Back to Journals » Patient Preference and Adherence » Volume 17
A Predictive Model for Identifying Low Medication Adherence Among Patients with Cirrhosis
Authors Wang N, Li P, Suo D, Wei H, Wei H, Guo R, Si W
Received 20 June 2023
Accepted for publication 10 October 2023
Published 1 November 2023 Volume 2023:17 Pages 2749—2760
DOI https://doi.org/10.2147/PPA.S426844
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Na Wang,1 Pei Li,1 Dandan Suo,1 Hongyan Wei,1 Huanhuan Wei,2 Run Guo,2 Wen Si2
1Department of Infectious Diseases, Tangdu Hospital, Air Force Medical University, Xi’an, People’s Republic of China; 2Department of General Practice Medicine, Tangdu Hospital, Air Force Medical University, Xi’an, People’s Republic of China
Correspondence: Wen Si, Department of General Practice Medicine, Tangdu Hospital, Air Force Medical University, 569 Xinsi Road, Baqiao District, Xi’an, Shaanxi Province, People’s Republic of China, Tel +86 15829643781, Email [email protected]
Purpose: This study aims to identify the novel risk predictors of low medication adherence of cirrhosis patients in a large cohort and construct an applicable predictive model to provide clinicians with a simple and precise personalized prediction tool.
Patients and Methods: Patients with cirrhosis were recruited from the inpatient populations at the Department of Infectious Diseases of Tangdu Hospital. Patients who did not meet the inclusion criteria were excluded. The primary outcome was medication adherence, which was analyzed by the medication possession ratio (MPR). Potential predictive factors, including demographics, the severity of cirrhosis, knowledge of disease and medical treatment, social support, self-care agency and pill burdens, were collected by questionnaires. Predictive factors were selected by univariable and multivariable logistic regression analysis. Then, a nomogram was constructed. The decision curve analysis (DCA), clinical application curve analysis, ROC curve analysis, Brier score and mean squared error (MSE) score were utilized to assess the performance of the model. In addition, the bootstrapping method was used for internal validation.
Results: Among the enrolled patients (460), most had good or moderate (344, 74.78%) medical adherence. The main risk factors for non-adherence include young age (≤ 50 years), low education level, low income, short duration of disease (< 10 years), low Child-Plush class, poor knowledge of disease and medical treatment, poor social support, low self-care agency and high pill burden. The nomogram comprised these factors showed good calibration and good discrimination (AUC = 0.938, 95% CI = 0.918– 0.956; Brier score = 0.14). In addition, the MSE value was 0.03, indicating no overfitting.
Conclusion: This study identified predictive factors regarding low medication adherence among patients with cirrhosis, and a predictive nomogram was constructed. This model could help clinicians identify patients with a high risk of low medication adherence and intervention measures can be taken in time.
Keywords: medication adherence, cirrhosis, nomogram, prediction model
Introduction
Liver cirrhosis accounts for over 2 million deaths annually and increasingly becomes a severe health burden worldwide.1 It is the end-point of many chronic liver diseases and is characterized by a long period of chronic injury and persistent inflammatory and liver fibrogenesis activation. Previous studies indicated many etiologies.2 The causes of liver cirrhosis include alcoholic liver disease, Hepatitis B (HBV) and hepatitis C-related viral (HCV) hepatitis, nonalcoholic steatohepatitis, and autoimmune and genetic disorders.3 According to its symptoms, cirrhosis can be classified into two categories, namely compensated (CC) and decompensated (DC).4 Patients with DC are usually accompanied by various life-threatening complications, including hepatic encephalopathy, hepatorenal syndrome, varicose bleeding and ascites.5 In addition, the median survival time of CC patients is 12 years, whereas the median survival time of DC patients is two years and nearly 30% of patients might die within one year.6
Nowadays, the development of antiviral medications and antibiotics has efficiently prevented disease deterioration and significantly improved the prognosis of cirrhosis patients.7 For example, pegylated interferon and nucleos(t)ide analogues, such as lamivudine, adefovir and entecavir, could improve liver inflammation and fibrosis and suppress the replication of HBV DNA.8 Long-term antibiotics such as norfloxacin could effectively prevent infection and sepsis.9 Apart from antibiotics and antivirals, other drugs, including β blockers,10 loop diuretics,11 spironolactone,12 lactulose,7 rifaximin13 and oral nutritional supplements,14 have shown great therapeutic efficacy. However, the oral drugs have no direct action on the cccDNA of the HBV-infected hepatocytes and a prolonged medication procedure is needed. Unfortunately, the long treatment period is often affected by increasing cost, side effects and loss-of-follow-up, which usually leads to low medication adherence and significantly influences the therapeutic effects.15
Medication adherence is critical in symptom control of cirrhosis.16 According to a case–control study conducted by Fu et al,17 the likelihood of complicated cirrhosis is significantly lower in more adherent patients and adherence to oral drug therapy is negatively related to decompensation and mortality. However, the medication adherence of cirrhosis patients is still low. Fu et al17 indicated that the 1-year medication possession ratio (MPR) is 0.65 in CC and 0.57 in DC patients. MPR represents the ratio of the number of days of medication supplied within the follow-up period and most studies use 0.80 as a cut-off point for determining good adherence. Polis18 evaluated the medication adherence of cirrhosis by using the Morisky adherence tool, which focuses on medication adherence in the last 30 days. Their study indicated that the proportion of low medication adherence was 25%. Similar results were reported by a survey conducted by Hayward et al,19 in which the low medication adherence proportion was 22%.
