surveys and consensus meetings with multi-stakeholders around China. We shared opinions about the LSS and preferred therapies with several LSS patients at an outpatient hospital. The comprehensive CM treatment has been optimized according to the Guidelines, experts’ experience, and patient preference (the findings will be published separately). In contrast, the comprehensive CC for LSS usually includes physiotherapy and analgesics, typically based on guidelines.11,14
Interventions in CMG
Patients with CMG will be treated with Chinese herbal cupping and bloodletting and Chinese herbal ointment massage for 30 to 40 min each time, twice a week, a total of 24 times for 3 months. The treatment course of autonomy and oral CHM will be determined by shared-decision making and administered by physicians.
In a thorough therapy, acupotomy is administered initially. At the 17 predetermined acupoints (Supplementary), acupotomy is done with a 0.50 × 50 mm sterile, disposable, stainless steel acupotomy with no needle retention. When an intrusive intervention is required, it is slowly placed while being watched to see whether the subjects experience discomfort along the neural path. The doctors chose the specifics of the depth, placement, and treatment strategy according to their medical judgments. The treatment will last about 5 min.
Cupping and bloodletting are a combination of cupping and traditional herbal medicine. The bamboo cups, thumb-like in diameter, are prepared and boiled in a stainless-steel pot with herbal decoction before treatment. After disinfecting the skin with 75% alcohol, the practitioner will puncture the skin with the injection needle vertically with the blood, then quickly pick up the cup, shack off the hot water on it, and then apply it to the bleeding spot. While the cup cools smoothly, it is sucked tightly onto the acupoints along the Du Jing, bladder Jing and Ashi points. The cup will drop or be taken away for nearly 20 min. The injection needles and bamboo cups will be immediately disposed of after treatment, and the herbal decoction will be used for half a day. The treatment will last about 25 min.
Tuina aims to release soft tissues around lesions where cups suction and to increase the lumbar spine’s flexibility, with CHM ointment reducing pain. The rubbing, pushing, and kneading methods combined with ointment are frequently applied on the acupuncture points or along the meridians (Supplementary). The contents of the ointment are the same as an herbal decoction for boiled cups. The treatment will last 7–10 min. CHM for herbal cupping and ointment is an herbal concoction consisting of 16 kinds of CHM in equal portions (Supplementary Files).
Oral Chinese herbal medicine (CHM) granules to individuals will be administered based on the CM pattern differentiation. Whether a patient should take oral CHM depends on the sharing decision. If this is the case, the patient must visit the doctor once a week to get a prescription for CHM or stop taking it at any time at the discretion of the patient and the doctor.
Interventions in CCG
CCG treatment is an ESI-centered therapy, meaning at least one injunction treatment. Furthermore, physical therapies, including near-infrared light therapy (NILT), Transcutaneous Electrical Nerve Stimulation (TENS), and soft tissue massage (STM) for 30–40 min each time, twice a week, for 24 sessions over a period of 3 months. If necessary, multi-ESIs and oral non-steroidal anti-inflammatory drugs (NSAID) will be administered after the first injection based on a mutual decision.
ESI is the primary therapy for participants in CCG before other treatments. The doctor decided upon transforaminal or interlaminar injection techniques, which stayed the same for each patient’s future injections. The injectable glucocorticoid solution included chemical betamethasone in 1 to 3 mL of 0.25% to 1% lidocaine (0.5 to 1 mL).15 Patients whose pain improvement is less than 50%, lasting less than a month after the first ESI, can undergo ESI up to three times within three months at the doctor’s discretion.
During the physical treatment, the TENS will be administered first. Practitioners get electrical pads that are self-adhesive and disposable placed over the patients’ para-spinal muscles from L3 to S1. The electrodes are linked to a participant-worn TENS device tucked away in a waist bag. The TENS machine was configured to deliver pulses with a width of 100–200 used at a frequency of 70–100 Hz modulated over 3 s intervals. According to preliminary tests, the current intensity was set at 3 mA, below the threshold that causes muscles to twitch.16 The treatment will last about 10 min.
