Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
A Prospective, Open-Label Study to Evaluate the Impact of VYC-12L Injection on Skin Quality Attributes in Healthy Volunteers
Authors Safa M , Natalizio A, Hee CK
Received 9 December 2021
Accepted for publication 23 February 2022
Published 10 March 2022 Volume 2022:15 Pages 411—426
DOI https://doi.org/10.2147/CCID.S352007
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Marva Safa,1 Audrey Natalizio,2 Christopher K Hee3
1La Jouvence, Neuchâtel, Switzerland; 2Eurofins Dermscan Pharmascan, Villeurbanne, France; 3Allergan Aesthetics, an AbbVie company, Irvine, CA, USA
Correspondence: Marva Safa, La Jouvence, Rue de l’Hôpital 18, 2000 Neuchâtel, Switzerland, Tel +41 32 710 19 07, Email [email protected]
Purpose: Age-related changes in skin structure and function can negatively impact skin quality. VYC-12L is a crosslinked hyaluronic acid filler for treating fine lines and improving hydration and elasticity. The goal of this study was to understand skin quality, histologic, and genomic changes underlying long-term clinical benefits of VYC-12L treatment.
Patients and Methods: In this prospective, nonrandomized, open-label study, 11 healthy men (n = 2) and women (n = 9) received intradermal VYC-12L treatment on the volar forearm. Clinical probes assessed skin quality at baseline and months 1 and 3 post-treatment. Punch biopsies were collected 1 and 3 months post-treatment to evaluate histologic and genomic changes. Safety was evaluated throughout.
Results: Participants had a mean age of 41 years and Fitzpatrick skin phototypes II (54.5%) and III (45.5%). At months 1 and 3, VYC-12L-treated skin had higher hydration in the stratum corneum than untreated skin. Cutometer measurements indicated treated skin that was firmer and more resistant to deformation. Histology showed increased epidermal AQP3 and Ki67 expression 1 and 3 months post-treatment and a qualitative increase in papillary dermal collagen I at month 3. Genomic analyses demonstrated treatment-related upregulation of genes involved in adipocyte differentiation, lipid metabolism, keratinocyte renewal, and dermal extracellular matrix (ECM) maintenance. Injection site reactions were mild-to-moderate in severity and resolved by month 1. Five participants reported 19 adverse events; most (68.4%) were related to the biopsy and none to VYC-12L.
Conclusion: VYC-12L produced changes in hydration, firmness, and ECM density and composition consistent with improved skin properties, demonstrating that VYC-12L can act as a substrate for tissue repair.
Keywords: hyaluronic acid filler, hydration, collagen, skin quality, elasticity, histology
Corrigendum for this paper has been published
Introduction
Aging is accompanied by changes in skin quality that are influenced by intrinsic (eg, age) and extrinsic (eg, UV light, pollution exposure) factors. As skin ages, extracellular matrix (ECM) dynamics are disrupted, leading to reductions in elastin, collagen, and glycosaminoglycans such as hyaluronic acid (HA).1–6 These age-related changes, which result in clinical signs such as wrinkles, are often accompanied by detrimental changes to skin quality attributes, such as hydration, elasticity, thickness, and firmness.2,7,8 Such visible signs of aging and poor skin quality can negatively impact self-perception and quality of life.9–12
HA fillers have typically been used to address appearance of facial aging by correcting the structure of the face through the physical space-occupying properties of the filler. More recently, evidence demonstrates that intradermal injection of HA-based products can also be used to provide improvements in skin quality. These positive effects are often attributed to the properties of the HA hydrogel to hold water and occupy space in the skin, leading to improved hydration and physical properties.13,14 Additionally, the space-occupying nature of the HA filler can result in physical interactions with the surrounding cells and tissues, serving as a scaffold for cell ingrowth (eg, intradermal fibroblasts) or physically stretching the cells leading to restoration of dermal matrix components, such as collagen.13–16
VYC-12L is a crosslinked HA hydrogel designed to correct superficial cutaneous depressions, such as fine lines, and for additional improvement in skin quality attributes, such as hydration. VYC-12L contains 12 mg/mL HA and 0.3% (w/w) lidocaine and is intended to be injected intradermally. In prior studies, treatment with VYC-12L has been shown to improve facial skin quality and topography, as assessed by digital image analysis and investigator ratings on photonumeric scales, as well as patient satisfaction with skin.17–19 However, clinical observations of degree of smoothness and patient satisfaction are not necessarily aligned. Indeed, real-world practice and outcomes show high patient satisfaction with VYC-12L treatment but without a great understanding of why. These positive effects have often been attributed to the presence of a hydrophilic product within the skin, but recent short-term ex vivo studies have also demonstrated treatment-related alterations in ECM (eg, collagen and fibrillin density) and hydration markers (eg, Aquaporin-3), resulting in a measurable change in hydration.20,21 However, extrapolation of these findings to longer-term clinical outcomes is limited based on the ex vivo study conditions.
The goal of this exploratory study was to further explore the outcomes from short-term (~8 day) ex vivo studies in a longer-term clinical model to better understand later post-treatment changes to skin quality attributes, as well as histologic and gene expression changes, that may explain the positive clinical benefits and high patient satisfaction reported by patients and physicians after VYC-12L treatment. To do this, skin quality attributes were assessed by physical probe measurements and histologic and genomic analyses after a single VYC-12L treatment in the forearm of healthy volunteers.
