Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
A Prospective Cohort Study of Elevated Serum NLRP1 Levels to Prognosticate Neurological Outcome After Acute Intracerebral Hemorrhage at a Single Academic Institution
Authors Li W, Wang J, Tang C, Lv X, Zhu S
Received 15 December 2023
Accepted for publication 26 March 2024
Published 29 March 2024 Volume 2024:20 Pages 737—753
DOI https://doi.org/10.2147/NDT.S455049
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Wei Li,1,2 Jun Wang,1,2 Chao Tang,1,2 Xuan Lv,1,2 Suijun Zhu1,2
1Department of Neurosurgery, First People’s Hospital of Linping District, Hangzhou, People’s Republic of China; 2Department of Neurosurgery, Linping Campus, the Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
Correspondence: Suijun Zhu, Department of Neurosurgery, First People’s Hospital of Linping District, Hangzhou, People’s Republic of China, Email [email protected]
Background: Nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 1 (NLRP1) participates in neuroinflammation. This study aimed to identify serum NLRP as a potential prognostic biomarker of acute intracerebral hemorrhage (ICH).
Methods: This prospective cohort study enrolled 145 patients with supratentorial ICH and 51 healthy controls. Serum NLRP1 levels were quantified on admission of all 145 patients, on days 1, 3, 5, 7, and 10 after stroke in 51 of 145 patients and at entry into the study of controls. Poststroke 6-month modified Rankin Scale (mRS) scores of 3– 6 signified a poor prognosis.
Results: Compared to controls, patients had prominently increased serum NLRP1 levels until day 10 after ICH, with the highest levels at days 1 and 3. Serum NLRP1 levels were independently correlated with National Institutes of Health Stroke Scale (NIHSS) scores, hematoma volume and six-month mRS scores, and independently predicted six-month bad prognosis. A linear relationship was observed between serum NLRP1 levels and the risk of poor prognosis in a restricted cubic spline. Under the receiver operating characteristic (ROC) curve, serum NLRP levels efficiently discriminated poor prognosis. Serum NLRP1, NIHSS, and hematoma volume were merged into a prognosis prediction model, which was portrayed using a nomogram. Good performance of the model was verified using calibration curve, decision curve, and ROC curve.
Conclusion: Serum NLRP1 levels are elevated during the early period following ICH and are independently related to hemorrhagic severity and poor prognosis, suggesting that serum NLRP1 may represent a promising prognostic biomarker of ICH.
Keywords: intracerebral hemorrhage, NLRP1, prognosis, severity, biomarkers
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a sort of very common cerebrovascular disorder that primarily affects the brain tissue surrounding the bleeding site and even remote locations in cases of intraventricular or subarachnoid extension.1 In China, the estimated prevalence, incidence, mortality rate, disability rate, recurrence rate of stroke are still high since 2015.2,3 Also, ICH has a high mortality and disability rate among survivors.4 Primary ICH is a multifactorial disease, with hypertension and cerebral amyloid angiopathy being the two main causes.5 Extensive research has yielded valuable insights into the pathophysiological mechanisms of secondary brain injury following ICH, highlighting the importance of inflammatory responses, oxidative stress reactions, and cellular death.6–8 Some severity scales, such as the National Institutes of Health Stroke Scale (NIHSS) and hematoma volume, have been used clinically for ICH.9–11 Notably, there is a growing amount of data showing that the detection of some valuable biomarkers can delve into the mechanisms of disease progression and aid in the severity evaluation and prognosis prediction of ICH.12–16
Inflammasomes are critical components of the early innate inflammatory response to injury.17–19 The nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 1 (NLRP1) inflammasome can activate caspase-1 and promote maturation of the inflammatory cytokine interleukin-1β and interleukin-18 mature, thereby augmenting the inflammatory response.20–22 By amplifying neuroinflammation, damaging the blood–brain barrier, increasing brain edema, and eliciting neuronal apoptosis, NLRP1 may participate in acute brain injury, including ICH.23–27 Upregulation of NLRP1 expression has been shown in animal cerebral cortices injured by ICH, subarachnoid hemorrhage, ischemia and trauma.23–27 In humans with aneurysmal subarachnoid hemorrhage or traumatic brain injury, NLRP1 expression in the cerebrospinal fluid is highly associated with poor prognosis.28,29 Likewise, serum NLRP1 levels were substantially elevated after acute ischemic stroke, which independently predicted poor prognosis three months after injury.30 Thus, serum NLRP1 may serve as a biomarker of acute brain injury. To the best of our knowledge, serum NLRP1 has never been studied in ICH, and this study was designed to investigate serum NLRP1 as a prognostic biomarker of ICH.