After TENS, the patient will get NILT, which employs the bio-stimulating principles, at a dosage of 120 J/cm2 equal to 3000 J for the 25 cm2 treated region. NILT is constantly administered for 20 min across the para-spinal musculature from L3 to S1, using a remote application followed by STM to release soft issues on the same areas of muscles for 7–10 min. Based on medical judgments made by the doctors, patients will be permitted to obtain analgesics as a rescue medication for appropriate situations.
Concomitant Treatment and Patient Drop-Out
Participants received an instruction pamphlet on self-management strategies17 at the first visit after randomization, including the precautions about everyday activities throughout three months of treatment, problem-solving techniques, pacing, and relaxation. Participants were instructed to adjust their bodies while standing and walking to minimize lumbar lordosis and increase spinal canal diameter.18 Instructions on the component of an organized home exercise regimen (Supplementary Files) were given to the participants,19 and they will practice on their own. Lumbar flexions are made possible through various exercises, such as muscle stretching and strengthening, to enhance general back and lower extremity fitness. The back belt is only a rescue method to relieve acute pain.
Adherence rates will be determined after withdrawal requirements are met. To promote participation, we set a baseline rate of 67% (at least two months with 16 treatments). Moreover, patients who fulfill this benchmark rate will be disqualified from the research. The case report form (CRF) will include specific information on the usage of rescue medication and the level of adherence for each patient.
Patients will be withdrawn from the study if any of the following occurs: (1) the onset of a severe illness; (2) the patient’s request to withdraw consent; (3) the occurrence of pregnancy during the study; (4) the patient’s performance status is too low to warrant the administration of an intervention, and (5) any other circumstance in which dropping out would be in the best interest of the patient.
Assessment and Safety
The primary outcome is the success rate measured by the Zurich Claudication Questionnaire (ZCQ) at 3 months (short-term) and 15 months (long-term) after randomization. The ZCQ, a validated 15-item LSS-specific patient-reported outcome questionnaire, quantifies symptom severity (items 1–7, scoring range 1–5), physical function (items 8–12, scoring range 1–5), and patient’s satisfaction after treatment (items 13–15, scoring range 1–5); higher score indicates worse symptoms, lower physical function, or lower satisfaction. The ZCQ was translated into Chinese and validated in 2014.20 Treatment is considered successful if at least 2.5 points reduce the satisfaction subscale score and at least 0.5 points reduce both the symptom severity and physical function score; the values are considered the most clinically important difference (MCID).21,22
The secondary outcome is the patient’s perception of the intensity of their lower back pain and any referred pain in the lower limbs, as evaluated by a numeric rating scale (NRS) with a range from 0 (no pain or discomfort) to 10 (the worst pain and discomfort conceivable). The Oswestry disability index (ODI), a validated functional disability questionnaire for evaluating low back pain, is also used to assess lumbar function.23,24 Scores may be between 0 and 5 on each item. The possible outcomes range from 0 (the best possible result) to 100 (a worse outcome). On the Health-related Quality of Life short-form 12 (SF-12), a higher score indicates a higher quality of life, which consists of 12 items across 8 areas.25 The adverse events and surgical incidence are recorded at any time during the trial and follow-up. Patients’ outcomes will be assessed by outcome assessors who are blind to the patients’ allocation using questionnaires patients complete on-site or over the phone during follow-up. Timeframe for outcome assessment is listed in Figure 2.
Recruitment
Social media and leaflet distribution at outpatient facilities are used to recruit participants. In addition, announcements are displayed on hospital bulletin boards. The recruitment search started on September 15, 2022.
Data Management and Monitoring
There is a clear distinction between a doctor’s duties and those of a researcher. The physician will evaluate each intervention group’s medical plan and then determine and implement the treatment plan. Immediately after each treatment, the researchers will complete the CRF with the relevant information. Involvement in the treatment process does not imply that the researcher is performing medical duties. Any medical or physiotherapeutic treatment that helps with LSS (such as pain in the lower back or legs) shall be quickly noted in the CRF.
