Back to Journals » Infection and Drug Resistance » Volume 15
A Preliminary Investigation on a Commercial Ovine Pasteurellosis Vaccine Using Clinical and Pathological Endpoints
Authors Asfaw M, Senbit M, Yesuf M , Dagnaw M , Birhan G, Abat AS , Ibrahim SM
Received 23 March 2022
Accepted for publication 27 May 2022
Published 8 June 2022 Volume 2022:15 Pages 2937—2948
DOI https://doi.org/10.2147/IDR.S365745
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Mersha Asfaw,1 Menur Senbit,2 Mohammed Yesuf,1 Melkie Dagnaw,3 Girma Birhan,1 Anmaw Shite Abat,1 Saddam Mohammed Ibrahim1
1Department of Veterinary Pathobiology, College of Veterinary Medicine and Animal Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Veterinary Epidemiology and Public Health, College of Veterinary Medicine and Animal Sciences, University of Gondar, Gondar, Ethiopia; 3Department of Veterinary Clinical Studies, College of Veterinary Medicine and Animal Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Saddam Mohammed Ibrahim, Tel +251 918150427, Email [email protected]
Introduction: In this study we aimed to provide preliminary evidence on the safety and efficacy of the currently used ovine pasteurellosis vaccine in Ethiopia using clinical and pathological endpoints.
Methods: Twenty, conventionally reared, apparently healthy, seronegative male lambs, were randomly classified into two groups of 10 animals as “vaccinated-challenged” and ”unvaccinated-challenged controls”. The first group received 1 mL of the licensed Pasteurella multocida biotype A based vaccine subcutaneously while the second group received phosphate-buffered saline as a placebo. Following vaccination, lambs were monitored for one month for potential vaccine adverse reactions. Five weeks postvaccination, all lambs were immunosuppressed using dexamethasone, and intratracheally challenged with 5.2× 109 CFU/mL live Mannheimia haemolytica A1 (clinical isolates). Then, all lambs were followed up for eight days for clinical examination and necropsied on the ninth day postchallenge for pathological investigation.
Results: There were no safety issues recorded during the study. In terms of clinical signs, lambs developed fever, depression, mucoid bilateral oculonasal discharge, coughing and sneezing regardless of their vaccination status. Fisher’s exact test between vaccination status and each clinical sign showed a statistically insignificant association (p> 0.05). The main pathological findings in both groups were pulmonary congestion, atelectasis, emphysema, and suppurative bronchopneumonia. Consolidation lung lesion score of +1 (5/10 of vaccinated, 6/10 of unvaccinated) and +2 (3/10 of vaccinated, 4/10 of unvaccinated) were recorded in a statistically indifferent manner among both vaccinated and nonvaccinated groups (p> 0.05).
Discussion and Conclusion: Collectively, the results suggested that the vaccine posed no safety concern and presumably lacks protective efficacy against local isolates. However, the study did not analyze antibody titer and their functionality using serum bactericidal assays. Further confirmatory studies could provide more evidence on the vaccine efficacy. Safety should further be assessed in a field setting involving a large number of animals to enable detection of rare vaccine adverse events.
Keywords: ovine pasteurellosis, pathological assessment, vaccine efficacy, vaccine safety
Introduction
Pneumonic pasteurellosis is a financially costly disease in sheep known for its high morbidity and mortality. The disease is mainly caused by Mannheimia haemolytica, Pasteurella multocida, and Bibersteinia trehalosi,1–3 with M. haemolytica being the most common agent incriminated in disease outbreaks.4–8 Microbiologically, these bacteria are nonmotile, nonsporing, aerobic, fermentative, gram-negative rod and coccobacilli usually having pleomorphic shape.9,10 These bacteria are normally carried up by the upper respiratory tract of healthy animals,11 causing serious diseases when immunity is suppressed due to several factors such as harsh weather, feed and water shortage, transportation, overcrowding, and concomitant infections.4,12,13 Ovine respiratory diseases are responsible for huge economic losses to the production sector due to high mortality rate, reduction in carcass values, exhaustive costs for treatment and prevention practices.14,15
Pneumonic pasteurellosis is also a serious concern for sheep producers in Ethiopia particularly for smallholder farmers which represent the largest group of poor people in the country.16 Outbreaks have been reported from different corners of the country5–8,17 underscoring the need for implementation of effective intervention urgently. Regardless of the existence of diverse serotypes of M. haemolytica, P. multocida, and B. trehalosi,5,6,18 a monoserotype vaccine consisting of formalin inactivated P. multocida biotype A (National Veterinary Institute, NVI, Bishoftu, Ethiopia) is currently in use as a means to control and prevent ovine pasteurellosis in Ethiopia.7 Similarly, the Amhara regional state of Ethiopia, a potential sheep producing area, implements mass vaccination on a regular basis prior to stress seasons to prevent occurrence of pneumonic pasteurellosis. However, the disease remained a challenge with increased complaints from farmers and district veterinarians about occurrence of the disease even among vaccinated flocks which is ascribed to “vaccine failure”. Yet, there are no published reports on the efficacy and safety of the currently used vaccine. In addition, the animal health bureau of the region reported a “safety issue” against the vaccine following claims of adverse reactions and death following vaccination (Amhara Livestock and Fishery Resource Development Office, unpublished report). Therefore, in this study we aimed to conduct a preliminary investigation on the safety and efficacy of the currently used ovine pasteurellosis vaccine experimentally against local isolates of M. haemolytica recovered from clinical cases in the Amhara region.