Materials and Methods
Study Plan and Ethical Consent
Between May 2019 and August 2022, a prospective cohort study was conducted at the First People’s Hospital of Linping District (Hangzhou, China) to investigate temporal changes in serum NLRP1 levels following ICH, and to further uncover its prognostic role as a biomarker of ICH. Serum NLRP1 levels were detected at admission in all patients and on days 1, 3, 5, 7, and 10 after stroke in some patients who agreed to blood collection at multiple time points. In addition, serum NLRP1 levels in the controls were measured at their entrance into the study. This study was performed in compliance with the guidelines of the Declaration of Helsinki and its later amendments, and was approved by the Ethics Committee at the First People’s Hospital of Linping District (NO. LPH2018012), and informed consent forms were signed by patients’ proxies or controls themselves.
Subject Enrollments
All adults who were hospitalized within 24 h after the onset of stroke symptoms because of new-onset primary supratentorial ICH and underwent conservative management of hematoma were consecutively recruited as clinical cases. We excluded patients with (1) other neurological diseases, such as cerebral ischemia, intracranial tumors, head trauma, central nervous system infections, degenerative diseases, and immune diseases; (2) severe illnesses in other organs, such as malignancies, liver cirrhosis, uremia, chronic heart failure, acute myocardial infarction, and ascites; and (3) specific conditions, such as pregnancy, use of immunosuppressive drugs, loss to follow-up, incomplete information, refusal to participate, and unavailable blood samples. Controls consisted of healthy volunteers who were free from some chronic diseases, such as hypertension, diabetes mellitus, and chronic cardiac, hepatic, pulmonary, and renal diseases, and showed normal results in conventional blood tests, such as blood leukocyte counts, blood glucose levels, and blood creatinine levels.
Information Collection
We inquired about patients’ age and sex. Vascular risk factors including hypertension, diabetes mellitus, hyperlipidemia, cigarette smoking, and alcohol consumption were recorded. The medication history included antilipidemic, anticoagulation, and antiplatelet therapies. Supratentorial hematomas were dichotomized into lobar and deep bleeding types. Hematoma volume was calculated using the following equation: ABC/2.31 Extension of the hematoma into the intraventricular or subarachnoid cavity was observed. Admission NIHSS scores were estimated to reflect the neurological function status. A four-score or greater increase in NIHSS score or death within 24 hours post-admission indicated early neurological deterioration.32 Based on poststroke six-month modified Rankin Scale (mRS), clinical outcomes were divided into poor and good prognosis, with scores of 3–6 and 0–2 respectively.33
Measurements of Serum NLRP1 Levels
According to the principle of voluntariness, some blood samples were obtained only at the time of admission, and other blood samples were acquired both at the admission of patients and on days 1, 3, 5, 7, and 10 after ICH. Controls were drawn based on their willingness to participate in the study. Via the antecubital vein, 5 mL of venous blood was drawn and placed in a gel-containing biochemical tube. After blood collection, samples were centrifuged at 2000 × g for 10 min. Thereafter, the supernatants were isolated, and serum was aliquoted into labelled Eppendorf tubes and preserved at - 80 ◦C condition until final quantification. Every three months, a batch of collected serum samples was melted. Serum NLRP1 levels were quantitatively measured using a human enzyme-linked immunosorbent assay kit (Catalog Number: MBS924094; MyBioSource). The detection range varied from 18.75 pg/mL to 1200 pg/mL, and the minimum detectable concentration was typically less than 4.67 pg/m, with intra-assay coefficients of variation < 8% and inter- assay coefficients of variation < 10%. The detections were performed in duplicate, following the manufacturer’s instructions, by the same skilled staff who were unfamiliar with the clinical materials. The two measurements were averaged for statistical analysis.