Previous studies have reported several risk factors associated with low medication adherence,20,21 such as medication beliefs, underlying diseases and disease-related knowledge levels. Nevertheless, these studies were based on a relatively small cohort and the relevant risk factors were still largely unknown. Moreover, these factors could hardly be applied in clinical practice and there were no applicable predictive models. Thus, improving medication adherence is still challenging in cirrhosis management and treatment.22
Therefore, this study aims to identify the novel risk predictors of low medication adherence of cirrhosis patients in a large cohort and construct an applicable predictive model to provide clinicians with a simple and precise personalized prediction tool.
Materials and Methods
Study Design and Participants
The study was observational, and the predictive model was constructed following the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) checklist.23 After the approval of the ethics committee of Tangdu Hospital, participants attending the Department of Infectious Diseases from 2022.06 to 2023.06 were recruited. All participants provided informed consent in accordance with the Declaration of Helsinki. The inclusion criteria were as follows: (1) Patients diagnosed with cirrhosis with a Child-Pugh score between 5 and 9; (2) Patients aged over 18 years; (3) Patients who had at least one hospital admission and had complete clinical data; (4) Patients with no major comorbidities, such as cardiac disease, renal disease, cognitive impairment (eg, dementia) or carcinoma; (5) Patients with no psychological disorders (eg, anxiety disorders, mood disorders, impulse-control disorders, and substance use disorders) and no description of anti-depression or other drugs which may influence the medication adherence; (6) Patients with no hepatic encephalopathy; (7) Patients must have been on medication for a minimum of 3 months.
Data Collection
Demographics include age, gender, etiology of cirrhosis, educational level, income and duration of disease (time since cirrhosis diagnosis). The education levels include high school and below and college and above. According to the data from the Chinese national statistics bureau,24 incomes were classified into three degrees according to family per capita monthly income: ≤2000 yuan (275 USD), 2000–5000 yuan (275–687 USD) and >5000 yuan (687 USD). Duration of disease was classified into two degrees: ≤10 years and >10 years.
The severity of cirrhosis was evaluated using the Child-Pugh score determined by senior medical officers.25 The classification system categorized enrolled patients into mild hepatic impairment (5–6) and moderate hepatic impairment (7–9).
Knowledge of disease and medical treatment was evaluated by the questionnaire developed by Polis et al,18 and some modifications were made. This questionnaire was developed based on evidence from the literature, patient experiences and consultation with experts in hepatology nursing. In this study, the items were modified to make them more accessible to our patients. The patients with accuracy higher than 70% were classified as having good knowledge of disease and medical treatment.
The Oslo Social Support Scale (OSSS-3) was used to measure social support.26 This scale includes three items; the total score is 3–14. Based on the score, patients were divided into three categories: poor support (3–8), moderate support (9–11) and strong support (12–14).
The Exercise of Self-Care Agency (ESCA) scale developed by Kearney et al27 was used in evaluating patients’ self-care agency. The scale comprised 43 items categorized into four dimensions: self-concept, self-nursing responsibility, self-care skills and health knowledge level. Each item has a 5-point Likert scale, ranging from 0 (very uncharacteristic of me) to 4 (very characteristic of me). Patients were classified according to the total score (172 points): low (≤56 scores), moderate (57–114) and high (≥115).