We shall keep all CRFs in a secure filing cabinet to avoid information loss. We will assign each piece of information a unique number to protect the subjects’ privacy. Data monitoring is the responsibility of the clinical research data management system. Only the principal investigator has direct access to the dataset, and the research team members need to know who the participants are based on the data provided.
Statistical Considerations
A total of 94 patients are expected to be included in the sample. Assuming a significant rate of 0.05, this sample size was calculated to offer 80% power. Our prospective case series study of 3-month blooding cupping therapy on LSS showed the success rate was 50% with ZCQ criteria at 3 months and 83.3% at 4 months,26 and the systematic review showed ESI alone, or ESI-centered therapy with a success rate of 40–65% from 3 to 6 months.27 No data exists at 15 months for both groups. In our study, the effect of acupotomy, Tuina combined with blooding cupping therapy is estimated with more improvement than any of them. Therefore, we predict a 30% gap between CMG and CCG after 3 months, which will remain for 15 months. According to this formula, there were a total of 44 people involved in the CMG and a total of 33 people involved in the CCG. Our sample size is 94, considering the 20% dropout rate. Power analysis showed that this sample size was sufficient to detect a difference in success rates of at least 30% between the two groups.
Intention-to-treat analysis is performed primarily. Patients who adhere to the treatment plan 67% of the time (16 sessions) also receives per-protocol comments. A mixed model for repeated measures will be used to examine the data for missing values. Multiple imputations and the last-observation-carried-forward technique will form the foundation of the sensitivity analysis. Descriptive statistics will be used to characterize each group’s demographic and social features. Data for each continuous variable will be given as mean ± standard deviations or median ± quartile ranges. Student’s t-test or Wilcoxon’s rank-sum test will be used to analyze the differences between the two groups. The chi-square or Fisher’s exact test will be used to analyze categorical variables, which will be shown as frequency and percentile (%). Each time point’s group effects were given as mean differences and 95% CIs. SPSS 20.0 will be used for all analyses, and a two-tailed, 5% significance level will be used for all tests.
If we cannot find a significant statistical advantage in testing the intervention hypothesis, we will switch to testing for non-inferiority. This difference in success rates between the two groups is 30%. Hence a non-inferiority margin of 0.1 has been predetermined. Comprehensive CM will be declared non-inferior to conventional care treatment if the difference in the success rate change between the two groups does not surpass the lower limit of the 95% confidence interval.
Patient and Public Involvement
The importance of patient and public engagement (PPI) cannot be overstated. The authors interviewed LSS patients to gather information before developing the therapy procedure. To consider patient preferences and requirements, the writers solicited their input and asked them to engage in the conversation. Further, LSS patients are encouraged to provide practical suggestions for gauging the intervention’s efficacy in terms of desired outcomes. The findings will be shared with interested PPI representatives and participants.
Discussion
There are surgical and non-surgical treatments for LSS. While the number of operations performed in this population is rising, up to 35% of patients may be dissatisfied with the results at the one-year mark.28 As an added downside, the advantages of a surgery over non-surgical therapy may not last for more than two to four years,29,30 and surgery has a risk of complications of 10–24%.31 Surgery may be necessary if non-operative treatment fails or if the patient’s condition worsens.
Function and mobility may be enhanced with several non-surgical treatments, and LSS symptoms may be alleviated,32 However, there is less evidence from scientific studies to back up their deployment.5 The most up-to-date recommendations for non-surgical therapy throughout the LSS illness range provide only vague direction due to a lack of supporting research data.11,33 However, these recommendations emphasized the need to take advice/education and exercises accessible to persons with LSS presenting with any LSS and any degree of symptom intensity. Therefore, our study considers advice/education and exercise therapy as the foundation of multimodal treatments.
A comprehensive CC of LSS usually includes analgesia and physical therapy.11,31 A variety of oral and injectable analgesics have been proposed. Substantial evidence is acquired from many relevant, high-quality RCTs for lumbar epidural injections, and the new guideline strongly recommends their use for managing persistent spinal pain.14 Pharmacotherapy is indicated to a limited extent that acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) are helpful. However, none is noticeably better than the other.34,35 NSAIDs will be used as a last resort in our trial since no standard therapy is presently prescribed for this condition.