Statistical Analysis
The three statistical software packages, including the Statistical Package for the Social Sciences 23.0 (SPSS Inc., Chicago, IL, USA), MedCalc 20 (MedCalc Software, Ltd, Ostend, Belgium) and R 3.5.1 (https://www.r-project.org), were used for data analysis, and GraphPad Prism 7.01 (GraphPad Software Inc., San Diego, California, USA) was used to plot graphs. The Kolmogorov–Smirnov test was used to determine the normal distribution of continuous variables. Categorical variables are shown as frequencies (proportions), normally distributed continuous variables as means (standard deviations, SDs), and non-normally distributed continuous variables as medians (25th – 75th percentiles). Statistical methods for intergroup comparison of data included the chi-square test, Fisher’s exact test, independent-sample Student’s t-test, and the Mann–Whitney U-test. The Kruskal–Wallis test was used to discern differences in serum NLRP1 levels among multiple groups. Spearman’s rank correlation coefficient was used to assess bivariate correlations. To determine whether serum NLRP levels were independently correlated with severity and 6-month mRS scores, a multivariate linear regression model was established, in which serum NLRP levels or 6-month mRS scores were selected as the dependent variable. All the significant variables in the univariate analysis (P<0.05) were included in the multivariate model. Under the restricted cubic spline, a linear relationship between serum NLRP1 levels and the risk of poor prognosis was observed. The receiver operating characteristic (ROC) curve was configured to evaluate discriminative efficiency. The cut-off value for serum NLRP1 levels was chosen using the Youden method. The patients were then dichotomized based on the cutoff values. In other words, serum NLRP1 was transformed into a categorical variable, which was used in the binary logistic regression model to investigate whether serum NLRP emerged as an independent predictor of a poor prognosis. In addition, all independent predictors of poor prognosis were integrated into a combined model, which was delineated using a nomogram. Furthermore, the model was evaluated using a series of statistical methods, namely ROC curve, decision curve, and calibration curve analyses. The sample size was calculated using the MedCalc 20 (MedCalc Software, Ltd, Ostend, Belgium). For bivariate correlation analysis, the least sample size was 59. For intergroup comparison, the least sample size was 62. Thus, a total of 145 patients were sufficient for the clinical analysis in this study. Statistical significance was set at P < 0.05.
Results
Subject Characteristics
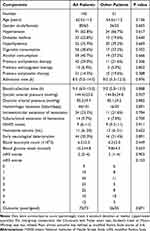
Altogether, 191 adult patients, who were hospitalized within 24 h following stroke due to first-ever primary supratentorial ICH, underwent conservative management of the hematoma. Subsequently, 46 patients were excluded for the reasons outlined in Figure 1. In total, 145 patients were included in the analysis. Among them, only 51 agreed to undergo blood collection at multiple time points. Table 1 shows that there were no significant differences in the demographic, clinical, radiological, and biochemical data between the 145 and 51 patients (all P>0.05). In addition, there were 51 controls, who were aged from 41 to 88 years (mean, 63.3 years; SD, 12.5 years), as well as included 28 males, 18 alcohol drinkers and 16 cigarette smokers. Age, sex, cigarette smoking, and alcohol consumption did not differ substantially between the 51 controls and 51 patients (all P>0.05).
3.2 Longitudinal change of serum NLRP1 levels after ICH, its prognostic predictive ability and its correlation with illness severity
As shown in Figure 2, serum NLRP1 levels were markedly increased at admission in 51 patients, peaked on day 1, plateaued on day 3, and then gradually decreased on day 5 until day 10 after ICH. In contrast to the controls, serum NLRP1 levels were substantially elevated at all designated time points (all P<0.05). Table 2 displays that, as for the prognostic predictive capability, there were no dramatic differences in the AUC between serum NLRP1 levels at admission and at other time points in the 51 patients with ICH (all P>0.05).