Pill burdens were collected from patient self-reports and confirmed using medical records.
Medication adherence was evaluated by medication possession ratio (MPR = total days’ supply/study time) of 3 months. High medication adherence was defined as MPR value of 0.8 or higher.28 Compared with subjective evaluation tools, such as self-report, MPR is more objective and is a standard measure of possession of filled prescription medication over time.29
Details of the knowledge of disease and medical treatment questionnaire, Oslo Social Support Scale and Exercise of Self-Care Agency scale were provided in the Supplementary Material 1-3. Filled questionnaires were checked immediately for completeness and accuracy. In addition, all cases with missing data were deleted.
Predictors and Outcome
Potential predictors were determined by previous studies and clinical practice.18 The predictors include demographics, the severity of cirrhosis, knowledge of disease and medical treatment, social support, self-care agency and pill burdens. The primary outcome measure was medication adherence evaluated by MPR.
Sample Size Calculation
As the guideline recommended,30 10 events per variable rules were used to calculate the sample size. In this study, approximately ten variables were included and at least 100 samples were needed for prediction model construction.
Statistical Analysis
Statistical analyses were performed using the R software (4.2.1). All medical adherence-related factors were evaluated by univariable logistic regression analysis. Variables with P value < 0.05 were considered candidate factors and were included in the multivariate logistic regression analysis. The collinearity of variables was determined by the Least Absolute Shrinkage and Selection Operator (LASSO) tests and variables with collinearity were excluded from model construction. A low medication adherence predictive nomogram was constructed using the R package “RMS”. The accuracy of the prediction model was evaluated by the Brier score and the calibration curve. The decision curve analysis (DCA) and clinical application curve analysis assessed the benefits of prediction model application at different thresholds. The mean squared error (MSE) was calculated to determine if overfitting occurs. The enumerative data were expressed as rates and percentages, whereas the quantitative variables were expressed as mean and standard deviation. For comparison, the chi-square test was used in enumerative data, and the student-t test was used in quantitative data. If not otherwise stated, P < 0.05 was considered statistical significance.
Results
Patient Characteristics
From 2022.06 to 2023.06, 540 patients with cirrhosis were admitted to the Department of Infectious Diseases of Tangdu Hospital. According to the inclusion and exclusion criteria, 460 eligible patients with complete information were selected. Patient characteristics are shown in Table 1; most were male (82.39%) and had an educational level of high school and below. For the etiology of cirrhosis, HBV infection was the leading cause of cirrhosis, followed by alcohol-associated liver disease, HCV, nonalcoholic steatohepatitis and autoimmune disease. Meanwhile, 60% of patients were classified as low-income (<2000 yuan/275 USD per month). For disease characteristics, 65% of patients had mild cirrhosis according to the Child-Pugh score, and 65.22% had a disease duration of less than ten years. In this cohort, the mean score of knowledge of disease and medical treatment was 6.99 ± 1.45, and 190 (41.30%) patients were categorized as “poor”. The mean social support score was 9.23 ± 1.65, and 148 (32.17%) patients had poor social support. The mean score of self-care agency was 88.41 ± 23.80, and 64 (13.91%) patients had poor self-care agency. In addition, patients reported taking 3.89 ± 2.31 pills per day. Regarding medication adherence, most patients had good or moderate (344, 74.78%) medical adherence. However, the proportion of low adherence was not low (116 patients, 25.22%), indicating that this problem was still prominent.
 |
Table 1 Demography and Medication Characteristics of Study Participants (N = 460) |
Predictive Factors Selection
As shown in Table 2, compared with the low adherence group, patients with high adherence were more likely to have a higher age, education level, income, duration of disease, Child-Pugh score, knowledge of disease and treatment, social support, and self-care agency. In addition, they usually took fewer pills per day. However, no significant differences in gender and medication type were found between the two groups. Then, potential predictive variables were included in multivariable logistic regression analysis. The following factors were identified as final predictors of low medication adherence, shown in Figure 1. The protective factors include age (0.552, 0.378−0.807), education level (0.306, 0.124−0.757), income (0.479, 0.343−0.670), duration of disease (0.358, 0.231−0.555), Child-Pugh class (0.458, 0.314−0.668), knowledge of disease and medical treatment (0.146, 0.095−0.224), social support (0.404, 0.292−0.559) and self-care agency (0.278, 0.199−0.389). The risk factor was pill burden (1.591, 1.094–2.315).