Materials and Methods
Study Site
This study was conducted at the animal facility of the College of Veterinary Medicine and Animal Sciences, University of Gondar, Gondar, Ethiopia. The facility is located near the veterinary clinic of the college making it ideal for close supervision and monitoring of the experimental animals. Gondar city is found in northwest Ethiopia, 727 km from the capital, Addis Ababa.
Experimental Animals and Their Management
A total of 20 (n=20) conventionally reared, apparently healthy, seronegative (to M. haemolytica antibodies on indirect hemagglutination test) male lambs less than one-year old, and with no history of vaccination against pneumonic pasteurellosis were included in the study. Lambs were individually identified with ear tags, and they were dewormed using ivermectin (0.2 mg/kg, Hebei, Chengshengtang Animal Pharmaceutical Co., Ltd) upon arrival. During the three weeks of adaptation period, food and water was given ad libitum and animals were allowed to move out during the day but ensuring no interaction with other animals. Animals were kept in four separate well ventilated rooms (five animals per room).
Study Design
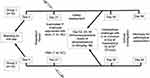
Study subjects were classified into two groups of 10 animals randomly. The first group received 1 mL of the licensed ovine pasteurellosis vaccine (formalin inactivated P. multocida biotype A adjuvanted with alum, NVI, Bishoftu, batch # 4–20) after three weeks of adaptation period subcutaneously, while animals in the second group were nonvaccinated but received only phosphate-buffered saline (1 mL, subcutaneously). Then, all lambs were intratracheally challenged with 5.2×109 CFU/mL live M. haemolytica serotype A1 confirmed to harbor the virulence associated genes, PHSSA (P. haemolytica serotype specific antigen) and Rpt2 (a gene coding for methyltransferase), using multiplex PCR (mPCR). The challenge took place five weeks postvaccination to allow sufficient time for adaptive immunity to develop (Figure 1).19
Infection Challenge
As stressful conditions are prerequisites for the occurrence of pneumonic pasteurellosis due to M. haemolytica A1, all animals were subjected to predisposing factors prior to infection challenge.20 Five weeks after vaccination,19 animals received dexamethasone (Dexalone®, Coophavet, France) intramuscularly at a dose of 0.04 mg/kg for three consecutive days to induce immunosuppression.21 All lambs were then challenged intratracheally with 4 mL of the inoculum described in the above section. It is important to mention that M. haemolytica is the most common agent isolated from outbreaks of pneumonic pasteurellosis in several parts of Ethiopia as well as the study region.5–8 Therefore, it is reasonable to assess the efficacy of the vaccine against these isolates.
Vaccine Safety Evaluation
One major concern according to Amhara Livestock and Fishery Resource Development Office were the reports of death following vaccination that were ascribed to the vaccine. In order to assess any potential link, sheep were closely followed up for a period of one month following vaccination and before challenge during which safety parameters were evaluated. In addition to mortality, parameters such as morbidity, fever, pain, redness, swelling at injection site, and anaphylactic reactions were used to assess safety of the vaccine.