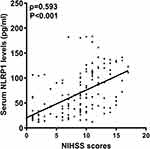
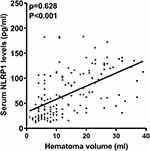
There was a close correlation between serum NLRP1 levels and NIHSS scores (P<0.001; Figure 3), between serum NLRP1 levels and hematoma volume (P<0.001; Figure 4), and between serum NLRP1 levels and other variables, including diabetes mellitus, intraventricular extension of hematoma, blood leukocyte counts, and blood glucose levels (all P<0.05; Table 3). The six significant variables were forced into the multivariate linear regression model, and it was confirmed that serum NLRP1 levels were independently correlated with NIHSS scores (beta, 2.848; 95% confidence interval, 0.563–5.134; VIF, 2.701; P=0.015) and hematoma volume (beta, 1.256; 95% confidence interval, 0.184–2.329; VIF, 2.898; P=0.022).
 |
Table 3 Factors in Correlation with Serum Nucleotide-Binding Oligomerization Domain-Like Receptor Family Pyrin Domain-Containing 1 Levels After Acute Intracerebral Hemorrhage |
Serum NLRP1 Levels and Six-Month Functional Outcome After ICH
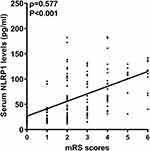
As shown in Figure 5, serum NLRP1 levels were dramatically elevated in the order of 6-month mRS scores, from 0 to 6 (P<0.001). Moreover, serum NLRP1 levels were positively correlated with six-month mRS scores (P<0.001; Figure 6). As listed in Table 4, six-month mRS scores were strongly correlated with intraventricular extension of the hematoma, NIHSS scores, hematoma volume, early neurological deterioration, blood glucose levels, and serum NLRP1 levels (all P<0.01). The six aforementioned significant factors were incorporated into the multivariate logistic regression model, in which it was revealed that serum NLRP1 levels (beta, 0.008; 95% confidence interval, 0.002–0.014; VIF, 1.575; P=0.005), NIHSS score (beta, 0.123; 95% confidence interval, 0.047–0.199; VIF, 2.631; P=0.002), and hematoma volume (beta, 0.071; 95% confidence interval, 0.026–0.116; VIF, 2.698; P=0.002) were independently correlated with poststroke 6-month mRS scores.
 |
Table 4 Factors in Relation to Six-Month Modified Rankin Scale Scores After Acute Intracerebral Hemorrhage |
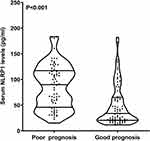
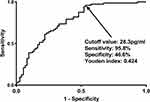
As outlined in Figure 7, patients with poor prognosis displayed substantially higher serum NLRP1 levels than those with good prognosis (P<0.001). The levels efficiently discriminated the risk of a poor prognosis (the maximum Youden index=0.424; Figure 8). As shown in Figure 9, serum NLRP1 levels were linearly correlated with the risk of poor prognosis (P=0.124). In contrast to patients with good prognosis, those with poor prognosis displayed substantially elevated percentages of intraventricular extension of hematoma, early neurological deterioration, and serum NLRP1 levels > 28.3 pg/mL, as well as markedly increased NIHSS scores, hematoma volume, and blood glucose levels (all P<0.05; Table 5). The preceding significant variables were entered into the binary logistic regression model and subsequently it was found that NIHSS scores (odds ratio, 1.286; 95% confidence interval, 1.082–1.528; P=0.004), hematoma volume (odds ratio, 1.058; 95% confidence interval, 1.005–1.114; P=0.033) and serum NLRP1 levels > 28.3 pg/mL (odds ratio, 7.429; 95% confidence interval, 1.919–28.758; P=0.004) were independently associated with poststroke six-month poor prognosis.
 |
Table 5 Factors Associated with Poor Prognosis at Six Months After Acute Intracerebral Hemorrhage |
The three preceding independent predictors of poor prognosis were merged into a prediction model described in the form of a nomogram (Figure 10). The model performed well under the calibration curve (Figure 11) and the decision curve (Figure 12). The AUCs were not significantly different when serum NLRP1 levels were compared with NIHSS scores and hematoma volume (both P>0.05; Figure 13). Intriguingly, the model had a more efficient prognostic predictive ability than the NIHSS score combined with hematoma volume (P<0.05; Figure 14).