 |
Table 2 Correlations of Demography, Disease Characteristics, Knowledge of Disease and Medical Treatment, Social Support, Self-Care Agency and Pill Burden with Medication Adherence |
 |
Figure 1 Multivariable logistic regression analysis. |
Nomogram Construction and Performance
In the LASSO regression test, no significant collinearity was found between the screened predictive factors. A nomogram was constructed, thereby providing a personalized and convenient tool for low medication adherence. As shown in Figure 2A, the total score of each patient was calculated by adding the scores of each item. The equation of each variable as follows: total point = age (>50 = 0, ≤50 = 21.65551) + educational level (college and above = 0, high school and below = 27.72299) + incomes (>5000 yuan/687 USD = 0, 2000–5000/275–687 USD = 27.88395, <2000 yuan/275 USD = 55.76789) + duration of disease (>10 = 0, ≤10 = 34.56261) + Child−Pugh class (moderate = 0, mild = 29.80692) + knowledge of disease and medical treatment (good = 0, poor = 66.65692) + social support (strong = 0, moderate = 33.82919, poor = 67.65838) + self-care agency (high = 0, moderate = 50, low = 100) + pill burden (≤3 = 0, >3 = 15.21446). Then, the risk of low medication adherence could be calculated by the following equation: Risk of low medication adherence ≈ −9.01e-07 * points ^3 + 0.000765903 * points ^2 - 0.204991306 * points + 17.596724457’.
 |
Figure 2 (A) Nomogram; (B) calibration curves of the nomogram; (C) decision curve of the nomogram; (D) clinical impact curve. |
Calibration curves of the nomogram are shown in Figure 2B; the performance of the model represented by the solid line was very close to an ideal model represented by the diagonal dotted line. In addition, the Brier score of the model was 0.14, with a reliability of 0.001 and a resolution of 0.015. A Brier score closer to 0 (and similar reliability and resolution) provided measurements of better calibration. The decision curve is shown in Figure 2C. The results indicated that using the nomogram to predict low medication adherence adds more benefit than the scheme. The clinical impact curve of the nomogram is shown in Figure 2D, the red curve represents the number of patients predicted by the nomogram, and the blue curve represents the number of patients who were actually low medication adherence. The results indicated that when the risk threshold exceeded 0.3, the estimated number was close to the actual number. In addition, the MSE value was 0.03, indicating no overfitting.
ROC Curve Analysis
According to the nomogram, the total score of each patient was calculated and the ROC curve was plotted (Figure 3). The area under the curve was 0.938 (0.918–0.956, red line). Then, the model was validated by the bootstrapping method, and the AUC of the validation cohort was 0.935 (0.922–0.952, blue line). These results indicated that the nomogram had an excellent prediction capability.
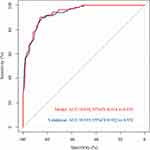 |
Figure 3 The ROC curve of the nomogram. |
Discussion
This study found that higher age, higher education level, higher income, longer duration of disease, higher Child-Pugh score, higher knowledge of disease and treatment, higher social support, higher self-care agency and lower pill burden could predict higher medication adherence. Combined with these predictive factors, a predictive model was constructed and exhibited good prediction capability. These findings were partially consistent with previous studies that illustrated predictive factors’ role in medication adherence.