Materials and Methods
Study Design
This was a prospective, nonrandomized, interventional, open-label study conducted on healthy volunteers at 1 site in Poland between October 8, 2019 and July 29, 2020. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki, in accordance with local regulatory requirements, approved by the Bioethics Committee of the Regional Chamber of Physicians in Gdansk (Gdansk, Poland), and registered at clinicaltrials.gov (NCT#04206293). Informed consent was obtained from all participants at screening.
The study comprised 7 visits, including screening. Participants received a single treatment and follow-up visits occurred 1 and 3 months after treatment, with intermediate follow-ups ~10 days after each of these visits to assess biopsy healing. Clinical probe measurements and biopsies were collected at the 1- and 3-month follow-up visits. Safety assessments were completed at all follow-up visits, and the final study visit was a safety-related visit conducted via phone 9 months after treatment.
Participants
Eligible participants were healthy men and women aged 30–50 years with Fitzpatrick skin types II or III. Participants had to be willing to receive VYC-12L in the volar forearm and agree to complete all study-related procedures, including not making changes to their usual skincare regimen on the forearms over the course of the study.
Exclusion criteria included a history of permanent or semi-permanent filling products in the treatment area; a known allergy or hypersensitivity to HA; or the presence of any skin disease on the treated zone that the investigator deemed as likely to interfere with measured parameters. Participants were also not eligible if they had undergone a topical treatment during the previous month or a physical treatment (eg, radiotherapy) during the 6 previous months on the biopsy zone.
Eleven participants were included to expect at least 10 analyzable participants.
Treatment
The single treatment session consisted of a specialist injector [Marva Safa, MD] disinfecting the treatment area with octenidine dihydrochloride (1 mg/mL)/phenoxyethanol (20 mg/mL) prior to injecting VYC-12L (Juvéderm® Volite; Allergan Aesthetics, an AbbVie company, Irvine, CA) according to the current CE-marked directions for use. Approximately 0.01 mL intradermal microboluses spaced 0.5–1 cm apart were injected over a 4×8 cm area of the left volar forearm, with a maximum of 1 mL total injected. The single-use 32G 1/2” needles that came with the prefilled syringes were used for all injections. Massage was performed post-injection.
Product Performance Endpoints
Clinical Probe Measurements and Biopsy Collection
Skin quality attributes were measured with a variety of clinical instruments (Table 1) to assess the differences in measurements between untreated and treated zones at months 1 and 3 compared to baseline. These measurements were used to assess hydration, elasticity, density, thickness, roughness, vascularity, brightness, color, and melanin content. Depending on the instrument, 1–5 repeat measurements were taken on each zone (Table 1); average values are reported. Clinical instrument measurements that were not significantly different based on treatment or time point are not reported.
 |
Table 1 Skin Quality Attributes and Measurement Tools |
To examine histologic and genomic changes in the skin following VYC-12L treatment, a trained clinician took 6 cutaneous biopsies from the volar forearm. Two biopsy samples per condition were collected 1 (VYC-12L and untreated) and 3 (VYC-12L only) months after treatment; 1 biopsy was used for histologic analysis and 1 for genomic analysis (Figure 1). For collection, after application of a local anesthetic, a single-use, 3-mm punch biopsy was used for each sampling. A single stitch and/or Steristrips® were applied before the site was covered with a protective dressing. A clinician removed the stitch 10 days after biopsy. Biopsies for histological analysis were placed in a vial immediately after collection and stored at −80°C. Biopsies for genomic analysis were placed in a tube with RNAlater® solution (Millipore Sigma) immediately after collection and stored at 4° for 1 day before being preserved at −20°C. Samples were shipped on dry ice to Laboratory BIO-EC (Longjumeau, France) for analysis.
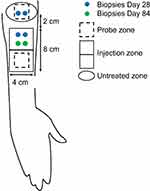 |
Figure 1 Schematic of treatment, assessment, and biopsy zones. |
Histological Analyses
Biopsy samples were fixed in formol before being frozen or dehydrated, impregnated in paraffin, and embedded. Prepared samples were cut into 5 µm sections and analyzed for staining of acidic glycosaminoglycans (GAGs) using alcian blue protocol; general morphology using Masson’s trichrome protocol; and immunostaining for aquaporin-3 (AQP3), Hyaluronic Acid Binding Protein (HABP), collagen I, collagen III, elastin, fibrillin-1, versican, platelet endothelial cell adhesion molecule 1 (PECAM-1), and Ki67. Analysis was performed at the epidermis, papillary dermis, and lower reticular dermis (LRD) depending on the ECM component of interest. Immunostaining was qualitatively evaluated and graded by an expert in skin histology. All images for each batch were quantitatively analyzed using Cell^D (Olympus Life Science) software for each depth of interest to evaluate the percent of positive stained area.