Discussion
In this clinical epidemiological investigation of patients with ICH, several multifactorial analyses were performed and some noteworthy findings were unveiled. First, there was a substantial enhancement in serum NLRP1 levels after acute ICH, with the highest levels at days 1 and 3, as well as compared to healthy controls, higher levels were observed during 10 days after stroke. Second, serum NLRP1 levels independently correlated with NIHSS scores and hematoma volume. Third, serum NLRP1 levels at different time points after ICH showed similar AUCs for predicting a poor prognosis. Fourth, serum NLRP1 was independently correlated with six-month mRS scores and remained independently associated with six-month poor prognosis after acute ICH. Lastly, the prognostic predictive value of serum NLRP1 levels was equivalent to that of NIHSS scores and hematoma volume, and the model containing serum NLRP1, NIHSS scores, and hematoma volume outperformed the combination of NIHSS scores and hematoma volume in predicting poor prognosis six months after acute ICH. Such data may provide sufficient evidence to support the assumption that serum NLRP1 represents a promising biochemical marker for assessing hemorrhagic severity and predicting functional outcomes in patients with ICH.
ICH is a common cerebrovascular disorder characterized by inflammation of both the central and peripheral systems.34–37 However, a comprehensive understanding of the pathophysiological mechanisms underlying ICH-related secondary brain injury remains elusive. The discovery of inflammasomes has provided a promising avenue for dissecting the complex cellular and molecular interactions that drive the inflammatory response following ICH.38,39 NLRP1 is the first described inflammasome.40 NLRP1 is mainly expressed in neurons and microglia.41 NLRP1 is associated with inflammation and pyroptosis after acute brain injury, thereby aggravating brain damage and worsening neurological impairment.21 Specifically, NLRP1 expression in neurons of the cerebral cortex was upregulated, and its therapeutic neutralization markedly reduced the innate immune response and improves histopathology in experimental traumatic brain injury.25 In addition, the expression levels of NLRP1 were significantly increased in ipsilateral brain tissues of cerebral ischemia/perfusion mice and stroke patients, and intravenous immunoglobulin treatment protected neurons against ischemic brain injury in mice by suppressing NLRP1 activity.26 In addition, using muramyl dipeptide (an activator of NLRP1), it was demonstrated that the inhibition of purinergic neurotransmission receptors may block the NLRP1/Caspase-1 pathway, thereby conferring a neuroprotective effect in mice with ICH.27 Collectively, NLRP1 may be a detrimental factor and NLRP1 can be considered a target for the treatment of brain injury.
In a previous clinical study of 10 controls and 24 patients with spontaneous subarachnoid hemorrhage, immunoblot analysis revealed that NLRP1 levels in the cerebrospinal fluid of patients were substantially increased, and the higher levels were tightly associated with increasing severity and poor outcome at three months after stroke.29 Alternatively, in patients with traumatic spinal cord or brain injury, exosomes derived from the cerebrospinal fluid contained NLRP1.42 Similarly, immunoblot analysis showed that NLRP1 expression was substantially elevated in the cerebrospinal fluid of 23 patients with traumatic brain injury, and these significantly elevated levels were closely associated with clinical outcomes indicated by the 5-month Glasgow Outcome Scale scores after head trauma.28 Although only a small number of patients were recruited in the two clinical investigations,28,29 such findings suggest that fluid NLRP1 may be a potential biomarker of acute brain injury.
Serum NLRP1 levels were substantially higher in patients with ischemic stroke than in controls.30 In our study, 51 of 145 patients agreed to undergo blood collection at multiple time points. Compared with all patients, this subgroup of patients had similar baseline demographic, clinical, radiological, and biochemical characteristics, indicating that this subgroup of patients could represent the whole group of patients. Moreover, our study showed that patients with ICH, in contrast to controls, exhibited significantly increased serum NLRP1 levels, with higher levels observed at least until day 10 after stroke. Given that NLRP1 expression in brain tissue and cerebrospinal fluid could be obviously enhanced in response to acute brain injury,23–29 it is believably conceived that serum NLRP1 may be at least partially derived from central nervous system. NLRP1 is produced by peripheral cells under pathological conditions.43,44 The systemic inflammatory response can be activated after acute ICH.34,35 Thus, serum NLRP may be secreted by peripheral cells. Injuries in both the central and peripheral systems are highly related to poor prognosis of ICH.45–47 Overall, elevated serum NLRP1 levels may be linked to poor clinical outcomes following ICH.