Age could affect medication adherence, but the results vary in different studies. Simeonova et al31 reported that older patients were more likely to adhere to medication and change their lifestyle than younger patients. Aggarwal et al32 noted that younger age could predict non-adherence to cholesterol and anti-hypertensive drugs. However, opposite results were reported by Panahi et al33 and Kimura et al.34 The reason for the discrepancy could be explained as follows. The younger population usually thought they had a low risk of severe diseases, but they wished to keep their illness and treatment a secret.35 The older population usually be troubled by memory loss, but they are more likely to pay attention to health maintenance.36 These factors make it difficult to evaluate the impact of age on medication adherence. In addition, other factors, such as income, social status and family support, could also work as confounding factors. Although the findings suggested that younger age was associated with low adherence, health education should be applied to patients of all ages. Educational level is another medication adherence-related factor. Higher-educated people understand the complications of not taking medication and not receiving medical instructions, which explains why education affects treatment adherence.37 Nevertheless, it is worth noticing that the medication and treatment procedure must be fully explained to these populations, preventing low adherence caused by fear of side effects.38 Lower income was associated with low medication adherence in our study. The result could be explained as follows. A lower-income patient may perceive it as a secondary concern compared with other problems or cannot afford to pay for their medication.39,40 Raffaa et al41 also indicated that patients with low income or without insurance showed less treatment adherence to heart failure medications. Medication adherence is a tracking problem in chronic disease due to the long duration of the disease. However, this study found that patients with a longer disease duration exhibited better medication adherence. It could be explained that patients with a long disease course are already accustomed to the lifestyle and have more disease-related knowledge.42 The Child−Pugh grading system was widely used to quantitatively evaluate liver reserve function in patients with cirrhosis. This study found that patients with better liver function showed worse medication adherence, indicating that the disease’s improvement might prompt them to change their medicine habits.43
The factors above were related to demography and disease, which are hard to interfere with. However, the findings could indicate the high-risk population. In addition, correlations between medication adherence and the knowledge of disease and medical treatment, social support, self-care agency and pill burden were found in this study. Previous studies indicated that targeted intervention of these factors could improve adherence to medication.44,45 A patient’s disease knowledge is thought to influence medication adherence, since patients who lack adequate knowledge of their disease or prescribed medications may not consistently adhere to their medication regimen. Volk et al46 reported that simple interventions by improving patient knowledge of cirrhosis can be an effective way for management of the disease. A positive correlation was found between social support and medication adherence, which is consistent with previous research. With adequate social support, patients are more likely to receive external objective support from family members, relatives, or institutions, which could promote their health and help them improve medication adherence.47 In addition, the HBV virus, the leading cause of cirrhosis, is highly infectious and HBV patients may receive social isolation and discrimination.43 Thus, these patients tend not to use social support networks well and may obtain less support subsequently. A lack of self-care agency also influenced medication adherence. Bible et al48 reported that more self-care activities were performed by patients with a higher level of self-care ability, resulting in greater well-being and improved medication adherence. Chen et al49 found that self-management education increased the likelihood of receiving appropriate health care and engaging in beneficial activities. This study also indicated that a high pill burden was associated with low medication adherence. The same conclusion was found in medical conditions such as hypertension, diabetes mellitus type 2, cardiovascular diseases, and HIV.50 By simplifying medication regimens and using pill management systems, medication non-adherence and errors can be reduced.51,52 However, only associations have been investigated in this study, so it is unclear if a lower pill burden will result in higher adherence.
After the identification of predictive factors, a nomogram was constructed. The results of DCA, clinical impact, calibration curves and ROC curves indicated that this model was precise and reliable. The nomogram was a widely used predictive model, and it was reported in the prediction of non-adherence in chronic kidney disease,53 hemodialysis,54 type 2 diabetes55 and rheumatoid arthritis.56 Our model had a higher AUC value than other disease models, indicating that the patients might benefit more from this model.
This study also had some limitations. Firstly, this was a single-center study. A multi-center might improve the generalizability of our findings. Secondly, the model was only validated by the bootstrapping method. Thus, an external cohort was still needed to verify the conclusions. Thirdly, the number of participants was relatively small, and large-scale validation studies were required. Fourthly, HBV was the primary etiology of cirrhosis. However, the severe acute exacerbation of chronic hepatitis B, which could occur with fatal consequences, was not scrutinized. Fifthly, this study did not elucidate whether variables correspond to positive or negative effects and did not ascertain the relative importance of different variables’ effects. Despite these limitations, this was still the first study that reported the application of a predictive model in cirrhosis. We hope that the limitations could be solved by our further studies, thus providing an applicable tool to improve the medication adherence of cirrhosis patients.
Conclusion
This study found that higher age, higher education level, higher income, longer duration of disease, higher Child-Pugh score, higher knowledge of disease and treatment, higher social support, higher self-care agency and lower pill burden could predict higher medication adherence. In addition, a predictive nomogram was constructed. This model could help clinicians identify patients with a high risk of low medication adherence, and intervention measures can be taken in time.
Disclosure
The authors declare that they have no competing interests.