Furthermore, physiotherapy reduces pain and encourages natural healing by increasing energy. However, the therapeutic effects of these modalities are short-term and are used in combination with other modalities for LSS. TENS can help LSS patients with at least a 30% improvement in their walking ability.16 Animal studies have shown that paraspinal areas benefit from increased blood flow when electrically stimulated.36 Pain caused by musculoskeletal problems can be relieved by NILT.37 Guidelines for lower back pain recommend TENS and NILT as part of multidisciplinary rehabilitation.38 Soft tissue technique is a kind of massage treatment for reducing muscular tension and discomfort by working on the more profound tissue components of the muscle and fascia.39,40 It is an optional aspect of multimodal care, as guidelines advise.11 Physicians should make individualized judgments on the best action, including a combination of conservative treatments.
Most European and North American guidelines exclude complementary medicine (ie, CM) and explain that there is little evidence since CM trials are always reported in Chinese. We reviewed trials comparing the treatment with CM alone or treatment including CM versus the control interventions involved in routine treatment (eg, injection therapy and physiotherapy) or a combination of routine treatment. There is insufficient evidence from the GRADE system that combined CM therapy leads to significant improvements in pain, physical function, and walking impairments over CM alone, conventional therapy alone, or combination routine therapy (articles will be published separately).
We chose the interventions based on literature evidence and patient and healthcare professionals’ feedback. Because of this, we have identified the two types of interventions we described earlier. All treatment modalities will follow patient-centered, evidence-based procedures. Based on interviews with primary care physicians, acupuncturists, chiropractors, orthopedic surgeons, rehabilitation physicians, nurses, and older adults with LSS, the “comprehensive CM treatment” approach to administering LSS combines acupuncture, bloodletting, potable herbal cupping, Tuina, and oral CHM. Patients with LSS, in particular, seemed to favor, believe in, and follow through with the CM therapies because they thought they would help them maintain their fitness and activity levels.
Most CM interventions are generally regarded as relieving mild to moderate symptoms. Still, invasive methods, like acupotomy and bloodletting cupping, significantly affect severe symptom relief. Our study enrolls patients with mild to severe LSS symptoms, failure of previous conservative treatment, or surgical rejection. Thus, a combination of acupotomy and bloodletting cupping, accompanied by Tuina and CHM, constitute the CM group.
A meta-analysis confirmed our hypothesis that combined massage therapy benefits patients with LBP.11 A pilot trial have reported improvements in pain and function at 3 and 6 months of 4-week multimodal combination therapy (acupuncture, Tuina, and physician consultation, with or without herbal modulation) compared to CC therapy (including prescription medications, ESI, and physical therapy).12 The multimodal integrative treatments without herbal concoctions versus conventional nonsurgical therapies have been protocolized for symptomatic lumbar spinal spondylolisthesis.41
Lumbar Acupotomy is helpful for soft tissue pathologies, including weakened muscles, ligaments, and articular capsules.42 Recent studies suggest that acupotomy may be an effective non-invasive treatment for LSS in a meta-analysis of pooled estimates.7 To alleviate pain and remove illness, acupotomy on points and collaterals, it is necessary to release tension in the soft tissues surrounding the spinal canal and slow the progression of dynamic and static spinal canal stenosis.