Genomic Analyses
Genomic analyses were conducted by GENEX laboratory. Briefly, RNA from punch biopsy samples was extracted (ReliaPrep™ RNA Miniprep, fibrous tissue protocol; Promega) and validated with microcapillary electrophoresis (Experion™ Automated Electrophoresis System; BioRad) and spectrophotometry (BioDrop; Biochrom, Ltd) before processing via microarray according to manufacturer’s instructions. Because of low quantity of extracted RNA in some samples, Ovation® Pico WTA System V2 kit (Tecan Genomics) followed with Clariom S human microarrays (Affymetrix) was used. Ten ng total RNA of each sample were used as starting material, except with sample #15 for which 1 ng was used. Amplification was initiated at the 3’ end and randomly (using universal primers) throughout the transcriptome in the sample using the Ovation® Pico WTA System V2. Labeled samples were hybridized on WT Clariom S microarrays, which contain 21,448 sequences derived from well-annotated human genes. To identify genes modulated by VYC-12L treatment, genes were first filtered based on a fold change (FC) ≥1.45 (ie, induced in response to treatment vs control) or a FC ≤0.65 (ie, repressed in response to treatment vs control) for each participant; genes were selected for further analysis if FC criteria were met for the majority (n=6) of participants at either month 1 and 3. Genes modulated in response to treatment were then submitted to PredictSearch® (Genex Laboratories) to determine the enrichment of the selected terms from a database of 20,800 genes. For each biological term, a normalized enrichment score (NES) was calculated as the ratio between the enrichment of the selected term among the set of genes found modulated and among the randomly selected genes. Only NESs ≥2 were considered significant. Functional gene networks were then created with PredictSearch®.
Safety Endpoints
The treating investigator evaluated injection site reactions (ISRs), including erythema, pain/tenderness, hardening, edema, lumps/bumps, bruising/hematoma, pruritus, and discoloration/pigmentation, immediately after injection and at the 1- and 3-month follow-ups. Participants also completed a daily log to grade ISRs for each of the 28 days following injection. Biopsy healing was evaluated 10 days after each collection. The investigator also reported any adverse events (AEs) that occurred during the study. ISRs were classified as AEs if they were still present after month 1, required medical treatment, or were judged abnormal by the investigator.
Statistical Analyses
Demographic data are summarized descriptively. Skin quality parameter data relative to baseline within treated or untreated areas are summarized descriptively and with an exploratory mixed model ANOVA with repeated measures to compare Zone (treated and control) and Time (baseline, month 1, and month 3). Histological findings are presented in tabular form and illustrated with representative photographs. If statistical analyses were required, a t-test was used. Safety data are summarized descriptively.
Results
Participants
Of 14 screened participants, 11 participants were selected to receive VYC-12L treatment, and 10 participants completed all study visits. One participant withdrew consent prior to the month 3 follow-up due to dissatisfaction with biopsy healing at month 1. No measurements or biopsies were collected for this participant at month 3. Treated participants were majority female (n=9; 81.8%) with a mean age of 41 years (range: 30–49) and an approximately equal distribution of Fitzpatrick skin types II (54.5%) and III (45.5%). One minor protocol deviation delayed the month 3 visit and its ~10-day follow-up by approximately 1 week due to altered investigator availability.
Product Performance Endpoints
Clinical Probe Parameters
Hydration
Skin hydration, as measured by Corneometer, was significantly higher in the stratum corneum (SC) on the VYC-12L-treated area relative to baseline than the untreated area relative to baseline at months 1 (p<0.001) and 3 (p<0.001; Table 2). MoisturemeterD measurement of hydration rate in the superficial dermis of treated skin (as measured by the XS probe) was significantly higher at month 1 than at baseline (p=0.047; Table 2). There was a trend for hydration in the superficial dermis to be higher in treated skin than untreated skin at month 1 (p=0.07). MoisturemeterD measurement of hydration rate in the deep dermis of treated skin (as measured by the S probe) was not significantly different in treated vs untreated skin at either time point.
 |
Table 2 Adjusted Mean Change from Baseline in Clinical Probe Measurements of Skin Quality |
Skin Biomechanical Properties
Skin elasticity was assessed with a Cutometer (Table 2). Ue, Uf, Ur, and Ua parameters were significantly lower on treated skin than on untreated skin at months 1 and 3 (p<0.001), indicating firmer skin after VYC-12L that persists 3 months after treatment. At month 3, Q2 was significantly decreased by VYC-12L treatment compared to untreated skin (p=0.02), and there were trends for Q1 to be decreased (p<0.1) and Q3 to be increased (p<0.1) by VYC-12L at month 3 compared to untreated skin. The Uv/Ue ratio was also significantly higher on treated skin than on untreated skin at months 1 (p=0.047) and 3 (p=0.006).
The melanin index, as measured by Mexameter, was significantly lower on untreated skin at months 1 (p=0.03) and 3 (p=0.004) than at baseline, indicating reduced pigmentation expected during winter months (Table 2). Pigmentation on the skin treated with VYC-12L was not significantly different from baseline at months 1 or 3. Relative to untreated, the treated skin maintained significantly more pigmentation at month 3 (p=0.0241).
Non-invasive imaging techniques were also used to assess skin density, thickness, roughness, and vascularity. At month 3, Vivosight® Dx OCT measurement of vessel diameter was significantly higher on treated skin than on untreated skin (p<0.01), and there was a trend for vessel density to be increased by VYC-12L at month 3 (p=0.09; Table 3). The optical attenuation coefficient was not significantly different between treated and untreated skin, relative to baseline, at either time point. There were no significant changes in skin roughness or epidermal thickness following VYC-12L treatment. SkinScanner measurements of the echogenic surface, or skin density, was significantly higher in skin treated with VYC-12L at month 3 than at baseline (p<0.01; Table 3). There was a nonsignificant increase in skin density of treated skin at month 1 compared to baseline (p>0.1), and there was a trend for treated skin to have increased density compared to untreated skin at this timepoint (p=0.08). Skin thickness was not significantly altered by VYC-12L treatment. No significant effects on other skin quality parameters were observed following VYC-12L treatment.
 |
Table 3 Adjusted Mean Change from Baseline in Non-Invasive Imaging Measurements of Skin Quality |
Histological Analyses
General Morphology of Biopsied Tissue
General morphology of the SC (ie, thickness and lamination) was comparable on untreated and treated skin for the majority (82%) of participants at month 1. At month 3, 30% of participants had weaker SC lamination and 20% of participants had increased SC thickness on the treated zone. Epidermal morphology was good for all participants, treatment areas, and time points. The number of epidermal layers was comparable between the treated and untreated skin for 91% of participants at month 1 and for 60% of participants at month 3. In 40% of participants at month 3, slight hyperplasic acanthosis was observed in treated skin compared to untreated skin.