References
1. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–171. doi:10.1016/j.jhep.2018.09.014
2. Wilson R, Williams DM. Cirrhosis. Med Clin North Am. 2022;106(3):437–446. doi:10.1016/j.mcna.2021.12.001
3. Romanelli RG, Stasi C. Recent Advancements in Diagnosis and Therapy of Liver Cirrhosis. Curr Drug Targets. 2016;17(15):1804–1817. doi:10.2174/1389450117666160613101413
4. Huang DQ, Terrault NA, Tacke F, et al. Global epidemiology of cirrhosis - aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20(6):388–398. doi:10.1038/s41575-023-00759-2
5. Jepsen P. Comorbidity in cirrhosis. World j Gastroenterol. 2014;20(23):7223–7230. doi:10.3748/wjg.v20.i23.7223
6. Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139(4):1246–1256, 1256.e1241–1245. doi:10.1053/j.gastro.2010.06.019
7. Smith A, Baumgartner K, Bositis C. Cirrhosis: diagnosis and Management. Am Fam Physician. 2019;100(12):759–770.
8. Tang LSY, Covert E, Wilson E, Kottilil S. Chronic Hepatitis B Infection: a Review. JAMA. 2018;319(17):1802–1813. doi:10.1001/jama.2018.3795
9. Kockerling D, Nathwani R, Forlano R, Manousou P, Mullish BH, Dhar A. Current and future pharmacological therapies for managing cirrhosis and its complications. World j Gastroenterol. 2019;25(8):888–908. doi:10.3748/wjg.v25.i8.888
10. Villanueva C, Albillos A, Genescà J, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393(10181):1597–1608. doi:10.1016/S0140-6736(18)31875-0
11. Tapper EB, Parikh ND. Diagnosis and Management of Cirrhosis and Its Complications: a Review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
12. Sehgal R, Singh H, Singh IP. Comparative study of spironolactone and eplerenone in management of ascites in patients of cirrhosis of liver. Eur J Gastroenterol Hepatol. 2020;32(4):535–539. doi:10.1097/MEG.0000000000001678
13. Caraceni P, Vargas V, Solà E, et al. The Use of Rifaximin in Patients With Cirrhosis. Hepatology. 2021;74(3):1660–1673. doi:10.1002/hep.31708
14. Espina S, Gonzalez-Irazabal Y, Sanz-Paris A, et al. Amino Acid Profile in Malnourished Patients with Liver Cirrhosis and Its Modification with Oral Nutritional Supplements: implications on Minimal Hepatic Encephalopathy. Nutrients. 2021;13(11):3764. doi:10.3390/nu13113764
15. Eberhard CM, Davis KA, Nisly SA. Adherence to goal-directed therapy for patients with cirrhosis hospitalized with spontaneous bacterial peritonitis. Am J Health Syst Pharm. 2022;79(Suppl 2):S27–s32. doi:10.1093/ajhp/zxac041
16. Mendys P, Zullig LL, Burkholder R, Granger BB, Bosworth HB. Medication adherence: process for implementation. Patient Prefer Adherence. 2014;8:1025–1034. doi:10.2147/PPA.S65041
17. Fu KY, Hsieh ML, Chen JA, Hsieh VC. Association between medication adherence and disease outcomes in patients with hepatitis B-related cirrhosis: a population-based case-control study. BMJ open. 2022;12(6):e059856. doi:10.1136/bmjopen-2021-059856
18. Polis S, Zang L, Mainali B, et al. Factors associated with medication adherence in patients living with cirrhosis. J Clin Nurs. 2016;25(1–2):204–212. doi:10.1111/jocn.13083
19. Hayward KL, Valery PC, Martin JH, et al. Medication beliefs predict medication adherence in ambulatory patients with decompensated cirrhosis. World j Gastroenterol. 2017;23(40):7321–7331. doi:10.3748/wjg.v23.i40.7321
20. Brown MT, Bussell J, Dutta S, Davis K, Strong S, Mathew S. Medication Adherence: truth and Consequences. Am J Med Sci. 2016;351(4):387–399. doi:10.1016/j.amjms.2016.01.010
21. McQuaid EL, Landier W. Cultural Issues in Medication Adherence: disparities and Directions. J Gen Intern Med. 2018;33(2):200–206. doi:10.1007/s11606-017-4199-3