Chinese manual therapy, known as Tuina, emphasizes anatomy and physiology when treating neuromusculoskeletal problems such as lower back pain. Tuina practitioners mix soft-tissue manipulation with spinal manipulation to treat low back pain.43 Based on the findings of comprehensive evaluations suggesting that manual treatments may be helpful for LBP,44 the guidelines have developed modest recommendations of massage, mobilization, and manipulation for LBP.39
Cupping therapy has positive effects on the pain and physical parameters of LBP.45 Cupping may be done either dry or wet. There is no blood loss during dry cupping. The process of wet cupping is intrusive and often involves bloodletting.46 Due to wet cupping, blood circulation is enhanced, waste is eliminated, neuroendocrine harmony is restored, oxygen delivery to tissues is improved, and tissue perfusion is enhanced. Patients with fibromyalgia in a case series47 and with post-herpetic neuralgia in an RCT48 experience significant pain relief after undergoing cupping therapy, which we define as a bamboo cup heated for 5 minutes using a herbal decoction. After receiving herbal cupping, the pain is significantly relieved, which we describe as a bamboo cup heated for 5 min using traditional CM decoction.47
CM believes blockages in Qi, blood, bodily fluids or the meridians are the source of pain, rather than Western medicine focusing on pain receptors and pathways. Pain may be triggered and exacerbated by cold while being relieved by heat.49 For LSS, CM identifies three main treatment principles for older patients: warming channels and dispelling wind-cold-dampness. Promoting qi, activating blood, and removing blood stasis; invigorating qi, nourishing liver, and kidney.50 In addition, clinical evidence from human trials has shown that there are few adverse reactions to the efficacy of herbal remedies for musculoskeletal pain, both topical and oral51,52 For the elderly, always taking lots of medicines for internal diseases, CHM external therapy placed on the back for pain relief is recommended for safety.
Moreover, traditional CM, directly on the affected area of massage or cooked water cup, can play a thermal role, make local tissue temperature, dilate capillaries, accelerate blood circulation, and enhance local metabolism, to achieve anti-inflammatory, swelling, and reflex muscle spasm effects. In addition, warming the meridian, increasing blood flow, and decreasing pain through external therapy is performed by transporting the active ingredients of traditional CM through the meridians and collaterals to the site of the disease and forming a higher concentration in the back area.52 The CHM used in our study is established by experts’ consensus based on the herbals extracted from reviews, according to the principle above (Supplementary).
Therefore, this study adopts a practical effectiveness design, using protocols that allow for customized variations, rather than the more rigorous monotherapy approach in effectiveness design, to collect real-world data to study the narrow optimal non-surgical treatment approach. Our strategy mimics the delivery of various treatment approaches to get more generalizable findings. All of the treatments are administered in outpatient clinics. Both groups will get the same amount of therapy over the same amount of time. Patients and doctors were interviewed to help shape the protocols and procedures used by each therapy group.
The present research is limited by its single-center design and tiny sample size. Future analysis of this kind may need to be larger and multi-center. The Comprehensive CM therapy for DLSS is a new treatment in clinics, and this study primarily focuses on its safety and effectiveness. Future examination of resource use in the healthcare system may also be taken into consideration. Our study also had the drawback that neither the doctor nor the patient could remain blinded throughout the process. However, we use a blinded assessor and a statistician to correct these drawbacks when analyzing the final result. Due to the pragmatic nature of the study design, it may not be possible to provide sufficient evidence for the experimental validity of each component of the combination therapy. However, the efficacy shown in a randomized, controlled trial does not necessarily manifest in a natural clinical environment. From a pragmatic vantage point, we will examine the treatment methods and tactics currently employed for stenosis in the actual clinical context, which may include a combination of therapies.
Conclusion
Comprehensive CM treatment will be compared to conservative care (physiotherapy, pharmacotherapy) in this pragmatic, randomized, controlled, parallel-group clinical trial. The findings of this study will guide the selection of the most effective non-surgical treatment options for patients with LSS and facilitate clinical or policy decisions.
Abbreviations
LSS, Lumbar spinal stenosis; NC, Neurogenic claudication; CC, conservative care; CM, Chinese medicine; CMG, CM group; CCG, CC group; CHM, Chinese herbal medicine; PPI, patient and public engagement; TENS, Transcutaneous Electrical Nerve Stimulation; ESI, epidural steroid injection; NSAID, Nonsteroidal anti-inflammatory drug; ZCQ, Zurich Claudication Questionnaire; NRS, Numeric Rating Scale; ODI, Oswestry Disability Index; SF-12, Short-Form 12 for Health-Related Quality of Life.
Ethical Approval
The study has been approved by the Ethics Committee the Dongzhimen hospital of Beijing University of Chinese Medicine (2021DZMEC-123-02).