Vaccine Efficacy Evaluation
Vaccine efficacy was assessed on the basis of predefined endpoints including clinical signs (such as fever, depression, oculonasal discharges, coughing and sneezing), mortality, and consolidation lung lesion score among vaccinated and unvaccinated lambs. All animals were monitored for eight consecutive days postinfection until they were sacrificed for pathological examination.22
Clinical Investigation and Necropsy
Following challenge, all lambs were monitored daily for signs of respiratory infection. Rectal temperature was recorded for each subject twice a day at 3:00 am and 5:00 pm for eight successive days. Lambs were considered febrile when the mean rectal temperature was >39.1°C.23
At the end of the of the experiment (ninth day postchallenge), animals were properly and procedurally necropsied and major organs in the respiratory system were inspected, palpated, removed, and incised to be examined in close detail for M. haemolytica induced lesions according to the method described by other authors.24
Consolidation Lung Lesion Scores
Lung lesion scoring was performed and used as a pathological efficacy endpoint. Based on the percentage of consolidated lung masses, lesions were scored as followed: 0 (lungs without lesion), +1 (lungs with 1–4% consolidation), +2 (lungs with 5–14% consolidation), and +3 (lungs with >15% consolidation) according to previous methods.25
Histopathological Examination
In addition to gross examination, lesions were characterized microscopically. Lung samples were collected immediately after postmortem examination. Samples were processed for histopathological analysis and M. haemolytica reisolation. Each lung sample was separately placed in sterile plastic bottles with 10% neutral buffered formalin for histopathology examination. Tissue samples of 2–3 mm thickness were fixed in 10% neutral buffered formalin and were dehydrated in graded ethanol and embedded in paraffin. Sections of 5 mm thickness were stained with hematoxylin and eosin.26 Finally, slides were mounted with mounting dibutyl-phthalate polystyrene xylene and examined under light microscope.
Bacterial Reisolation
To confirm the role of M. haemolytica in the experimental development of pneumonic pasteurellosis, samples were collected with a sterile swab, placed in nutrient broth, and transferred to bacteriology laboratory of the college on an icebox. Specimens were processed for M. haemolytica reisolation as per the standard bacteriological techniques.9,27 Samples were streaked onto MacConkey agar plates to get pure cultures for further reisolation. In culture-positive plates, typical suspected colonies (at least four colonies from a single plate) were picked and Gram’s stain reaction was determined. Colonies with Gram’s reaction and colony morphology consistent to M. haemolytica were further subcultured on nutrient agar. Pure cultures of single colony type from MacConkey agar were transferred onto nutrient agar for a series of primary and secondary biochemical tests for bacterial identification as described elsewhere.9,28
Statistical Analysis
Data generated from this study was stored in Microsoft Excel spreadsheet (Microsoft Excel 2016, Microsoft Corporate, USA). Statistical analysis was performed using STATA versions 14 (Stata Corp., College Station, TX, USA). Descriptive statistics was used to summarize clinical signs and pathological findings. Fisher’s exact test was performed to determine association between vaccination status and clinical signs. Independent t-test was used to compare lung lesion scores among vaccinated and unvaccinated control groups. A p-value of less than 0.05 was considered statistically significant.
Results
Indirect Hemaglutination
Serum was collected from all apparently healthy lambs at the start of the experiment to confirm for seronegativity against M. haemolytica. Serum was analyzed using indirect hemaglutination at NVI, Debrezeit, Ethiopia. Accordingly, all samples tested negative.
Clinical Investigation
Prior to vaccination, all experimental lambs were free from clinical signs of pneumonia. All vaccinated and unvaccinated lambs were daily monitored and clinical signs recorded. Of the total (n=20) lambs (vaccinated and unvaccinated) challenged with live M. haemolytica, 18 (90%) developed symptoms of pneumonia and various pathological lung lesions, ie all (100%) of lambs from the unvaccinated and eight (80%) from the vaccinated group developed the disease. The clinical signs observed were bilateral ocular lacrimation (50% of vaccinated and 80% of unvaccinated lambs), sneezing and coughing (80% of vaccinated and 100% of unvaccinated lambs), watery to mucoid bilateral nasal discharges (80% of vaccinated and 100% of unvaccinated lambs), and dullness or depression (80% of vaccinated and 100% of unvaccinated lambs). However, no death was observed in any of the study animals. The clinical signs persisted until the end of the experiment where animals were necropsied. An association test between vaccination status and each clinical sign observed show no statistically significant result (p>0.05) suggesting lack protective role of the vaccine (Table 1). Prior to challenge, the mean rectal temperature of vaccinated and unvaccinated lambs was 38.9°C and 39°C respectively which was within the normal range. As stated above, rectal temperature was recorded daily for eight consecutive days following challenge. The mean rectal temperature was found to be raised during the first three days postchallenge in both groups. Then, mean rectal temperature declined and return to normal range at day eight postchallenge (Figure 2).
 |
Table 1 Clinical Signs Observed Following Intratracheal Challenge of Lambs with M. Haemolytica A1. Fisher’s Exact Test was Used to Test for Association of Vaccination Status with Each Clinical Sign |
Gross and Histopathology
At the end of the experiment, all lambs were necropsied and postmortem examination conducted following standard procedures. Affected lungs were assessed macroscopically and samples were taken for histopathological examination. In this study, different types of pulmonary lesions were detected on both vaccinated and unvaccinated lambs in a comparable manner. Macroscopically, lungs showed one or more of the lesions such as pulmonary congestion, atelectasis, pulmonary emphysema, and suppurative bronchopneumonia (Table 2).
