Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
A Prospective Study of Dermoscopic and Ultrastructural Features of Vitiligo-Associated Leukotrichia
Authors Li M , Wang F , Li X , Ding X, Du J
Received 20 August 2023
Accepted for publication 9 December 2023
Published 21 December 2023 Volume 2023:16 Pages 3673—3680
DOI https://doi.org/10.2147/CCID.S435900
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Man Li,* Fang Wang,* Xinxin Li, Xiaolan Ding, Juan Du
Department of Dermatology, Peking University People’s Hospital, Beijing, 100044, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fang Wang; Juan Du, Department of Dermatology, Peking University People’s Hospital, No. 11 Xizhimen South Street, Xicheng District, Beijing, 100044, People’s Republic of China, Fax +861088325474, Email [email protected]; [email protected]
Background: Dermoscopic and ultrastructural features of vitiligo-associated leukotrichia (VAL) have not been well studied. This study is aimed at the dermoscopic and ultrastructural features of VAL.
Methods: We present a cross-sectional study of VAL-related dermoscopic signs and their relationship with disease stages and duration of leukotrichia. Characteristics of hair surface and finer details including melanosomes, macrofibrils, and remnant nucleus were observed under Electron Microscopy (EM).
Results: One hundred and forty samples on distinct sites from 100 patients were collected. Among 75 VAL from the scalp, the prevalence of diameter diversity of leukotrichia (52.6% vs 8.1%), Pohl-Pinkus constrictions (34.2% vs 0), and depigmented hair roots signs (34.2% vs 8.1%) in patients with progressive vitiligo was much higher than that in patients with stable vitiligo (all P< 0.05). The EM result of VAL showed that melanosomes were smaller with vesicles formation, reduced counts, and incomplete shape, and macrofibrils were irregularly arranged with widened spaces and vesicles formation.
Limitations: No conclusions about the histopathologic characteristics or dermoscopic-histopathologic correlation of the VAL.
Conclusion: The dermoscopic signs of diameter diversity of leukotrichia, Pohl-Pinkus constrictions, and depigmented hair roots are related to progressive vitiligo. The process of melanin synthesis and formation of VAL are impaired at the early stage of VAL under Electron microscopy.
Keywords: vitiligo, leukotrichia, dermoscopy, electron microscopy, diameter diversity, Pohl-Pinkus constrictions, melanosomes
Introduction
Vitiligo is an acquired skin disease characterized by depigmentation of the skin, mucosae, and hairs. Vitiligo-associated leukotrichia (VAL) is present in approximately 9.2–46.5% of vitiligo patients,1,2 reflecting the insufficient melanocyte reservoir of hair follicles.3–5 Leukotrichia is also a predictor of poor response to conventional treatments for vitiligo.6–8 Therefore, early detection of VAL is essential for the diagnosis and treatment decision-making of vitiligo. Dermoscopy has been used in the treatment of vitiligo in addition to Wood’s lamp and skin computed tomography (CT), and previous studies have reported the potential use of dermoscopy for the evaluation of vitiligo activity.9–11 In clinics, we observed that some VALs were shorter, thinner, and shedding earlier than the black hairs in unaffected areas. However, literature on the dermoscopic and ultrastructural features of VAL is scarce. In this study, we described the dermoscopic and ultrastructural features of VAL and analyzed the relationship between dermoscopic observations and disease stage, with the aim to investigate the potential pathological mechanism of VAL.
Methods
Patients and Ethical Approval
This cross-sectional study was conducted in the outpatient clinic of the Department of Dermatology at Peking University People’s Hospital between December 2020 and March 2022. The study protocol was approved by Ethics Committee of Peking University People’s Hospital (No. 2021PHB022-001) and all participants signed consent forms. Patients with confirmed diagnosis of vitiligo, who presented with leukotrichia in vitiliginous patches were enrolled. Patients who had a history of hair colouring within 90 days or white hair due to other causes (eg, premature hair graying, piebaldism, alopecia areata (AA), chemotherapy, or radiotherapy) were excluded. Questionnaires were completed for each patient after a complete examination of leukoderma and leukotrichia under natural light, Wood’s lamp (Sigma SW-10) and dermoscopy (FotoFinder Systems GmbH, GE). Demographic and clinical characteristics were collected using questionnaires (Figure S1), including age, sex, age at vitiligo disease onset, duration of leukotrichia, disease stage by VIDA (Vitiligo Disease Activity) score and sites of leukotrichia involvement.