Consent
Written informed consent will be obtained from all participants. All personal information about potential and enrolled participants will be confidential.
Acknowledgments
We acknowledge all the colleagues and staff at Tuina and pain management department of Dongzhimen Hospital. This study was funded by the National Natural Science Foundation of China (Grant No. 81803956); Capital Health Development Research Project (No. 2020-4-4195); Seed Funding of Golden Bridge Project of Beijing Municipal Science and Technology Commission (No. ZZ21053).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. Changhe Yu is funded for this work by a the National Natural Science Foundation of China (Grant No. 81803956); Capital Health Development Research Project (No. 2020-4-4195); Seed Funding of Golden Bridge Project of Beijing Municipal Science and Technology Commission (No. ZZ21053). The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the article; or in the decision to publish the results.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Jensen RK, Lauridsen HH, Andresen A, Mieritz RM, Schiottz-Christensen B, Vach W. Diagnostic screening for lumbar spinal stenosis. Clin Epidemiol. 2020;12:891–905. doi:10.2147/CLEP.S263646
2. Katz JN, Zimmerman ZE, Mass H, Makhni MC. Diagnosis and management of lumbar spinal stenosis: a review. JAMA. 2022;327:1688–1699. doi:10.1001/jama.2022.5921
3. Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ. 2016;352:h6234.
4. Kirker K, Masaracchio MF, Loghmani P, Torres-Panchame RE, Mattia M, States R. Management of lumbar spinal stenosis: a systematic review and meta-analysis of rehabilitation, surgical, injection, and medication interventions. Physiother Theory Pract. 2022;20:1–46.
5. Ammendolia C, Stuber KJ, Rok E, et al. Nonoperative treatment for lumbar spinal stenosis with neurogenic claudication. Cochrane Database Syst Rev. 2013;2013:D10712.
6. Ammendolia C, Hofkirchner C, Plener J, et al. Non-operative treatment for lumbar spinal stenosis with neurogenic claudication: an updated systematic review. BMJ Open. 2022;12:e57724.
7. Kwon CY, Yoon SH, Lee B, Leem J. Acupotomy for the treatment of lumbar spinal stenosis: a systematic review and meta-analysis. Medicine. 2019;98:e16662. doi:10.1097/MD.0000000000016662
8. Kim KH, Kim TH, Lee BR, et al. Acupuncture for lumbar spinal stenosis: a systematic review and meta-analysis. Complement Ther Med. 2013;21:535–556.
9. Chen X, Zheng Z, Lin J. Clinical effectiveness of conservative treatments on lumbar spinal stenosis: a network meta-analysis. Front Pharmacol. 2022;13:859296. doi:10.3389/fphar.2022.859296
10. Jacobi S, Beynon A, Dombrowski SU, Wedderkopp N, Witherspoon R, Hebert JJ. Effectiveness of conservative nonpharmacologic therapies for pain, disability, physical capacity, and physical activity behavior in patients with degenerative lumbar spinal stenosis: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2021;102:2247–2260. doi:10.1016/j.apmr.2021.03.033
11. Bussieres A, Cancelliere C, Ammendolia C, et al. Non-surgical interventions for lumbar spinal stenosis leading to neurogenic claudication: a clinical practice guideline. J Pain. 2021;22:1015–1039. doi:10.1016/j.jpain.2021.03.147
12. Kim K, Shin KM, Hunt CL, et al. Nonsurgical integrative inpatient treatments for symptomatic lumbar spinal stenosis: a multi-arm randomized controlled pilot trial. J Pain Res. 2019;12:1103–1113. doi:10.2147/JPR.S173178
13. World Federation of Chinese Medicine Associations, Chinese Society of Traditional Chinese Medicine. International clinical practice guidelines for traditional Chinese medicine for degenerative lumbar spinal stenosis. PLoS One. 2021;16:2371–2374.
14. Manchikanti L, Knezevic NN, Navani A, et al. Epidural interventions in the management of chronic spinal pain: American Society of Interventional Pain Physicians (ASIPP) comprehensive evidence-based guidelines. Pain Physician. 2021;24:S27–208.