No change of epidermal junction morphology was observed at any time point. Papillary dermis density was comparable between treated and untreated skin for 64% and 60% of participants at months 1 and 3, respectively. Compared to the treated zone, 20% of participants had increased papillary dermis density and 20% had decreased density at both time points. Density of the LRD was lower in the treated skin than untreated skin in 82% of participants at month 1 and similar decreases were observed in 60% of participants at month 3.
Immunohistochemical Staining
Qualitative immunostaining scoring showed that 91% and 90% of patients at months 1 and 3, respectively, had increased AQP3 immunostaining on treated skin compared to untreated skin and 70% of patients had increased AQP3 staining on treated skin at month 3 compared to month 1 (Figure 2). Quantitative image analysis (evaluating percent of stained tissue) supported these findings; AQP3 immunostaining was significantly higher on treated skin than untreated skin at months 1 (p=0.02) and 3 (p<0.01) and was significantly higher at month 3 than month 1 in the epidermis of skin treated with VYC-12L (p<0.01).
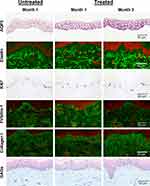 |
Figure 2 Histological changes 1 and 3 months after VYC-12L treatment. Representative images from histologic assessment at months 1 and 3 after treatment. Abbreviation: GAGs, glycosaminoglycans. |
Ki67 immunostaining (Figure 2) was significantly higher in the epidermis of treated skin than untreated skin at month 1 (p=0.02). In contrast, Ki67 was significantly lower in treated vs untreated skin of the dermis at months 1 (p<0.01) and 3 (p<0.01). Qualitative analysis of fibrillin-1 immunostaining (Figure 2) showed that 90% of participants had less fibrillin-1 staining in the papillary dermis of treated skin than untreated skin at month 3 and fibrillin-1 decreased on treated skin between months 1 and 3, with 60% having decreased immunostaining scoring and 40% having no change. Quantitative image analysis supported these data; fibrillin-1 was significantly lower in the papillary dermis of treated skin than untreated skin at month 3 (p<0.001) and was significantly lower at month 3 than month 1 on treated skin (p<0.01). However, the global fibrillin-1 network was not disrupted by VYC-12L treatment.
Elastin immunostaining (Figure 2) in the papillary dermis of skin treated with VYC-12L was significantly lower at month 3 than month 1 (p=0.01), an effect observed in 90% of patients with microscopic observations in papillary dermis and in 60% of patients in LRD, but there were no significant treatment effects on elastin expression.
No significant quantitative differences were observed in collagen I (Figure 2) or collagen III (not shown) immunostaining between treated and untreated skin; however, in the papillary dermis there was a trend for increased collagen I staining on treated skin at month 3 compared to month 1 (p=0.07), as assessed by image analysis. This effect was observed qualitatively in 70% of patients with microscopic observations in papillary dermis and in 60% of patients in LRD.
GAGs immunostaining (Figure 2) in the LRD of skin treated with VYC-12L was significantly lower than untreated skin at month 3 (p=0.03). This decrease was observed on 80% of participants with microscopic observations in both papillary dermis and LRD. GAGs immunostaining was also significantly lower in treated skin at month 3 than month 1 for both papillary dermis (p=0.02) and LRD (p=0.03), an effect observed in 60% of participants in both papillary dermis and LRD.
Genomic Analyses
Gene enrichment was deduced from 157 annotated induced genes and 60 annotated repressed genes at months 1 and 3 in response to treatment (Supplemental Table 1); biological terms associated with treatment-related gene upregulation are summarized in Figure 3. Functional transcriptomic analysis identified 4 gene networks induced by VYC-12L treatment (Figure 4).
Network 1 illustrates the treatment-associated induction of multiple peroxisome proliferator receptor (PPAR) and peroxisome proliferator response elements (PPREs) genes (Figure 4A). At day 28, upregulated genes in this network included PPARg and PPARa as well as PPRE genes involved in lipid accumulation and adipogenesis (FABP4, SCD, ADIPOQ, LPL, FADS1, PLIN1, FABP7, RBP4) which were negatively controlled by WNT signaling (SOSTDC1, WLS, DKK3) 3 months after treatment. Other PPREs upregulated by treatment at month 1 included FABP7, RBP4, SLCA3, SLC1A2/GLT1, and KRT18, which encode for proteins that can lead to keratinocyte differentiation.
Network 2 describes correlations between genes involved in lipogenesis and their biological functions induced by VYC-12L treatment (Figure 4B). Genes upregulated by treatment in this network at month 1 included PPARg, PPARa, GMAP/GPAT1, LPL, SCD, FABP4, RBP4, and PLIN1. At month 3, LPIN1, FADS2, FABP7, ALOX15B, and ANGEL2/CCR4 were upregulated by treatment, while FABP4 was downregulated.