Dermoscopic Examination
Dermoscopic images were captured and recorded in polarized light and non-infiltration solution mode at 20 and 50 magnifications. Determination and interpretation of dermoscopic signs of hair shaft and perifollicular changes were based on literature and dermatologists’ expertise. Dermoscopic signs of hair shaft included diameter diversity of leukotrichia (defined as >20% of hair with a diameter <30μm), Pohl-Pinkus constrictions, depigmented hair roots, repigmented leukotrichia, and clustered leukotrichia. Other features included perifollicular depigmentation, residual perifollicular pigmentation, telangiectasia, red area, white area, scales, yellow dots, and other unknown features.
Electron Microscopy (EM) Examination
VALs on the scalp were collected from three patients with progressive nonsegmental vitiligo who had newly onset leukotrichia within 3-month and were treatment-naive. For each patient, 10 white hairs in the vitiliginous area were collected using ophthalmic forceps, and black hairs 2cm away from perilesional areas were collected as controls. For scanning electron microscopy (SEM) (INSPECT S5, FEI), 10 hairs were aligned by root side and fixed by a conductive gel. Hair surface details including hair thickness and cuticle appearance were observed. For transmission electron microscopy (TEM) (TECNAI SPIRIT, FEI), the collected hairs were preserved in 1.5% glutaraldehyde solution at 4°C. Cross-sections were performed at a site 1 cm above the hair roots. Hair finer details of internal structure including melanosomes, macrofibrils (Mf), and remnant nucleus (RN) were recorded.
Statistical Analysis
All statistical analyses were performed using SPSS 26.0. Patient characteristics and dermoscopic signs were described. Continuous variables were presented as Mean ± standard deviation (SD) or Median (Min to max), and categorical variables were presented as numbers (n) and percentages (%). Dermoscopic signs were compared between patients with progressive and stable vitiligo using Chi-square or Fisher’s exact test. Duration of leukotrichia was compared between patients with and without the presence of different dermoscopic signs using an independent two-sample t-test. All tests were two-sided and a p value less than 0.05 (P<0.05) was considered statistically significant.
Results
Clinical Characteristics of the Patients
A total of 100 patients with VAL were enrolled, and 140 samples of vitiligo patches on distinct sites were collected. Demographic and clinical characteristics of patients are shown in Table 1. According to hair types and sites, 109 vitiligo patches were covered with terminal hairs (75 from the scalp, 21 from eyebrows, 13 from eyelashes) and 31 patches with tiny vellus hairs on the body. The mean ±SD (Min-Max) age at onset of vitiligo was 21.9 ±14.1 years (2–65 years), and the mean duration of leukotrichia was 82.9 ±98.7 months (0.3–456 months). Fifty-five percentage of patients with vitiligo were in progressive stage (VIDA≥1) and 44% were in stable stage (VIDA=0). Type of vitiligo was dominated by non-segmental type (56%) and segmental type (36%), followed by undefined type (6%) and mixed type (2%).
 |
Table 1 Patient Characteristics |
Dermoscopic Signs of VAL
Terminal (109 patches) and vellus leukotrichia (31 patches) were described and analyzed separately considering the morphologic variation.
Terminal Hairs
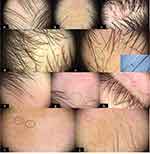
In 75 vitiligo patches of scalp terminal leukotrichia, the most common dermoscopic signs of VAL were perifollicular depigmentation (53/75, 70.7%), followed by clustered leukotrichia (39/75, 52%), white area (31/75, 41.3%), telangiectasia (31/75, 41.3%), diameter diversity of leukotrichia (23/75, 30.7%), red area (23/75, 30.7%), residual perifollicular pigmentation (20/75, 26.7%), depigmented hair roots (16/75, 21.3%), Pohl-Pinkus constrictions (13/75, 17.3%), repigmented leukotrichia (13/75, 17.3%), scales (8/75, 10.7%), and yellow dots (4/75, 5.3%) (See in Figure 1a-e).
In the terminal hair of eyebrows and eyelashes, the hair shaft change was not analyzed considering the dominant diameter diversity of healthy eyebrows and eyelashes. The dermoscopic signs of 21 eyebrow vitiligo patches included perifollicular depigmentation (19/21, 90.5%), red area (13/21, 61.9%), telangiectasia (11/21, 52.4%), residual perifollicular pigmentation (10/21, 46.6%), and repigmented leukotrichia (2/21 9.5%). The perifollicular change of eyelashes was not noticeable due to lack of pigment at the eyelid margin (Figure 1f-h).
