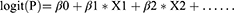Back to Journals » International Journal of Women's Health » Volume 15
A Predictive Model for Endometrial Carcinoma Based on Hysteroscopic Data
Authors Wu H, Chen Q , Liu Y, Tang Y, Zhao Y, Zhang X, Chen X, Ying X, Xu B
Received 8 June 2023
Accepted for publication 7 October 2023
Published 30 October 2023 Volume 2023:15 Pages 1651—1659
DOI https://doi.org/10.2147/IJWH.S416864
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Hao Wu,1,2,* Qianyu Chen,2,3,* Yanxin Liu,4 Yingdan Tang,5 Yang Zhao,5 Xueying Zhang,6 Xun Chen,2 Xiaoyan Ying,1 Boqun Xu1
1Department of Obstetrics and Gynecology, the Second Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 2Department of Obstetrics and Gynecology, the Affiliated Sir Run Run Hospital of Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 3Department of Obstetrics and Gynecology, Jiangsu Province Hospital of Chinese Medicine, Nanjing, Jiangsu, People’s Republic of China; 4Department of Obstetrics and Gynecology, Pukou Branch of Jiangsu People’s Hospital, Nanjing, Jiangsu, People’s Republic of China; 5Department of Statistics, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 6Department of Obstetrics and Gynecology, the Affiliated Jiangning Hospital of Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Boqun Xu, Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Nanjing Medical University, 121 Jiangjiayuan Road, Nanjing, Jiangsu, 210000, People’s Republic of China, Tel +86-13805164016, Email [email protected]
Objective: The purpose is to establish a model to predict endometrial carcinoma and assess its value in the preliminary diagnosis of endometrial carcinoma.
Methods: The data of 381 patients undergoing hysteroscopy were incorporated into the model, including 282 cases in the training cohort and 99 cases in the validation cohort. Significant morphological indexes were selected using the chi-square test and subjected to the binary logistic regression analysis. Besides, the scoring interval was set, and the nomogram of the prediction model was established. Model calibration curves were drawn using the data from the validation cohort. The study was approved by the Ethics Committee of the Affiliated Sir Run Run Hospital of Nanjing Medical University, and written informed consent was obtained from the patients.
Results: The sensitivity, specificity, positive predictive value, and negative predictive value of the model were 96.7%, 92.3%, 77.3%, and 99.0%, respectively. Analysis of the receiver operating characteristic curve in the training cohort showed an area under the curve of 0.984 (95% CI: 0.974– 0.995). The receiver operating characteristic curve in the validation cohort revealed an area under the curve of 0.976 (95% CI: 0.950– 1.000). The calibration curve indicated that the probability in the actual setting was consistent with that predicted by the nomogram in the training cohort.
Conclusion: Our model has high sensitivity and specificity in predicting endometrial carcinoma, and helps clinicians to make accurate diagnosis.
Keywords: hysteroscopy, endometrial carcinoma, morphology, prediction model
Introduction
Hysteroscopy is being widely applied in the diagnosis of endometrial carcinoma, due to its abilities to completely visualize the lesion in the uterine cavity.1 More accurate than vaginal ultrasound and diagnostic curettage, hysteroscopy can define an area for effective biopsy, thus improving the diagnostic rate of endometrial cancer, especially local and early-stage lesions.2,3 Hysteroscopic biopsy has become the gold standard for the diagnosis of endometrial carcinoma.4,5
As the learning curve of hysteroscopy is easy to be improved, the number of low-seniority and high-seniority hysteroscopic operators is increasing.6
Endometrial morphology under hysteroscopy is the diagnostic basis for endometrial cancer. In the clinic, the nature of endometrial lesions is preliminarily determined according to their morphological characteristics. Previous studies have analyzed the correlation between some hysteroscopic features and endometrial cancer, but no hysteroscopic features have been universalized as diagnostic criteria. Up to now, the criteria for the diagnosis of endometrial carcinoma by hysteroscopy are based on the subjective evaluation of the operator after observing the uterine cavity, and there is no objective standard based on morphology.7 Most clinicians make the diagnosis based on personal experiences, which are always heterogeneous.
In this study, clinical and hysteroscopic data were collected. Using significant indexes, a model was constructed to predict the risk of endometrial carcinoma.