22. Skladany L, Vnencakova J, Laffers L, et al. Adherence to Oral Nutritional Supplements After Being Discharged from the Hospital is Low but Improves Outcome in Patients with Advanced Chronic Liver Disease. Patient Prefer Adherence. 2020;14:2559–2572. doi:10.2147/PPA.S283034
23. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. doi:10.1136/bmj.g7594
24. Shen Z, Fan W, Hu J. Income inequality, consumption, and the debt ratio of Chinese households. PLoS One. 2022;17(5):e0265851. doi:10.1371/journal.pone.0265851
25. Jamil Z, Perveen S, Khalid S, et al. Child-Pugh Score, MELD Score and Glasgow Blatchford Score to Predict the In-Hospital Outcome of Portal Hypertensive Patients Presenting with Upper Gastrointestinal Bleeding: an Experience from Tertiary Healthcare System. J Clin Med. 2022;11(22):6654. doi:10.3390/jcm11226654
26. Chu HY, Huang HC, Huang CY, et al. A predictive model for identifying low medication adherence among older adults with hypertension: a classification and regression tree model. Geriatric Nursing. 2021;42(6):1309–1315. doi:10.1016/j.gerinurse.2021.08.011
27. Kearney BY, Fleischer BJ. Development of an instrument to measure exercise of self-care agency. Res Nurs Health. 1979;2(1):25–34. doi:10.1002/nur.4770020105
28. Kim J, Ozzoude M, Nakajima S, et al. Insight and medication adherence in schizophrenia: an analysis of the CATIE trial. Neuropharmacology. 2020;168:107634. doi:10.1016/j.neuropharm.2019.05.011
29. Vink NM, Klungel OH, Stolk RP, Denig P. Comparison of various measures for assessing medication refill adherence using prescription data. Pharmacoepidemiol Drug Saf. 2009;18(2):159–165. doi:10.1002/pds.1698
30. Riley RD, Ensor J, Snell KIE, et al. Calculating the sample size required for developing a clinical prediction model. BMJ. 2020;368:m441. doi:10.1136/bmj.m441
31. Simeonova E. Doctors, patients and the racial mortality gap. J Health Econ. 2013;32(5):895–908. doi:10.1016/j.jhealeco.2013.07.002
32. Aggarwal B, Mosca L. Lifestyle and psychosocial risk factors predict non-adherence to medication. Ann Behav Med. 2010;40(2):228–233. doi:10.1007/s12160-010-9212-6
33. Panahi S, Rathi N, Hurley J, Sundrud J, Lucero M, Kamimura A. Patient Adherence to Health Care Provider Recommendations and Medication among Free Clinic Patients. J Patient Exp. 2022;9:23743735221077523. doi:10.1177/23743735221077523
34. Kimura M, Usami E, Iwai M, et al. Oral anticancer agent medication adherence by outpatients. Oncol Lett. 2014;8(5):2318–2324. doi:10.3892/ol.2014.2480
35. Walker AN, Zhang T, Peng XQ, Ge JJ, Gu H, You H. Vaccine Acceptance and Its Influencing Factors: an Online Cross-Sectional Study among International College Students Studying in China. Vaccines. 2021;9(6):585. doi:10.3390/vaccines9060585
36. Jin H, Kim Y, Rhie SJ. Factors affecting medication adherence in elderly people. Patient Prefer Adherence. 2016;10:2117–2125. doi:10.2147/PPA.S118121
37. Pourhabibi N, Mohebbi B, Sadeghi R, et al. Factors associated with treatment adherence to treatment among in patients with type 2 diabetes in Iran: a cross-sectional study. Front Public Health. 2022;10:976888. doi:10.3389/fpubh.2022.976888
38. Alema NM, Semagn G, Melesse S, et al. Patterns and determinants of prescribed drug use among pregnant women in Adigrat general hospital, northern Ethiopia: a cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):624. doi:10.1186/s12884-020-03327-7
39. Algarni MA, Althobiti MS, Alghamdi SA, et al. Medication Non-Adherence among Patients with Chronic Diseases in Makkah Region. Pharmaceutics. 2022;14(10):2010. doi:10.3390/pharmaceutics14102010
40. Aziz H, Hatah E, Bakry MM, Islahudin F. How payment scheme affects patients’ adherence to medications? A systematic review. Patient Prefer Adherence. 2016;10:837–+. doi:10.2147/PPA.S103057