Network 3 describes treatment-related stimulation of adiponectin and its role in the synthesis and maintenance of the dermal matrix (Figure 4C). Genes upregulated at month 1 included PPARg, SLC2A3, ADIPOQ, TIMP4, COL1A1, and COL3A1. At month 3, ADIPOQ and HAS2 were downregulated by treatment, while IGF1R, COL1A1, and COL3A1 were upregulated.
Network 4 describes genes involved in the ECM network and their relation to stem cell regulation during cell differentiation and tissue regeneration (Figure 4D). At month 1, genes upregulated by treatment in this network included S100A7, S100A7A, DES, KRT18, and SLC1A2/GLT1. At month 3, upregulated genes included PXN, FMN1, LAMC3, TNC, MYO10, ITGA3, MARVELD1, LIRG1, DSG2, KRT6C, KRTAP9-4, KRTCAP3, and RPTN.
Safety
Immediately after injection, ISRs included erythema (n=11), lumps/bumps (n=8), pain/tenderness (n=7), edema (n=7), and hematoma (n=7). All ISRs were mild in severity, except for n=1 participant with moderate hematoma. No ISRs were reported at the months 1 or 3 follow-ups.
Five participants reported 19 AEs that were mild (63%) or moderate (37%) in severity. Of the 19 reported AEs, 31.6% (6/19) were considered not related to the product or protocol, and 68.4% (13/19) were considered related to the biopsy procedure (eg, edema, pain, skin tightness, pruritus). Of all reported AEs, 31.6% (6/19) (ie, headache, toothache, diarrhea, herpes) required a corrective treatment; none of the AEs requiring corrective treatment were related to VYC-12L treatment. All AEs were resolved by study end. One participant withdrew prior to the month 3 visit due to dissatisfaction with biopsy healing.
Discussion
VYC-12L treatment has previously been shown to improve facial skin quality and topography, as well as patient satisfaction with skin.17–19 Indeed, patient satisfaction and skin hydration improvements are maintained for up to 9 months following a single VYC-12L treatment.17,18 Other measures of skin quality (eg, decreased roughness and improved topography) are also improved by VYC-12L, but for shorter periods post-treatment, suggesting the contribution of other factors to the overall satisfaction. Thus, the goal of this study was to expand on recent ex vivo studies that examined early (~8 days) post-treatment histologic changes20 to identify potential changes to gene and protein expression at later timepoints (1 and 3 months) following VYC-12L treatment when the HA gel is still present in the skin that may explain the gap between long-lasting high patient satisfaction and shorter-term changes in subjective (ie, investigator ratings using photonumeric scales17) and objective physical measurements. This study showed that a single VYC-12L treatment in the volar forearm provides lasting increases in skin hydration and firmness that correspond to skin being more resistant to deformation. In addition, VYC-12L treatment was associated with increased AQP3, a marker of hydration, as well as increased ECM density and collagen I levels. Gene networks involved in adipocyte differentiation, lipid metabolism, keratinocyte renewal, and restoration of the cellular and dermal matrix were also influenced by VYC-12L. Taken together, these data suggest that skin quality improvements from VYC-12L treatment are due to underlying changes in the tissue following physical interactions between the filler and surrounding cells and tissue (dermis and subdermal adipose tissue), supporting the observations of long-term satisfaction.
The studied treatment area differs from where VYC-12L is typically administered (ie, face and neck); however, the volar forearm was chosen as the injection site because it shares properties with facial skin (eg, structure, thickness, amount of sun exposure, etc.). Although wrinkles are not observed as commonly on forearm skin as they are seen on the face and neck, studying the effects of VYC-12L on the forearm allowed for post-treatment biopsies, and histologic and genomic analyses provide information for a deeper understanding of how VYC-12L treatment may result in underlying changes in the composition of the skin, leading to improvements in skin quality attributes. A no-treatment control was used as a means of comparison in an attempt to understand the changes from baseline due to administration of VYC-12L. Although microneedling has been shown to elicit changes in collagen and elastin remodeling,22–25 the administration of the hydrogel in this evaluation is different from these studies in terms of number of injections and spacing (0.5–1 mm apart). Additionally, no evidence of different responses around needle injection points26 were observed in histological sections following VYC-12L treatment. Although this study was performed on a limited number of samples, the combination of both clinical measurements and biopsy analysis allowed for a multifactorial examination of VYC-12L’s effects on skin quality, including the interactions between VYC-12L and surrounding cells and tissues that may explain lasting improvements in skin quality and patient satisfaction observed in previous clinical studies.17,18 The present findings may also translate to better outcomes on facial skin, since the face has more subdermal fat and injection precision is easier.