 |
Table 2 Frequency of Pulmonary Lesions Recorded in Both Vaccinated and Unvaccinated Lambs |
Pulmonary congestion occurred in nearly one-third (6/20) of all challenged lambs regardless of their vaccination status. Grossly, wet lung with pinpoint to ecchymotic dark red hemorrhagic areas were observed on the cranial, middle, accessory and caudal lobes of lungs. However, congestion was prominent on the middle lobes. In most cases, lungs appeared notably dark red as shown in Figure 3A. Microscopically, blood vessels and capillaries were engorged with blood. The presence of large number of red blood cells inside blood vessels lead to rupture of blood vasculature and leakage of the blood cells in the alveolar space and septa. There were many hemosiderin-laden macrophages from breakdown of RBC’s which phagocytize the leaking plasma transudate and red blood cells (Figure 3B).
Similarly, atelectasis was observed in 20% (4/20) of lambs of both groups. Macroscopically, affected lungs appeared collapsed and depressed with considerable volume loss. The affected area was flabby and firm in texture. The pattern was lobular and prominently observed on the caudal lobes of the lung (Figure 4A). Microscopically, slit-like collapsed alveoli with narrow alveolar space, lumen and emphysematous foci in the adjacent areas were also observed. Alveolar walls appeared parallel and in apposition with no inflammatory change either in the alveolar or interstitial space. In addition, the alveolar septa were thick due to diffuse congestion (Figure 4B).
In the present study, pulmonary emphysema was another pathological lesion encountered in 45% (9/20) of all lambs. Grossly, sharply raised, defined, multifocal foci of pale and enlarged emphysematous areas involving one or more lobes of lungs projecting from the neighboring areas showing crepitation and depression were observed (Figure 5A). Microscopically, sections of lung revealed distended alveoli and ruptured interalveolar septa forming giant alveoli (Figure 5B).
 |
Figure 5 Lung with air inflated areas (A). Histologic section showing giant alveoli (wide arrow) accompanied by destruction of alveolar walls (long arrows) H&E ×10 (B). |
The other pathological finding was suppurative bronchopneumonia which was detected at a rate of 85% (17/20). Grossly, there was consolidation of affected lungs with multifocal and raised nodules in both right and left caudal lobes (Figure 6A). Microscopically, abundant neutrophils, and cellular debris were observed within the lumen of the bronchi, bronchioles and alveoli (Figure 6B).
 |
Figure 6 Multifocal raised darkish nodules in both right and left caudal lobes (star) (A). Neutrophilic exudation in bronchial lumina and alveolar space with cellular debris (arrow) H&E ×10 (B). |
Consolidation Lung Lesion Score
In this study, consolidation lung lesion score was used as a pathological endpoint to assess vaccine efficacy according to the method described by Akan et al (2006).25 Lesions were scored as “0”, “+1” and “+2” depending on the degree of consolidated areas of lung, ie “0” for lungs with no gross lesions, “+1” for lungs with 1–4% consolidation, and “+2” for lungs with 5–14% consolidation. Of all lambs in the vaccinated group, 80% (8/10) showed lesion score of +1 (5/8, 62.5%) or +2 (3/8, 37.5%). Similarly, 60% (6/10) and 40% (4/10) of lambs in the unvaccinated group showed lesion scores of +1 and +2 respectively (Table 3). However, there was no statistically significant difference (p>0.05) among the two groups regarding lung lesion scores (Table 4).
 |
Table 3 Consolidation Lung Lesion Score Following Intratracheal Challenge with Live M. Haemolytica (5.2×109cfu/Ml) |
 |
Table 4 Independent t-Test Comparison of Consolidation Lung Lesion Scores for Vaccinated and Unvaccinated Lambs |
Bacterial Reisolation
Samples were taken from affected tissue such as lung, mediastinal lymph nodes, and thoracic fluid from each lamb immediately after necropsy and were processed for reisolation of M. haemolytica. Accordingly, M. haemolytica was recovered from all pneumonic lesions in both vaccinated and unvaccinated lambs. The bacteriological/biochemical profile of isolates identified as M. haemolytica is presented in Table 5.
 |
Table 5 Biochemical Characteristics of Isolates Identified as M. Haemolytica |
Characteristics of Isolates
Bacteriologically, isolates were gram-negative, pink, short ovoid rods with an occasional tendency to bipolar staining. In addition, isolates grew on nutrient agar and showed a white to pale pinpoint colonies arranged in chain as shown in Figure 7. Upon culture on MacConkey agar, isolates appeared pink to red small pinpoint colonies. Biochemically, the isolates fermented glucose, lactose, sucrose and were negative for indole production.
 |
Figure 7 Culture positive plates, grossly, white to pale pinpoint cells arranged in chain (A). Microscopic view of gram-negative colonies with pink color and short ovoid rods in shape (B). |
Vaccine Safety
Vaccinated lambs were monitored for the presence of potential injection site reactions, fever, anaphylactic reactions, and death at time of vaccination and for a period of one month post vaccination. However, there were no adverse reactions recorded in all the vaccinated lambs.