In a recent prospective observational study of 196 ischemic stroke patients, elevated serum NLRP1 levels were significantly correlated with NIHSS scores and infarct volume, and were independently associated with poor prognosis (mRS scores 3–6) at 90 days after stroke.30 In our study, the AUCs of serum NLRP1 levels at multiple time points did not significantly differ for predicting poor prognosis; therefore, serum NLRP1 levels at admission were selected for further statistical analysis. Multivariate analysis showed that serum NLRP1 levels, which were independently correlated with NIHSS scores, hematoma volume and six-month mRS scores, were independently associated with poor prognosis six months after acute ICH. A prognosis prediction model in which serum NLRP1, NIHSS scores, and hematoma volume were merged was visually delineated using the nomogram. The model performed well using a series of statistical methods, such as ROC curve, calibration curve, and decision curve analyses. Taken together, serum NLRP1 levels may be potential biomarkers for assessing disease severity and predicting functional outcomes after ICH. However, C-reactive protein, interleukin-6 and tumor necrosis factor-alpha are all conventionally used. Admittedly, it may be of clinical value that next studies would be done to compare their prognostic predictive abilities in future.
Conclusions
After acute ICH, serum NLRP1 levels substantially increased during the early period and are significantly higher during ten days than those of the controls. Admission serum NLRP1 levels are independently related to illness severity, as reflected by NIHSS scores and hematoma volume, and are independently associated with six-month poor prognosis after ICH. In addition, the combination model integrating serum NLRP1 levels, NIHSS scores, and hematoma volume displays a significant prognostic predictive efficiency. Overall, serum NLRP1 may be of clinical significance in assessing severity and predicting neurological outcomes after ICH.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available because they are personal data, but are available from the corresponding author upon reasonable request.
Acknowledgments
We gratefully thank all study participants, their relatives, and the staff at the recruitment centers for their invaluable contributions.
Disclosure
The authors declared no potential conflicts of interest in this work.
References
1. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344(19):1450–1460. doi:10.1056/NEJM200105103441907
2. Tu WJ, Zhao Z, Yin P, et al. Estimated burden of stroke in China in 2020. JAMA Network Open. 2023;6(3):e231455. doi:10.1001/jamanetworkopen.2023.1455
3. Tu WJ, Wang LD; Special Writing Group of China Stroke Surveillance Report. China stroke surveillance report 2021. Mil Med Res. 2023;10(1):33. doi:10.1186/s40779-023-00463-x
4. Morotti A, Goldstein JN. Diagnosis and management of acute intracerebral hemorrhage. Emerg Med Clin North Am. 2016;34(4):883–899. doi:10.1016/j.emc.2016.06.010
5. Mendiola JMF, Arboix A, García-Eroles L, Sánchez-López MJ. Acute spontaneous lobar cerebral hemorrhages present a different clinical profile and a more severe early prognosis than deep subcortical intracerebral hemorrhages-A Hospital-Based Stroke Registry Study. Biomedicines. 2023;11(1):223. doi:10.3390/biomedicines11010223
6. Tschoe C, Bushnell CD, Duncan PW, Alexander-Miller MA, Wolfe SQ. Neuroinflammation after intracerebral hemorrhage and potential therapeutic targets. J Stroke. 2020;22(1):29–46. doi:10.5853/jos.2019.02236
7. Sheth KN. Spontaneous intracerebral hemorrhage. N Engl J Med. 2022;387(17):1589–1596. doi:10.1056/NEJMra2201449