15. Friedly JL, Comstock BA, Turner JA, et al. A randomized trial of epidural glucocorticoid injections for spinal stenosis. N Engl J Med. 2014;371:11–21.
16. Ammendolia C, Cote P, Rampersaud YR, et al. Effect of active TENS versus de-tuned TENS on walking capacity in patients with lumbar spinal stenosis: a randomized controlled trial. Chiropr Man Therap. 2019;27:24. doi:10.1186/s12998-019-0245-z
17. Linton SJ, Andersson T. Can chronic disability be prevented? A randomized trial of a cognitive-behavior intervention and two forms of information for patients with spinal pain. Spine. 2000;2825:2824.
18. Chung SS, Lee CS, Kim SH, Chung MW, Ahn JM. Effect of low back posture on the morphology of the spinal canal. Skeletal Radiol. 2000;29:217–223. doi:10.1007/s002560050596
19. Ammendolia C, Cote P, Southerst D, et al. Comprehensive nonsurgical treatment versus self-directed care to improve walking ability in lumbar spinal stenosis: a randomized trial. Arch Phys Med Rehabil. 2018;99:2408–2419. doi:10.1016/j.apmr.2018.05.014
20. Yi H, Wei X, Zhang W, et al. Reliability and validity of simplified Chinese version of Swiss Spinal Stenosis Questionnaire for patients with degenerative lumbar spinal stenosis. Spine. 2014;39:820–825. doi:10.1097/BRS.0000000000000273
21. Stucki G, Liang MH, Fossel AH, Katz JN. Relative responsiveness of condition-specific and generic health status measures in degenerative lumbar spinal stenosis. J Clin Epidemiol. 1995;48:1369–1378. doi:10.1016/0895-4356(95)00054-2
22. Zucherman JF, Hsu KY, Hartjen CA, et al. A prospective randomized multi-center study for the treatment of lumbar spinal stenosis with the X STOP interspinous implant: 1-year results. Eur Spine J. 2004;13:22–31. doi:10.1007/s00586-003-0581-4
23. Liu HTao H, Luo Z. Validation of the simplified Chinese version of the Oswestry Disability Index. Spine. 2009;1211:1217.
24. Lue YJ, Hsieh CL, Huang MH, Lin GT, Lu YM. Development of a Chinese version of the Oswestry Disability Index version 2.1. Spine. 2008;33:2354–2360. doi:10.1097/BRS.0b013e31818018d8
25. Su SW, Wang D. The reliability and validity of short form-12 health survey version 2 for Chinese older adults. Iran J Public Health. 2019;48:1014–1024.
26. Liu C, Wang X, Wang X, et al. Blood-letting and herbal-cupping therapy for lumbar spinal stenosis: prospective case series study. Int J Trad Chin Med. 2018;40:799–804.
27. Kreiner DS, Shaffer WO, Baisden JL, et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update). Spine J. 2013;13:734–743.
28. Deyo RA. Treatment of lumbar spinal stenosis: a balancing act. Spine J. 2010;10:625–627. doi:10.1016/j.spinee.2010.05.006
29. Lurie JD, Tosteson TD, Tosteson A, et al. Long-term outcomes of lumbar spinal stenosis: eight-year results of the Spine Patient Outcomes Research Trial (SPORT). Spine. 2015;40:63–76. doi:10.1097/BRS.0000000000000731
30. Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT). Spine. 2008;33:2789–2800. doi:10.1097/BRS.0b013e31818ed8f4
31. Zaina F, Tomkins-Lane C, Carragee E, Negrini S. Surgical versus nonsurgical treatment for lumbar spinal stenosis. Spine. 2016;41:E857–68. doi:10.1097/BRS.0000000000001635
32. Comer CM, Redmond AC, Bird HA, Conaghan PG. Assessment and management of neurogenic claudication associated with lumbar spinal stenosis in a UK primary care musculoskeletal service: a survey of current practice among physiotherapists. BMC Musculoskelet Disord. 2009;10:121.