Vellus Hairs
In 31 vitiligo patches of vellus leukotrichia from the body, the dermoscopic signs of perifollicular depigmentation (25/31, 80.6%), residual perifollicular pigmentation (13/31, 41.9%), telangiectasia (8/31, 25.8%), honeycomb-type hyperpigmentation (5/31, 16.1%) were found. The translucence vellus hair shaft was difficult to detect even at 20× magnification (Figure 1i and j).
Dermoscopic Signs of VAL by Disease Stages
A total of 75 VAL patches on the scalp were collected from 75 patients, 38 of whom were in progressive stage and 37 were in stable stage. In progressive stage, the probability of diameter diversity of leukotrichia was as high as 52.6%, followed by Pohl-Pinkus constrictions (34.2%) and depigmented hair roots (34.2%), all of which were significantly higher than those in patients of stable stage (P<0.05). Clustered leukotrichia was presented in 64.9% patients in stable vitiligo, which was higher than that (39.5%) in progressive vitiligo (P<0.05). The dermoscopic signs of hair shaft are shown in Table 2.
 |
Table 2 The Dermoscopic Signs of Hair Shaft Changes and Perifollicular Changes by Disease Stages |
Dermoscopic signs of perifollicular changes by stage are shown in Table 2. The positive rate of residual perifollicular pigmentation in progressive stage was 42.1%, which was significantly higher than that (10.8%) in stable stage (P<0.05). The prevalence of residual perifollicular pigmentation, white areas, red areas, and telangiectasia signs was similar between progressive and stable stages (P>0.05).
Correlation of Dermoscopic Signs and Duration of Leukotrichia
Median duration of leukotrichia by the presence of dermoscopic signs is shown in Table 3. Median duration of leukotrichia was as short as 2 months in patients with Pohl-Pinkus constrictions (n = 13), depigmented hair roots (n = 16), and diameter diversity of leukotrichia (n = 23), which were in contrast to a median of 58 months, 6 months, and 65 months, respectively, in patients without the corresponding sign. Median (range) duration of leukotrichia in patients with clustered leukotrichia was 60 (12–180) months, which was longer than the 6 (2–77.75) months in patients without the sign (P<0.05). Median duration of leukotrichia showed no difference in patients with and without a regimented leukotrichia sign (P=0.655>0.05).
 |
Table 3 Comparison of the Duration of Leukotrichia by Dermoscopic Signs |
Electron Microscopic Manifestations of Leukotrichia in Progressive Vitiligo
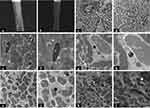
SEM was performed on the hair shafts from three patients with progressive vitiligo. The duration of leukotrichia was within 3 months, and healthy black hairs were collected as controls. SEM showed that the white hair shaft was thinner than the black ones, and the ability to reflect electrons of the VAL was lower than that of the black hair. As a result, it was difficult to observe the morphology of hair cuticles (Figure 2a and b).
TEM showed that VAL lacked mature type IV melanosomes (Figure 2c and d), and melanosomes in leukotrichia were smaller with vesicles formation, reduced counts, and incomplete shape. Macrofibrils in VAL are irregularly arranged around the remnant nucleus with widened spaces and vesicles formation (Figure 2e-l).
Discussion
Our study reported the dermoscopic signs of VAL and their correlations with both the disease stages and duration of leukotrichia, and we also described the early ultrastructural characteristics of VAL under electron microscopy, suggesting the possible pathogenesis of VAL.
The dermoscopic signs of vitiligous patches observed in our study were in consistent with those reported in previous literature, mainly including residual perifollicular pigmentation and perifollicular depigmentation.9 Besides, there are two case reports about the dermoscopic features of VAL12 and follicular vitiligo,13 the former reported a case of repigmented eyebrows in patient with vitiligo under dermoscopy, and the latter reported the dermoscopic features of a follicular vitiligo, which showed a complete depigmentation of the eyelashes including the cortex and the medulla of the hair, without any surrounding skin involvement.