8. Zhu H, Wang Z, Yu J, et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog Neurobiol. 2019;178:101610. doi:10.1016/j.pneurobio.2019.03.003
9. Weimar C, Kleine-Borgmann J. Epidemiology, prognosis and prevention of non-traumatic intracerebral hemorrhage. Curr Pharm Des. 2017;23(15):2193–2196. doi:10.2174/1381612822666161027152234
10. Kwah LK, Diong J. National Institutes of Health Stroke Scale (NIHSS). J Physiother. 2014;60(1):61. doi:10.1016/j.jphys.2013.12.012
11. LoPresti MA, Bruce SS, Camacho E, et al. Hematoma volume as the major determinant of outcomes after intracerebral hemorrhage. J Neurol Sci. 2014;345(1–2):3–7. doi:10.1016/j.jns.2014.06.057
12. Wu X, Liu M, Yan T, et al. Plasma PRPC levels correlate with severity and prognosis of intracerebral hemorrhage. Front Neurol. 2022;13:913926. doi:10.3389/fneur.2022.913926
13. Wu X, Yan T, Wang Z, et al. Role of plasma Apo-J as a biomarker of severity and outcome after intracerebral hemorrhage: a prospective and cohort study. Clin Chim Acta. 2022;533:148–155. doi:10.1016/j.cca.2022.06.018
14. Yan T, Wang ZF, Wu XY, et al. Plasma SIRT3 as a biomarker of severity and prognosis after acute intracerebral hemorrhage: a prospective cohort study. Neuropsychiatr Dis Treat. 2022;18:2199–2210. doi:10.2147/NDT.S376717
15. Wang Z, Wu X, Yan T, et al. Elevated plasma complement C1q levels contribute to a poor prognosis after acute primary intracerebral hemorrhage: a prospective cohort study. Front Immunol. 2022;13:920754. doi:10.3389/fimmu.2022.920754
16. Cai Y, Zhuang YK, Wu XY, et al. Serum hypoxia-inducible factor 1alpha levels correlate with outcomes after intracerebral hemorrhage. Ther Clin Risk Manag. 2021;17:717–726. doi:10.2147/TCRM.S313433
17. Rathinam VA, Fitzgerald KA. Inflammasome complexes: emerging mechanisms and effector functions. Cell. 2016;165(4):792–800. doi:10.1016/j.cell.2016.03.046
18. de Carvalho Ribeiro M, Szabo G. Role of the inflammasome in liver disease. Annu Rev Pathol. 2024;17:345–365. doi:10.1146/annurev-pathmechdis-032521-102529
19. Chung C, Seo W, Silwal P, Jo EK. Crosstalks between inflammasome and autophagy in cancer. J Hematol Oncol. 2020;13(1):100. doi:10.1186/s13045-020-00936-9
20. Chavarría-Smith J, Vance RE. The NLRP1 inflammasomes. Immunol Rev. 2015;265(1):22–34. doi:10.1111/imr.12283
21. Mi L, Min X, Chai Y, Zhang J, Chen X. NLRP1 inflammasomes: a potential target for the treatment of several types of brain injury. Front Immunol. 2022;13:863774. doi:10.3389/fimmu.2022.863774
22. Bauernfried S, Hornung V. Human NLRP1: from the shadows to center stage. J Exp Med. 2022;219(1):e20211405. doi:10.1084/jem.20211405
23. Puleo MG, Miceli S, Di Chiara T, et al. Molecular mechanisms of inflammasome in ischemic stroke pathogenesis. Pharmaceuticals. 2022;15(10):1168. doi:10.3390/ph15101168
24. Chen J, Zhang C, Yan T, et al. Atorvastatin ameliorates early brain injury after subarachnoid hemorrhage via inhibition of pyroptosis and neuroinflammation. J Cell Physiol. 2021;236(10):6920–6931. doi:10.1002/jcp.30351
25. de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29(7):1251–1261. doi:10.1038/jcbfm.2009.46
26. Fann DY, Lee SY, Manzanero S, et al. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 2013;4(9):e790. doi:10.1038/cddis.2013.326
27. Wu Y, Huang X, Yang L, Liu Y. Purinergic neurotransmission receptor P2X4 silencing alleviates intracerebral hemorrhage-induced neuroinflammation by blocking the NLRP1/Caspase-1 pathway. Sci Rep. 2023;13(1):14288. doi:10.1038/s41598-023-40748-8
28. Adamczak S, Dale G, de Rivero Vaccari JP, Bullock MR, Dietrich WD, Keane RW. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J Neurosurg. 2012;117(6):1119–1125. doi:10.3171/2012.9.JNS12815