Vaccine Efficacy
In the current study, respiratory symptoms following intratracheal challenge were recorded comparably in both vaccinated and unvaccinated lambs. The majority (80%) of vaccinated lambs and all (100%) unvaccinated lambs developed respiratory symptoms (section: “clinical investigation”) pertinent to pneumonic pasteurellosis in response to intratracheal challenge (Table 6). As already mentioned, consolidation lung lesion score did not show a statistically significant difference among vaccinated and unvaccinated groups (Table 6). In addition, M. haemolytica was successfully reisolated from lesions (of affected lungs) of both groups equally.
 |
Table 6 Comparison of Vaccinated and Unvaccinated Lambs Using Clinical and Pathological Endpoints |
Discussion
Ovine pasteurellosis remained a major concern to the sheep production sector and smallholder farmers in Ethiopia.5–8,16 Presently, vaccination with killed P. multocida biotype A adjuvanted with alum, is routinely practiced to control and prevent pneumonic pasteurellosis in Ethiopia. Repeated complaints of pneumonic pasteurellosis and safety concerns among vaccinated flocks in several districts of the Amhara region formed the basis of this study. Accordingly, we aimed to provide preliminary evidence on the safety and efficacy of the currently used ovine pasteurellosis vaccine experimentally which in turn provides the foundation for large-scale studies.
To assess safety concerns, we vaccinated a group of 10 (n=10) male lambs of less than one year old with 1 mL of the commercial vaccine preparation subcutaneously. Lambs were then followed up for a period of one month during which temperature and other adverse reactions were monitored. It is well known that vaccine safety studies are usually conducted in the field setting where a large number of subjects are vaccinated and safety data collected in either active or passive surveillance or reporting system.29,30 This method enables detection of some rare vaccine adverse events such as death and other complications over a relatively longer period of time.31 However, the practicality of this approach in veterinary medicine particularly in resource limited countries is quite challenging. First, food or production animals have a rapid population turnover (eg small ruminants) staying for a relatively short period of time in the flock due to market and household consumption making the task of animal follow-up postvaccination impractical.32 Second, there are no national or district vaccine safety monitoring systems in place to report adverse events after vaccination in Ethiopia. Third, animals in Ethiopia are mainly managed in an extensive farming system33,34 with no proper documentation and recording which makes it difficult to determine the type of vaccines and time of administration in a given flock. For these reasons, studying vaccine safety in a controlled environmental setting is methodologically feasible in our setting and can provide a clue in assessing potential vaccine adverse events.
In this study, none of the lambs showed adverse reactions related to vaccination and none were febrile during the one month follow-up period. This finding seems to contradict the reports of vaccine-induced death from the animal health bureau of the Amhara regional state. This might be due to differences in the immunogenetics of animals with some animals being genetically susceptible to anaphylactic reactions to one or more of the vaccine components.35 In addition, severe vaccine adverse events such as death have a very rare frequency of occurrence thus their detection requires vaccination of a large number of animals.36,37 In addition, it is also important to note that adverse events could not necessarily be vaccine-induced. It could occur in response to technical errors in vaccine storage, preparation, handling, and administration or due to coincidental events that happen by chance or result from some underlying illness.31
In this study, efficacy of the licensed ovine pasteurellosis vaccine was assessed on the basis of clinical and pathological endpoints following experimental challenge and necropsy respectively. Accordingly, majority (80%) of lambs from the vaccinated and all (100%) lambs from the unvaccinated group developed clinical signs such as anorexia, bilateral watery to mucoid oculonasal discharge, dullness, coughing and sneezing in line with the literature description of pneumonic pasteurellosis due to M. haemolytica.14,22 The bacteriological and biochemical profile of isolates recovered from lesions of lambs was typical of M. haemolytica.9 The fact that M. haemolytica was reisolated from the lower respiratory tracts (LRT) of affected lambs corroborates the role of the bacteria in the induction of the disease. It is well established that M. haemolytica is a normal inhabitant of the upper respiratory tract (URT) of healthy animals extending to the LRT only when immunity of animals is compromised due to several factors.11–13 As mentioned before, all lambs were necropsied the end of the experiment for macroscopic and microscopic analysis of M. haemolytica induced lesions. The majority (18/20) of lambs developed lesions of +1 and +2 score (consolidation lung lesion score) regardless of their vaccination status. This finding coupled with the results of the clinical investigation, is suggestive of lack of protective efficacy of the vaccine. This is coinciding with the reports of the regional animal health bureau that the vaccine is not providing adequate protection against the disease. The apparent absence of protection by vaccine could be due to lack of cross-reactive immunity thus cross protection between the vaccinal strain and the field isolate.12,13,38 The most common pathological findings in this study were pulmonary congestion (30%), atelectasis (20%), pulmonary emphysema (45%), and suppurative bronchopneumonia (85%) which occurred in all lambs irrespective of their vaccination status. Other studies have also reported similar pathological findings in natural or experimentally induced pneumonic pasteurellosis.39–43