A key finding of our study, not previously reported in patients with vitiligo, is the dermoscopic signs of diameter diversity of leukotrichia and Pohl-Pinkus constrictions. According to previous literature, increased diameter diversity and Pohl-Pinkus constrictions, reflecting consistent and rapid suppression of metabolic and mitotic activities in the hair bulb, occurs more frequently in patients with androgenic alopecia (AGA)14 and AA,15 respectively. Hence, the appearance of those signs probably indicates that the formation process of hair shaft is impaired. Beyond that, the abnormal pigmentation process reflected by the depigmented hair roots and repigmented leukotrichia signs directly suggests the impact of the disease on the activity of hair follicle melanocytes (HFMs). The dermoscopic sign of repigmented leukotrichia, which was reported by Bae12 et al, could also be found in our study.
Further, we analyzed the relationship between disease stages, duration of leukotrichia and dermoscopic signs. Our study showed that except for perifollicular changes reported by previous studies, the dermoscopic signs of hair shaft changes, such as diameter diversity of leukotrichia, Pohl-Pinkus constrictions, and depigmented hair roots were also related to progressive vitiligo (especially the sign of Pohl-Pinkus constrictions, mostly presenting within 2 months of leukotrichia onset). Meanwhile, clustered leukotrichia was more common in those with stable vitiligo. But actually, clustered leukotrichia that is hard to get repigmented can be completely found in patients with progressive vitiligo. This is probably because the persistent or burst (such as SV) course of the disease impaired the hair follicle, then leading to the clustered white hairs present in patients with progressive vitiligo. Therefore, even with our results, we still consider that the sign of clustered leukotrichia is not related to stable stage. Early prevention of leukotrichia is critical, and the phenomenon of repigmented leukotrichia suggests the possibility of hair repigmentation at initial stage of hair graying. The application of dermoscopy could significantly facilitate clinical recognition of early VAL and might be helpful in the treatment of VAL.
The hair cortex fills the core of the strand and consists mostly of bundles of keratin intermediate filaments called macrofibrils (Mf) and melanosomes. Mf, melanosomes, remnant nucleus (RN), and cell membrane complex (CMC) are the main structures that could be observed under TEM.16 Our study showed that melanosomes in leukotrichia were smaller with vesicles formation, reduced counts, and incomplete in shape. Similar characteristics were also reported on the white hairs of patients with AA.17 In 2016, Gan18 et al analyzed the histopathological changes of 8 patients with follicular vitiligo (with gray hair as the first manifestation, followed by leukoderma). This study found that hair follicles but not epidermal melanocytes were attacked by lymphocytes, suggesting that follicular vitiligo might be a missing link between vitiligo and AA. Our cross-sectional images of hair shaft under TEM also showed abnormal cornification changes in VAL, which can be reflected by incomplete macrofibrils and widened space. The vesicles existing in macrofibrils can cause a lower hair density, which can probably explain their fragility. Hence, both our dermoscopic and ultrastructural findings show that the microscopic signs of VAL are similar to those in patients with AA, suggesting that potential shared pathways between the two diseases, and future studies are needed to explore the underlying physiopathological mechanisms.
Our study has several limitations. First, we relied on the clinical diagnosis of VAL, and we did not do the histopathologic test to confirm the diagnosis. Thus, no conclusions about the histopathologic characteristics or dermoscopic-histopathologic correlation of the VAL can be provided. Second, we did not analyse the dermoscopic signs by disease types (non-segmental vitiligo and segmental vitiligo) due to the insufficient sample size. Finally, a larger population would improve statistical power.
In summary, our study investigates the dermoscopic signs and ultrastructural features of VAL, and in patients with VAL, dermoscopic signs including diameter diversity of leukotrichia, Pohl-Pinkus constrictions, and depigmented hair roots were highly related to progressive vitiligo. Melanin and keratin synthesis could both be impaired at the early progressive stage of VAL. There may be certain common mechanisms between VAL and AA, and such a hypothesis needs to be tested in future studies.
Abbreviation
VAL, vitiligo-associated leukotrichia; EM, Electron Microscopy; CT, computed tomography; SEM, scanning electron microscopy; TEM, transmission electron microscopy; Mf, macrofibrils; RN, remnant nucleus.
Compliance of Ethics Guidelines
This study complies with the Declaration of Helsinki and has received permission from the Ethics Committee of Peking University People’s Hospital (No. 2021PHB022-001).
Funding
This work was supported by the National Natural Science Foundation of China (No.82173403).
Disclosure
The authors have no conflict of interest to declare.