41. Raffaa HSM, Alasmari BA, Abadi SA, et al. Adherence of heart failure patients to heart failure medications and its determinants in the Aseer region, Southern Saudi Arabia. J Family Med Primary Care. 2020;9(9):5041–5045. doi:10.4103/jfmpc.jfmpc_904_20
42. Conn VS, Ruppar TM. Medication adherence outcomes of 771 intervention trials: systematic review and meta-analysis. Prev Med. 2017;99:269–276. doi:10.1016/j.ypmed.2017.03.008
43. Mohamed R, Ng CJ, Tong WT, Abidin SZ, Wong LP, Low WY. Knowledge, attitudes and practices among people with chronic hepatitis B attending a hepatology clinic in Malaysia: a cross sectional study. BMC Public Health. 2012;12(1):601. doi:10.1186/1471-2458-12-601
44. Neiheisel MB, Wheeler KJ, Roberts ME. Medication adherence part one: understanding and assessing the problem. J Am Assoc Nurse Pract. 2014;26(1):49–55. doi:10.1002/2327-6924.12099
45. Cheen MHH, Tan YZ, Oh LF, Wee HL, Thumboo J. Prevalence of and factors associated with primary medication non-adherence in chronic disease: a systematic review and meta-analysis. Int J Clin Pract. 2019;73(6):e13350. doi:10.1111/ijcp.13350
46. Volk ML, Fisher N, Fontana RJ. Patient knowledge about disease self-management in cirrhosis. Am J Gastroenterol. 2013;108(3):302–305. doi:10.1038/ajg.2012.214
47. Zhou Y, Huo QW, Du SY, et al. Social Support and Self-Efficacy as Mediating Factors Affecting the Association Between Depression and Medication Adherence in Older Patients with Coronary Heart Disease: a Multiple Mediator Model with a Cross-Sectional Study. Patient Prefer Adherence. 2022;16:285–295. doi:10.2147/PPA.S337634
48. Bible LJ, Casper KA, Seifert JL, Porter KA. Assessment of self-care and medication adherence in individuals with mental health conditions. J Am Pharm Assoc. 2017;57(3):S203–S210.e203. doi:10.1016/j.japh.2017.02.023
49. Chen R, Cheadle A, Johnson D, Duran B. US trends in receipt of appropriate diabetes clinical and self-care from 2001 to 2010 and racial/ethnic disparities in care. Diabetes Educ. 2014;40(6):756–766. doi:10.1177/0145721714546721
50. Baumgartner A, Drame K, Geutjens S, Airaksinen M. Does the Polypill Improve Patient Adherence Compared to Its Individual Formulations? A Systematic Review. Pharmaceutics. 2020;12(2):190. doi:10.3390/pharmaceutics12020190
51. Stone VE, Jordan J, Tolson J, Miller R, Pilon T. Perspectives on adherence and simplicity for HIV-infected patients on antiretroviral therapy: self-report of the relative importance of multiple attributes of highly active antiretroviral therapy (HAART) regimens in predicting adherence. J Acquir Immune Defic Syndr. 2004;36(3):808–816. doi:10.1097/00126334-200407010-00007
52. Seals AB, Duffy VG. Toward development of a computer-based methodology for evaluating and reducing medication administration errors. Ergonomics. 2005;48(9):1151–1168. doi:10.1080/00140130500193566
53. Wu X, Tang F, Li H, et al. Development and validation of a nomogram model for medication non-adherence in patients with chronic kidney disease. J Psychosom Res. 2023;171:111385. doi:10.1016/j.jpsychores.2023.111385
54. Wang Y, Yao Y, Hu J, Lin Y, Cai C, Zhao Y. Development of a Predictive Nomogram for Estimating Medication Nonadherence in Hemodialysis Patients. Med Sci Monit. 2022;28:e934482. doi:10.12659/MSM.934482
55. QiMuge N, Fang X, Chang B, Li DM, Li Y. Predicting population: development and validation of a new predictive nomogram for evaluating medication nonadherence risk in a type 2 diabetes. PeerJ. 2022;10:e13102. doi:10.7717/peerj.13102
56. Liu Z, Ge R, Yang T, et al. Development of a nomogram to predict medication nonadherence risk in patients with rheumatoid arthritis. Am J Transl Res. 2022;14(12):9057–9065.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