Pulmonary congestion develops due to accumulation of fluid in the lungs, resulting in impaired gas exchange and arterial hypoxemia. It has been shown that pulmonary congestion occurs sequentially: first developing in the hilar region of the lungs, followed by filling of the interstitial space and finally, in the most severe form, by alveolar flooding.44 Atelectasis is common when collateral ventilation is less.45 The possible cause is occlusion of the bronchus or bronchiole, which supplies the lung parenchyma. This mostly results from a plug of mucus or purulent exudates. The air contained at the time the bronchus is closed could be absorbed in a short time as entrapped gases which leads to alveolar collapse under surrounding pressure.45 In addition, sepsis induces an inflammatory cascade that affects the function of endothelial cells in the alveolar septa and promoting capillary thrombosis. It also causes surfactant dysfunction which in turn provokes abnormal surface tensions in the alveoli that physically damage the type I pneumocyte leading to formation of atelectasis.46
In animals, pulmonary emphysema is always secondary to obstruction of outflow of air or is agonal at slaughter. Secondary pulmonary emphysema occurs frequently in animals with bronchopneumonia, in which exudate plugging bronchi and bronchioles causes an airflow imbalance where the volume of air entering exceeds the volume leaving the lung.20,47
Suppurative bronchopneumonia results when organisms are deposited in the epithelium of peripheral airways (distal bronchi or bronchioles), resulting in epithelial ulcerations and formation of a peribronchiolar exudate. The inflammatory process spreads through the airway to involve the peribronchiolar alveoli, which become filled with edema and pus.40,43,48
In conclusion, the results of this study suggested that the currently used ovine pasteurellosis vaccine seem to provide no protection against strains of M. haemolytica circulating in Amhara region. Regarding safety, there were no adverse events recorded in association with the vaccine. As a limitation, this study did not analyze antibody titer and their functionality using tests such as serum bactericidal assays. Further confirmatory studies (involving immunogenicity endpoints) could provide more evidence on the vaccine efficacy. Safety should better be assessed in a field setting involving large number of animals to enable detection of rare vaccine adverse events. Moreover, development of a vaccine that incorporates the currently circulating field isolates will offer the desired vaccine efficacy or effectiveness.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
The experiment has been conducted as per the guidelines of the Institutional Animal Ethics Committee, College of Veterinary Medicine and Animal Sciences, University of Gondar. The study was approved by the Institutional Review Board of the University of Gondar.
Acknowledgments
We are grateful to the staff and technical assistants at vet microbiology and vet pathology laboratories of the College of Veterinary Medicine at the University of Gondar.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was financially supported by university of Gondar (Office of the vice president for research and community service) with budget code, VPRCS 6223, for the year 2019/20-–2020/21.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Aitken ID. Diseases of Sheep. John Wiley & Sons; 2008.
2. Bell S. Respiratory disease in sheep: 1. Differential diagnosis and epidemiology. In Pract. 2008;30(4):200–207. doi:10.1136/inpract.30.4.200
3. Bruère AN, West DM. The Sheep: Health, Disease & Production. 1993.
4. Gay CC, Hinchcliff DC, Kenneth W, Radostits OM. Veterinary Medicine; a Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. Elsevier Health Sciences; 2000.
5. Sisay T, Zerihun A. Diversity of Mannheimia haemolytica and Pasteurella trehalosi serotypes from apparently healthy sheep and abattoir specimens in the highlands of Wollo, North East Ethiopia. Vet Res Commun. 2003;27(1):3–14. doi:10.1023/A:1022088005887
6. Legesse A, Abayneh T, Mamo G, et al. Molecular characterization of Mannheimia haemolytica isolates associated with pneumonic cases of sheep in selected areas of Central Ethiopia. BMC Microbiol. 2018;18(1):1–10. doi:10.1186/s12866-018-1338-x
7. Ayelet Gelagay LY, Gelaye E, Tariku S, Asmare K. Epidemiologic and Serologic Investigation of Multifactorial Respiratory Disease of Sheep in the Central Highland of Ethiopia. Int J Appl Res Vet Med. 2004;2(4):274–278.