Improvements in hydration were observed in the clinical study for VYC-12L, with longer lasting effect than the change in skin roughness.17 Similarly, a significant increase in hydration was observed at months 1 and 3 after treatment, as measured by Corneometer, and a trend toward significance was observed with the MoistureMeter at more superficial depths (XS probe) and no difference observed with the MoistureMeter at deeper depths (S probe), indicating a decreasing impact on hydration with increasing depth. The hydrophilic nature of HA fillers has been proposed as a mechanism for improving hydration of the skin, due to the presence of the filler and its ability to increase local water content. Although increased water content may improve Corneometer measures of hydration, GAG staining was observed to be decreased in treated skin. As GAG staining with alcian blue would also stain the implanted gel, observations of decreased GAG staining indicate that the presence of HA filler was not solely responsible for the observed increases in hydration, as measured through the probes. The decreased GAG content may be a result of negative feedback on HA synthesis, as hyaluronic acid synthase (HA2) was decreased at the month 3 time point, although this did not correlate with a decrease in the measured hydration. Interestingly, we saw a progressive, VYC-12L-related increase in protein expression of AQP3, which is a membrane transporter of water and glycerol, a marker of hydration in the skin, and a key component of various processes involved in keratinocyte function. The observation of a progressive increase in AQP3 expression in the skin confirms the observations seen in the ex vivo study20 and, combined with the decreased GAG staining, indicate that increased hydration is not solely due to the hydrophilic HA in the skin, but at least partially due to a change in how the skin regulates transport of water.
The physical properties of the skin are another important skin quality attribute related to how the tissue may look and feel. Different probes have been used to measure the physical properties of the skin; however, one commonly used probe is the Cutometer.27–30 The Cutometer uses suction to apply a force to the skin and uses measurement of the resulting deformation to understand components of the skin's physical properties. One such property is elasticity, or the ability of the skin to recover, or spring back, following the application of a force. Another important property is firmness, or how much the skin deforms when a force is applied. In this study, skin elasticity was significantly decreased following VYC-12L treatment; however, a significant increase was observed for the firmness of the tissue. Although the observed decreases in Cutometer measurements of skin elasticity may seem undesirable, this may be a terminology consideration. Indeed, skin that is more elastic may be more similar to younger skin; however, when combined with tissue that is less firm, this may give the appearance of a tissue that is less resistant to deformation, or stretchy. Firmer, tighter skin that does not appear to be lax or stretch and is resilient is ultimately a desired outcome for patients, regardless of the changes in elasticity, as it may relate more to how the tissue looks and feels.
The decline of elasticity and firmness with aging is associated with changes to the composition and organization of ECM components, including collagen, elastin, and other matrix proteins.1–6 Physical interactions with HA hydrogels, including stretching of dermal fibroblasts, have been shown to be able to change expression of these ECM proteins.15,16,21 Therefore, an evaluation of the interaction of cells and tissues with an implanted biomaterial like an HA filler is important to better understand the relationship between the skin tissue and the resulting properties. In addition to the physical properties, composition and organization were evaluated with probe measurements (ie, ultrasound, OCT) and histology. Interestingly, ultrasound measurements showed a trend for an increase in density while the skin thickness was maintained. This suggests that the changes in physical properties of the skin are not solely associated with the presence of the gel in the dermis, but rather may be related to changes to the composition or organization, as residual gel would present as hypoechoic (ie, lower density) and would increase the thickness of the skin though its space-occupying properties. In contrast to the trend for increased density of the dermis measured by ultrasound, the density was observed to be decreased in the LRD by histology. Although a decrease in density in a portion of the dermis was unexpected given the ultrasound outcomes, it did not result in decreased mechanical properties overall. The ultrasound results are also consistent with other histological observations. For example, a qualitative increase in collagen I protein in the papillary dermis was observed, suggesting that an interaction between the cells and tissues with the gel matrix manifested as a change to the physical properties of the tissue. Although collagen I was not increased when quantified by Cell^D software, this is consistent with the results observed by non-invasive imaging. The qualitative evaluation evaluated both area of staining (ie, distribution) and intensity of staining (ie, density), while the quantitative assessment only looked at area of staining. The differences between the two evaluations support the increased density observation from ultrasound, while indicating that the overall stained area in the dermis remained consistent. In contrast, expression of other proteins in treated skin, as measured by histology, was either not changed (ie, collagen III) or decreased (ie, elastin, fibrillin-1) compared to baseline levels, although this was only significant for fibrillin-1 between treated and untreated skin at month 3. These findings differ slightly from recent ex vivo data showing increases in collagen density and fibrillin-1 expression after VYC-12L treatment.20 Although the reasons for the difference in observations of fibrillin-1 is not known, it may be related to differences in the in vivo vs ex vivo regulation of elastic fiber formation or methodological considerations, including time of observation (7 days vs 1 and 3 months). Future studies to characterize the time course of changes for fibrillin-1 may help resolve the apparent differences between the ex vivo and in vivo histological changes following HA filler treatment, as well as any role this may play in skin properties (eg, elasticity vs firmness) over time.