References
1. S E, Deepali A, Krishna V. Dermatology MYJIJo. Vitiligo: Clinical profiles in Vadodara, Gujarat; 2006.
2. Mogawer RM, Elmasry MF, Mostafa WZ. New insights into leukotrichia in nonsegmental vitiligo: a cross-sectional study. Indian j Dermatol Venereol Leprol. 2019;85(4):374–379. doi:10.4103/ijdvl.IJDVL_49_18
3. Seleit I, Bakry OA, Abdou AG, Dawoud NM. Immunohistochemical evaluation of vitiliginous hair follicle melanocyte reservoir: is it retained? J Eur Acad Dermatol Venereol. 2015;29(3):444–451. doi:10.1111/jdv.12573
4. Song HJ, Choi GS, Shin JH. Preservation of melanoblasts of white hair follicles of segmental vitiligo lesions: a preliminary study. J Eur Acad Dermatol Venereol. 2011;25(2):240–242. doi:10.1111/j.1468-3083.2010.03710.x
5. Anbar TS, Abdel-Raouf H, Awad SS, Ragaie MH, Abdel-Rahman AT. The hair follicle melanocytes in vitiligo in relation to disease duration. J Eur Acad Dermatol Venereol. 2009;23(8):934–939. doi:10.1111/j.1468-3083.2009.03233.x
6. Fa Y, Lin Y, Chi XJ, et al. Treatment of vitiligo with 308-nm excimer laser: our experience from a 2-year follow-up of 979 Chinese patients. J Eur Acad Dermatol Venereol. 2017;31(2):337–340. doi:10.1111/jdv.13917
7. Lee DY, Park JH, Lee JH, Yang JM, Lee ES. Poor response of phototherapy in segmental vitiligo with leukotrichia: role of digital microscopy. Int J Dermatol. 2012;51(7):873–875. doi:10.1111/j.1365-4632.2010.04609.x
8. Lee DY, Kim CR, Park JH, Lee JH. The incidence of leukotrichia in segmental vitiligo: implication of poor response to medical treatment. Int J Dermatol. 2011;50(8):925–927. doi:10.1111/j.1365-4632.2011.04914.x
9. Jha AK, Sonthalia S, Lallas A. Dermoscopy as an evolving tool to assess vitiligo activity. J Am Acad Dermatol. 2018;78(5):1017–1019. doi:10.1016/j.jaad.2017.12.009
10. Ibrahim S, Hegazy RA, Gawdat HI, et al. Differentiating active from stable vitiligo: the role of dermoscopic findings and their relation to CXCL10. J Cosmet Dermatol. 2022;21(10):4651–4658. doi:10.1111/jocd.14922
11. Thatte SS, Khopkar US. The utility of dermoscopy in the diagnosis of evolving lesions of vitiligo. Indian J Dermatol Venereol Leprol. 2014;80(6):505–508. doi:10.4103/0378-6323.144144
12. Bae JM, Kwon HS, Lee JH, Kim GM. Repigmentation of poliosis in a patient with segmental vitiligo. J Am Acad Dermatol. 2016;75(1):e23–4. doi:10.1016/j.jaad.2016.01.056
13. Cabrera R, Recule F, Hojman L, Larrondo J. Follicular vitiligo: dermatoscopic features of a new subtype of vitiligo. An Bras Dermatol. 2019;94(1):120–121. doi:10.1590/abd1806-4841.20198086
14. de Lacharriere O, Deloche C, Misciali C, et al. Hair diameter diversity: a clinical sign reflecting the follicle miniaturization. Arch Dermatol. 2001;137(5):641–646.
15. Rudnicka L, Rakowska A, Kerzeja M, Olszewska M. Hair shafts in trichoscopy: clues for diagnosis of hair and scalp diseases. Dermatol Clin. 2013;31(4):695–708, x. doi:10.1016/j.det.2013.06.007
16. Richena M, Rezende CA. Structure of photo-damaged white and naturally pigmented human hair. J Photochem Photobiol B. 2020;202:111673. doi:10.1016/j.jphotobiol.2019.111673
17. Coroaba A, Chiriac AE, Sacarescu L, et al. New insights into human hair: SAXS, SEM, TEM and EDX for Alopecia Areata investigations. PeerJ. 2020;8:e8376.
18. Gan EY, Cario-Andre M, Pain C, et al. Follicular vitiligo: a report of 8 cases. J Am Acad Dermatol. 2016;74(6):1178–1184. doi:10.1016/j.jaad.2015.12.049
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.