8. Marru HD, Anijajo TT, Hassen AA. A study on Ovine pneumonic pasteurellosis: isolation and Identification of Pasteurellae and their antibiogram susceptibility pattern in Haramaya District, Eastern Hararghe, Ethiopia. BMC Vet Res. 2013;9(1):1–8. doi:10.1186/1746-6148-9-239
9. Quinn P, Morkey B, Carter M, Donnelly W, Lenard F, Maguire D. Pasteurella Species and Mannheimia Haemolytica. Veterinary Microbiology and Microbial Diseases.
10. Chen HI, Hulten K, Clarridge III JE. Taxonomic subgroups of Pasteurella multocida correlate with clinical presentation. J Clin Microbiol. 2002;40(9):3438–3441. doi:10.1128/JCM.40.9.3438-3441.2002
11. Richard Y, Borges E, Favier C, Oudar J. Nasal and pulmonary flora in the goat. Ann Rech Vet. 1989;20(3):269–276.
12. Thompson D, Fraser J, Gilmour N. Serotypes of Pasteurella haemolytica in ovine pasteurellosis. Res Vet Sci. 1977;22(1):130–131. doi:10.1016/S0034-5288(18)33329-0
13. Gilmour N, Gilmour J. Pasteurellosis of Sheep. Pasteurella and Pasteurellosis. Adlam C, Rutter JM, eds. London, UK: Academic Press; 1989.
14. Hussain R, Mahmood F, Ali HM, Siddique AB. Bacterial, PCR and clinico-pathological diagnosis of naturally occurring pneumonic pasturellosis (mannheimiosis) during subtropical climate in sheep. Microb Pathog. 2017;112:176–181. doi:10.1016/j.micpath.2017.09.061
15. Laishevtsev A Mannheimiosis of cattle, sheep and goats.
16. Alemu B, Desta H, Kinati W, Mulema AA, Gizaw S, Wieland B. Application of mixed methods to identify small ruminant disease priorities in Ethiopia. Front Vet Sci. 2019;6:417. doi:10.3389/fvets.2019.00417
17. Deressa A, Asfaw Y, Lubke B, Kyule M, Tefera G, Zessin K. Molecular detection of Pasteurella multocida and Mannheimia haemolytica in sheep respiratory infections in Ethiopia. Int J Appl Res Vet Med. 2010;8(2):101.
18. Berhe K, Weldeselassie G, Bettridge J, Christley RM, Abdi RD. Small ruminant pasteurellosis in Tigray region, Ethiopia: marked serotype diversity may affect vaccine efficacy. Epidemiol Infect. 2017;145(7):1326–1338. doi:10.1017/S095026881600337X
19. Sabri M, Zamri-Saad M, Mutalib A, Israf D, Muniandy N. Efficacy of an outer membrane protein of Pasteurella haemolytica A2, A7 or A9-enriched vaccine against intratracheal challenge exposure in sheep. Vet Microbiol. 2000;73(1):13–23. doi:10.1016/S0378-1135(99)00205-9
20. Miller MA, Zachary JF. Mechanisms and morphology of cellular injury, adaptation, and death. Pathologic Basis of Veterinary Disease. 2017;2:2154.
21. Charley B. Immunomodulation in Domestic Food Animals: Advances in Veterinary Science and Comparative Medicine. Academic Press; 1990:29.
22. Tesfaw L, Jenberie S, Sori H, Sisay T, Negussie H. Efficacy of Mannheimia haemolytica A2, A7, and A2 and A7 combined expressing iron regulated outer membrane protein as a vaccine against intratracheal challenge exposure in sheep. Afr J Microbiol Res. 2014;8(11):1237–1244. doi:10.5897/AJMR2013.6371
23. Robertshaw D. Temperature Regulation and Thermal Environment in Dukes’ Physiology of Domestic Animals. Copyright; 2004.
24. King JM. The Necropsy Book: New York State College of Veterinary Medicine. Cornell University; 1989.
25. Akan M, Öncel T, Sareyyüpoğlu B, Hazıroğlu R, Tel OY, Cantekin Z. Vaccination studies of lambs against experimental Mannheimia (Pasteurella) haemolytica infection. Small Rumin Res. 2006;65(1–2):44–50. doi:10.1016/j.smallrumres.2005.05.020
26. Luna LG. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. Elsevier Health Sciences; 1968.
27. Quinn PJ, Markey BK, Leonard FC, Hartigan P, Fanning S, Fitzpatrick E. Veterinary Microbiology and Microbial Disease. John Wiley & Sons; 2011.