As a means to better understand the interaction of cells and tissue with the HA filler, a functional genomic analysis was used to evaluate changes in expression for over 20,000 genes, and PredictSearch® text mining was used to integrate this data to better understand the pathways which were involved. This analysis showed that a single VYC-12L treatment resulted in changes to gene pathways associated with adipogenesis, lipid accumulation, epidermal renewal, and restoration of the cellular and dermal matrix. These effects are likely the result of the physical properties of VYC-12L allowing for close physical interactions (eg, stretching) between the gel and surrounding cells,15,16,21 rather than the prior thinking that skin quality improvements after VYC-12L treatment were solely due to the presence of the product in the skin. This analysis suggests that interaction of the HA filler with the subdermal adipose tissue and associated cells may result in the changes to hydration, firmness, and ECM composition that were observed. Our analyses show that, at month 1 in response to treatment, lipogenic and adipogenic genes such as PPARs and adiponectin, are increased. This is followed by a negative regulation of these genes by WNT signaling at month 3. Such factors are known to increase adipogenesis, lipid metabolism, and transfer of fatty acids to the epidermis, leading to epidermal renewal. Other PPREs induced by treatment (eg, FABP7, KRT18, SLC2A3) code for genes involved in keratinocyte differentiation, which may contribute to the activation of stratum corneum renewal to maintain skin barrier homeostasis.31,32 In addition to the observed changes related to hydration effects (AQP3 expression in the epidermis), an increase in keratinocyte proliferation (Ki67 staining) was also observed, suggesting changes within the epidermis related to epidermal renewal. Interestingly, decreases in melanin/pigmentation (as observed in the untreated zone), which were expected based on the timing of the study (ie, winter months), were not seen in skin treated with VYC-12L, although it is not clear whether this was due to the previously described dermal/epidermal changes. Future studies assessing maintenance of melanin and pigmentation following HA filler treatments could evaluate the mechanisms involved in these changes. In addition to the changes in the epidermis, these gene pathways can also lead to the synthesis of HAs and the production of collagen (COL1A1 induced at months 1 and 3) in fibroblasts. These observations correlated with the collagen I observations in this study and with a study demonstrating that subcutaneous adipocytes influence dermal condition through upregulating collagen and HA production by dermal fibroblasts via adiponectin secretion.33 Moreover, the repression of adiponectin and its target gene HAS2 at month 3 suggest a feedback control of the HA level, which shows that feedback mechanisms in the gene expression do not result in uncontrolled treatment-associated stimulation of HA or other ECM components. Additionally, genes such as SCD, ADIPOQ, and PXN that are typically repressed during aging were induced by VYC-12L treatment, which, coupled with other observed genomic responses that may lead to restoration of the cellular matrix, further suggests that VYC-12L can impact properties of the tissue normally observed with aging, providing support for the observed clinical satisfaction outcomes.17–19
Conclusion
Taken together, the findings from this study provide evidence to help explain long-lasting skin quality changes following VYC-12L treatment. That is, VYC-12L improves not only skin smoothness,17 but can also produce changes at the histologic and genomic levels (through interaction with the dermal/subdermal cells and tissues) that do not require the product to remain directly in the dermis over time. This may also help explain prior observations that VYC-12L treatment is associated with lasting, high levels of patient satisfaction after skin smoothness tended to return to baseline.17–19 Overall, histologic and genomic interactions between VYC-12L and subdermal adipose tissue produce lasting improvements in skin quality that include increased skin hydration and firmness.
Data Sharing Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Acknowledgments
This study was sponsored by Allergan plc (prior to its acquisition by AbbVie Inc). AbbVie participated in the interpretation of data, review, and approval of the publication. The authors wish to thank Dr. Agnieszka Cegielska and the team of Dermscan Poland who performed participant recruitment, skin quality measurements, and biopsies. Writing and editorial assistance were provided to the authors by Sarah J. Cross, PhD, of AbbVie Inc, and funded by AbbVie Inc.
Author Contributions
M Safa served as the treating investigator and performed all VYC-12L injections. A Natalizio participated in clinical study management and data analysis. CK Hee participated in study design and data analysis. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
M Safa was a consultant for Allergan plc (prior to its acquisition by AbbVie Inc). A Natalizio is an employee of Eurofins Dermscan Pharmascan (Villeurbanne, France), a clinical research organization that received funding from Allergan plc (prior to its acquisition by AbbVie Inc). CK Hee is an employee of AbbVie Inc and may own stock/stock options in the company. The authors report no other conflicts of interest in this work.
References
1. Haydont V, Bernard BA, Fortunel NO. Age-related evolutions of the dermis: clinical signs, fibroblast and extracellular matrix dynamics. Mech Ageing Dev. 2019;177:150–156. doi:10.1016/j.mad.2018.03.006
2. Mayrovitz HN, Corbitt K, Grammenos A, Abello A, Mammino J. Skin indentation firmness and tissue dielectric constant assessed in face, neck, and arm skin of young healthy women. Skin Res Technol. 2017;23(1):112–120. doi:10.1111/srt.12310
3. Okano Y, Masaki H, Sakurai H. Dysfunction of dermal fibroblasts induced by advanced glycation end-products (AGEs) and the contribution of a nonspecific interaction with cell membrane and AGEs. J Dermatol Sci. 2002;29(3):171–180. doi:10.1016/s0923-1811(02)00021-x
4. Agren UM, Tammi RH, Tammi MI. Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ culture. Free Radic Biol Med. 1997;23(7):996–1001. doi:10.1016/s0891-5849(97)00098-1
5. Baroni E, Biondo-Simões M, Auersvald A, et al. Influence of aging on the quality of the skin of white women: the role of collagen. Acta Cir Bras. 2012;27(10):736–740. doi:10.1590/s0102-86502012001000012
6. El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11(5):398–405. doi:10.1034/j.1600-0625.2002.110502.x
7. Choi JW, Kwon SH, Huh CH, Park KC, Youn SW. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: a comprehensive and objective approach. Skin Res Technol. 2013;19(1):e349–355. doi:10.1111/j.1600-0846.2012.00650.x
8. Langton AK, Alessi S, Hann M, et al. Aging in skin of color: disruption to elastic fiber organization is detrimental to skin’s biomechanical function. J Invest Dermatol. 2019;139(4):779–788. doi:10.1016/j.jid.2018.10.026
9. Balkrishnan R, McMichael AJ, Hu JY, et al. Correlates of health-related quality of life in women with severe facial blemishes. Int J Dermatol. 2006;45(2):111–115. doi:10.1111/j.1365-4632.2004.02371.x
10. Dayan S, Rivkin A, Sykes JM, et al. Aesthetic treatment positively impacts social perception: analysis of subjects from the HARMONY study. Aesthet Surg J. 2019;39(12):1380–1389. doi:10.1093/asj/sjy239
11. Imadojemu S, Sarwer DB, Percec I, et al. Influence of surgical and minimally invasive facial cosmetic procedures on psychosocial outcomes: a systematic review. JAMA Dermatol. 2013;149(11):1325–1333. doi:10.1001/jamadermatol.2013.6812
12. Farage MA, Miller KW, Berardesca E, Maibach HI. Psychological and social implications of aging skin: normal aging and the effects of cutaneous disease. In: Farage MA, Miller KW, Maibach HI, editors. Textbook of Aging Skin. Berlin, Heidelberg: Springer Berlin Heidelberg; 2010:949–957.