29. Wu Q, Wang XL, Yu Q, et al. Inflammasome proteins in cerebrospinal fluid of patients with subarachnoid hemorrhage are biomarkers of early brain injury and functional outcome. World Neurosurg. 2016;94:472–479. doi:10.1016/j.wneu.2016.07.039
30. Tao C, Wang Y, Xiao S. Clinical significance of CT angiographic assessment of collateral circulation combined with serum NLRP1 levels in ischemic stroke patients. Medicine (Baltimore). 2023;102(13):e33433. doi:10.1097/MD.0000000000033433
31. Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304–1305. doi:10.1161/01.str.27.8.1304
32. You S, Zheng D, Delcourt C, et al. Determinants of early versus delayed neurological deterioration in intracerebral hemorrhage. Stroke. 2019;50(6):1409–1414. doi:10.1161/STROKEAHA.118.024403
33. Zhao Y, Yang J, Zhao H, Ding Y, Zhou J, Zhang Y. The association between hyperglycemia and the prognosis of acute spontaneous intracerebral hemorrhage. Neurol Res. 2017;39(2):152–157. doi:10.1080/01616412.2016.1270575
34. Fonseca S, Costa F, Seabra M, Dias R, Soares A, Dias C. Systemic inflammation status at admission affects the outcome of intracerebral hemorrhage by increasing perihematomal edema but not the hematoma growth. Acta Neurol Belg. 2021;121(3):649–659. doi:10.1007/s13760-019-01269-2
35. Zhu Y, Xie Z, Shen J, et al. Association between systemic inflammatory response syndrome and hematoma expansion in intracerebral hemorrhage. Adv Clin Exp Med. 2022;31(5):489–498. doi:10.17219/acem/145852
36. Xue M, Yong VW. Neuroinflammation in intracerebral haemorrhage: immunotherapies with potential for translation. Lancet Neurol. 2020;19(12):1023–1032. doi:10.1016/S1474-4422(20)30364-1
37. Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurol. 2019;18(11):1058–1066. doi:10.1016/S1474-4422(19)30078-X
38. Xiao L, Zheng H, Li J, Wang Q, Sun H. Neuroinflammation mediated by NLRP3 inflammasome after intracerebral hemorrhage and potential therapeutic targets. Mol Neurobiol. 2020;57(12):5130–5149. doi:10.1007/s12035-020-02082-2
39. Gan H, Zhang L, Chen H, et al. The pivotal role of the NLRC4 inflammasome in neuroinflammation after intracerebral hemorrhage in rats. Exp Mol Med. 2021;53(11):1807–1818. doi:10.1038/s12276-021-00702-y
40. Fahy RJ, Exline MC, Gavrilin MA, et al. Inflammasome mRNA expression in human monocytes during early septic shock. Am J Respir Crit Care Med. 2008;177(9):983–988. doi:10.1164/rccm.200703-418OC
41. de Rivero Vaccari JP, Dietrich WD, Keane RW. Therapeutics targeting the inflammasome after central nervous system injury. Transl Res. 2016;167(1):35–45. doi:10.1016/j.trsl.2015.05.003
42. de Rivero Vaccari JP, Brand F, Adamczak S, et al. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem. 2016;136 Suppl 1(1):39–48. doi:10.1111/jnc.13036
43. Taabazuing CY, Griswold AR, Bachovchin DA. The NLRP1 and CARD8 inflammasomes. Immunol Rev. 2020;297(1):13–25. doi:10.1111/imr.12884
44. Bauernfried S, Scherr MJ, Pichlmair A, Duderstadt KE, Hornung V. Human NLRP1 is a sensor for double-stranded RNA. Science. 2021;371(6528):eabd0811. doi:10.1126/science.abd0811
45. Boehme AK, Hays AN, Kicielinski KP, et al. Systemic inflammatory response syndrome and outcomes in intracerebral hemorrhage. Neurocrit Care. 2016;25(1):133–140. doi:10.1007/s12028-016-0255-9
46. Hagen M, Sembill JA, Sprügel MI, et al. Systemic inflammatory response syndrome and long-term outcome after intracerebral hemorrhage. Neurol Neuroimmunol Neuroinflamm. 2019;6(5):e588. doi:10.1212/NXI.0000000000000588
47. Hu YY, Dong XQ, Yu WH, Zhang ZY. Change in plasma S100B level after acute spontaneous basal ganglia hemorrhage. Shock. 2010;33(2):134–140. doi:10.1097/SHK.0b013e3181ad5c88
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.