28. Ahmed WA, Mohammed RJ, Khalaf IA. Molecular and phenotypical characterization of mannheimia haemolytica isolated from goats in Baghdad province. Adv Microbiol. 2017;7(04):304. doi:10.4236/aim.2017.74025
29. Munnoch S-A, Cashman P, Peel R, Attia J, Hure A, Durrheim DN. Participant-Centered online active surveillance for adverse events following vaccination in a large clinical trial: feasibility and usability study. J Med Internet Res. 2019;21(10):e14791. doi:10.2196/14791
30. Liu Z, Meng R, Yang Y, et al. Active vaccine safety surveillance: global trends and challenges in China. Health Data Science. 2021;2021:1–10. doi:10.34133/2021/9851067
31. Tizard IR. Adverse consequences of vaccination. Vaccines Veterinarians. 2021;1:115.
32. Yirga A, Jemberu WT, Lyons N, Gebru A, Akililu F, Rushton J. Post-vaccination herd immunity against peste des petits ruminants and inter-vaccination population turnover in small ruminant flocks in northwest Ethiopia. Prev Vet Med. 2020;174:104850. doi:10.1016/j.prevetmed.2019.104850
33. Edea Z, Haile A, Tibbo M, Sharma A, Sölkner J, Wurzinger M. Sheep production systems and breeding practices of smallholders in western and south-western Ethiopia: implications for designing community-based breeding strategies. Livest Res Rural Dev. 2012;24(7):2012.
34. Mekuriaw Z, Harris-Coble L, Duong M, et al. Ethiopia’s Livestock Systems: Overview and Areas of Inquiry. Int J Med. 2021;1:25.
35. Kennedy RB, Ovsyannikova IG, Palese P, Poland GA. Current challenges in vaccinology. Front Immunol. 2020;11:1181. doi:10.3389/fimmu.2020.01181
36. Moro PL, Arana J, Cano M, Lewis P, Shimabukuro TT. Deaths reported to the vaccine adverse event reporting system, United States, 1997–2013. Clin Infect Dis. 2015;61(6):980–987. doi:10.1093/cid/civ423
37. Hu R, Peng S, Liu Y, et al. The characteristics and trend of adverse events following immunization reported by information system in Jiangsu province, China, 2015–2018. BMC Public Health. 2021;21(1):1–8. doi:10.1186/s12889-021-11387-3
38. Odugbo MO, Odama LE, Umoh JU, Lombin LH. The comparative pathogenicity of strains of eight serovars and untypable strains of Mannheimia haemolytica in experimental pneumonia of sheep. Vet Res. 2004;35(6):661–669. doi:10.1051/vetres:2004044
39. Hashemnia M, Chalechale A, Malmir E. Pulmonary lesions in slaughtered sheep in Western Iran: gross and histopathological findings. Vet Ital. 2019;55:47–56. doi:10.12834/VetIt.785.3795.3
40. Mekibib B, Mikir T, Fekadu A, Abebe R. Prevalence of pneumonia in sheep and goats slaughtered at Elfora Bishoftu export abattoir, Ethiopia: a pathological investigation. J Vet Med. 2019;2019:1–10. doi:10.1155/2019/5169040
41. Kamil SA. Pathological Studies on Ovine Pneumonia with Particular Reference to Pasteurella Haemolytica Infection. IVRI; 1989.
42. Ramesh KP. Studies on pathology of ovine pneumonia and experimental Pastueurella multocida infection in rabbits. Indian J Vet Pathol. 2005;29:153.
43. Oruc E. The pathologic and bacteriologic comparison of pneumonia in lambs. Turk J Vet Anim Sci. 2007;30(6):593–599.
44. Cotter G, Metra M, Milo‐Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure—re‐distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008;10(2):165–169. doi:10.1016/j.ejheart.2008.01.007
45. McGavin MD, Carlton WW, Zachary JF. Thomson’s special veterinary pathology. J Cardiol. 2001;2:587.
46. Jubb KVF, Kennedy PC, Palmer N. Pathology of Domestic Animals. Academic press; 2012.
47. Constable PD, Hinchcliff KW, Done SH, Grünberg W. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. Elsevier Health Sciences; 2016.
48. McLoud TC, Boiselle PM. Pulmonary infections in the normal host. Thoracic Radiology. 2010;1:80.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.