13. Landau M, Fagien S. Science of hyaluronic acid beyond filling: fibroblasts and their response to the extracellular matrix. Plast Reconstr Surg. 2015;136(5Suppl):188S–195S. doi:10.1097/PRS.0000000000001823
14. Streker M, Reuther T, Krueger N, Kerscher M. Stabilized hyaluronic acid-based gel of non-animal origin for skin rejuvenation: face, hand, and décolletage. J Drugs Dermatol. 2013;12(9):990–994.
15. Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143(2):155–163. doi:10.1001/archderm.143.2.155
16. Quan T, Wang F, Shao Y, et al. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Invest Dermatol. 2013;133(3):658–667. doi:10.1038/jid.2012.364
17. Niforos F, Ogilvie P, Cavallini M, et al. VYC-12 injectable gel is safe and effective for improvement of facial skin topography: a prospective study. Clin Cosmet Investig Dermatol. 2019;12:791–798. doi:10.2147/CCID.S216222
18. Ogilvie P, Safa M, Chantrey J, et al. Improvements in satisfaction with skin after treatment of facial fine lines with VYC-12 injectable gel: patient-reported outcomes from a prospective study. J Cosmet Dermatol. 2020;19(5):1065–1070. doi:10.1111/jocd.13129
19. Cavallini M, Papagni M, Ryder TJ, Patalano M. Skin quality improvement with VYC-12, a new injectable hyaluronic acid: objective results using digital analysis. Dermatol Surg. 2019;45(12):1598–1604. doi:10.1097/DSS.0000000000001932
20. Nakab L, Hee CK, Guetta O. Improvements in skin quality biological markers in skin explants using hyaluronic acid filler VYC-12L. Plast Reconstr Surg Glob Open. 2020;8(3):e2723. doi:10.1097/GOX.0000000000002723
21. Paliwal S, Fagien S, Sun X, et al. Skin extracellular matrix stimulation following injection of a hyaluronic acid-based dermal filler in a rat model. Plast Reconstr Surg. 2014;134(6):1224–1233. doi:10.1097/PRS.0000000000000753
22. El-Domyati M, Barakat M, Awad S, Medhat W, El-Fakahany H, Farag H. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8(7):36–42.
23. El-Domyati M, Barakat M, Awad S, Medhat W, El-Fakahany H, Farag H. Multiple microneedling sessions for minimally invasive facial rejuvenation: an objective assessment. Int J Dermatol. 2015;54(12):1361–1369.
24. Moftah NH, El Khayyat MAM, Ragai MH, Alaa H. Carboxytherapy versus skin microneedling in treatment of atrophic postacne scars: a comparative clinical, histopathological, and histometrical study. Dermatol Surg. 2018;44(10):1332–1341.
25. Zeitter S, Sikora Z, Jahn S, et al. Microneedling: matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration. Burns. 2014;40(5):966–973.
26. Zheng Z, Goo B, Kim DY, Kang JS, Cho SB. Histometric analysis of skin-radiofrequency interaction using a fractionated microneedle delivery system. Dermatol Surg. 2014;40(2):134–141.
27. Dobrev H. Use of Cutometer to assess epidermal hydration. Skin Res Technol. 2000;6(4):239–244. doi:10.1034/j.1600-0846.2000.006004239.x
28. Qu D, Seehra GP. Improving the accuracy of skin elasticity measurement by using Q-parameters in Cutometer. J Cosmet Sci. 2016;67(1):37–44.
29. Luebberding S, Krueger N, Kerscher M. Mechanical properties of human skin in vivo: a comparative evaluation in 300 men and women. Skin Res Technol. 2014;20(2):127–135. doi:10.1111/srt.12094
30. Ryu HS, Joo YH, Kim SO, Park KC, Youn SW. Influence of age and regional differences on skin elasticity as measured by the Cutometer. Skin Res Technol. 2008;14(3):354–358. doi:10.1111/j.1600-0846.2008.00302.x
31. Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125(2):183–200. doi:10.1111/j.0022-202X.2005.23668.x
32. Marangoni RG, Masui Y, Fang F, et al. Adiponectin is an endogenous anti-fibrotic mediator and therapeutic target. Sci Rep. 2017;7(1):4397. doi:10.1038/s41598-017-04162-1
33. Ezure T, Amano S. Adiponectin and leptin up-regulate extracellular matrix production by dermal fibroblasts. Biofactors. 2007;31(3–4):229–236. doi:10.1002/biof.5520310310
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