33. Rousing R, Jensen RK, Fruensgaard S, et al. Danish national clinical guidelines for surgical and nonsurgical treatment of patients with lumbar spinal stenosis. Eur Spine J. 2019;28:1386–1396. doi:10.1007/s00586-019-05987-2
34. Haig AJ, Tong HC, Yamakawa KS, et al. Predictors of pain and function in persons with spinal stenosis, low back pain, and no back pain. Spine. 2006;31:2950–2957.
35. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA. 2018;319:872–882. doi:10.1001/jama.2018.0899
36. Budgell BS, Sovak G, Soave D. TENS augments blood flow in somatotopically linked spinal cord segments and mitigates compressive ischemia. Spinal Cord. 2014;52:744–748. doi:10.1038/sc.2014.120
37. Clijsen R, Brunner A, Barbero M, Clarys P, Taeymans J. Effects of low-level laser therapy on pain in patients with musculoskeletal disorders: a systematic review and meta-analysis. Eur J Phys Rehabil Med. 2017;53:603–610. doi:10.23736/S1973-9087.17.04432-X
38. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514–530. doi:10.7326/M16-2367
39. Furlan AD, Giraldo M, Baskwill A, Irvin E, Imamura M. Massage for low-back pain. Cochrane Database Syst Rev. 2015;2015:D1929.
40. Kong LJ, Fang M, Zhan HS, et al. Tuina-focused integrative Chinese medical therapies for inpatients with low back pain: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2012;2012:578305. doi:10.1155/2012/578305
41. Kim K, Youn Y, Lee SH, et al. The effectiveness and safety of nonsurgical integrative interventions for symptomatic lumbar spinal spondylolisthesis: a randomized controlled multinational, multicenter trial protocol. Medicine. 2018;97:e667.
42. Zhao H, Liu B, Liu Z, et al. Clinical practice guidelines of using acupuncture for low back pain. World J Acupunct Moxibust. 2016;26:1–13. doi:10.1016/S1003-5257(17)30016-8
43. Pei XZhan H, Li Y. Classification of Chinese spinal Tuina. In: Li Y, Ye G, editors. Chinese Spinal Tuina Encyclopedia. Beijing, China: Military Medical Science Press; 2005:12–16.
44. Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478–491. doi:10.7326/0003-4819-147-7-200710020-00006
45. Moura CC, Chaves E, Cardoso A, Nogueira DA, Correa HP, Chianca T. Cupping therapy and chronic back pain: systematic review and meta-analysis. Rev Lat Am Enfermagem. 2018;26:e3094.
46. Furhad S, Bokhari AA. Cupping Therapy. StatPearls Publishing; 2022.
47. Cao H, Hu H, Colagiuri B, Liu J. Medicinal cupping therapy in 30 patients with fibromyalgia: a case series observation. Forsch Komplementmed. 2011;18:122–126. doi:10.1159/000329329
48. Wu X, Hu H, Guo L, Wang H. 药罐治疗带状疱疹后遗神经痛临床观察 [Clinical observation of post-herpetic neuralgia treated with TCM herbal cupping therapy]. Zhongguo Zhen Jiu. 2013;33:141–144. Chinese.
49. Luo Y, Wang CZ, Sawadogo R, Tan T, Yuan CS. Effects of herbal medicines on pain management. Am J Chin Med. 2020;48:1–16. doi:10.1142/S0192415X20500019
50. Wu J, Su S. Analysis of medication rule in treating lumbar spinal stenosis with traditional Chinese medicine based on data mining. Asia Pacific Trad Med. 2021;17:161–165.
51. Jahromi B, Pirvulescu I, Candido KD, Knezevic NN. Herbal medicine for pain management: efficacy and drug interactions. Pharmaceutics. 2021;13:251. doi:10.3390/pharmaceutics13020251
52. Ding X, Wu J, Shen Q, Xu J, Mo W. Clinical control study of traditional Chinese medicine hot compress combined with traction in the treatment of cervical spondylotic radiculopathy: study protocol. Medicine. 2021;100:e23880. doi:10.1097/MD.0000000000023880
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.