Materials and Methods
Included were patients who had undergone hysteroscopy for endometrial thickening or abnormal uterine bleeding and no history of malignancy and tamoxifen administration. Patients with poor-quality hysteroscopic images were excluded. Each patient enrolled in the study had an endometrial biopsy pathology, based on which we divided all patients into two groups: one group of benign endometrial lesions (including simple hyperplasia, polypoid hyperplasia, and submucous myoma), and the other group of endometrial carcinoma.
A total of 282 patients in the Second Affiliated Hospital of Nanjing Medical University from October 2013 to October 2019 were selected for model development and internal validation. A total of 99 patients in Pukou Branch of Jiangsu People’s Hospital from January 2020 to March 2021 were selected for external validation.
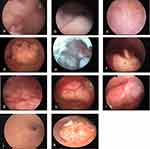
Two experienced physicians blind to the patient’s medical history and histological diagnosis analyzed the hysteroscopic images of each patient to record the following morphological characteristics: endometrial thickening (widespread or localized),8,9 polyposis (multiple or single),10–12 irregular branched vessels,13,14 irregular distribution of vessels,15 irregular vascular diameters (dilation, stenosis or interruption),14,16 abnormal endometrial color,13,17 increased brittleness of tissue (worse fragility),12,18 dilated glandular orifices,19 and calcification13 (shown in Figure 1). The negative result was given 0 and the positive result 1. These morphological indexes were recorded to assess the risk of endometrial carcinoma.
Descriptive statistics were reported as frequencies and proportions for categorical variables and means and SDs for continuous variables. t-test was used to assess the differences between means and χ²-test to proportions. Variables clinically relevant for endometrial carcinoma by χ²-test from the training cohort were put into the binary logistic regression model, and a backward stepwise selection was performed. The final model takes the form as
where β is the regression coefficient obtained from the logistic model and X is the reported value of the covariate showing association in binary regression. A nomogram based on the final model was constructed from the overall data of the training cohort. The formula for calculating the probability of endometrial cancer in the nomogram was calculated with the R software package nomogramEX.
We used SPSS to calculate the area under the receiver-operating curve (AUC) and 95% CIs to assess the predictive ability of the nomogram model for endometrial carcinoma. We generated 1000 bootstrap samples and created calibration curves to evaluate the agreement between nomogram-predicted and actually observed probabilities in the training cohort. We applied the predictive model to the dataset from Pukou Branch of Jiangsu People’s Hospital for external validation. All the statistical analyses were performed with R 4.0.5 (http://www.r-project.org) and SPSS version 25 (IBM, Armonk, NY, USA), and a p value of less than 0.05 was considered to indicate statistical significance.
Statement of Ethics: This study was approved by the Ethics Committee of the Second Affiliated Hospital of the Nanjing Medical University ([2022]-KY-001-01) and the Ethics Committee of the Affiliated Sir Run Run Hospital of Nanjing Medical University (2021-SR-S036). The objectives of the trial were explained to patients who were scheduled to undergo hysteroscopy, and their written informed consent was obtained. The subjects were free to discontinue their participation at any time. All personal data were treated confidentially and only reported in collective form.
Results
A total of 282 patients undergoing hysteroscopy in the Second Affiliated Hospital of Nanjing Medical University were enrolled for modeling, including 222 cases of benign endometrial lesions (105 cases of simple hyperplasia, 107 cases of polypoid hyperplasia, and 10 cases of submucous myoma), and 60 cases of endometrial carcinoma. The age was 61.23 ± 10.9 years in the endometrial carcinoma group and 43.10 ± 11 years in the benign endometrial lesion group, with statistical difference (p < 0.001) (Table 1). The data of 99 cases receiving hysteroscopy in Pukou Branch of Jiangsu People’s Hospital were used to verify the accuracy of the prediction model, including 80 cases of benign endometrial lesions (46 cases of polypoid hyperplasia, 29 cases of simple hyperplasia, and 5 cases of submucous myoma), and 19 cases of endometrial carcinoma. The age was 61.06 ± 13.5 years in the endometrial carcinoma group, and 48.64 ± 11.9 years in the benign endometrial lesion group, with statistical difference (p < 0.001) (Table 2).
 |
Table 1 Age and Endometrial Histological Results of Patients in the Second Affiliated Hospital of Nanjing Medical University |
 |
Table 2 Age and Endometrial Histological Results of Patients in Pukou Branch of Jiangsu People’s Hospital |
Compared with benign endometrial lesions, the proportion of patients with widespread endometrial thickening, multiple endometrial polyps, abnormal endometrial color, irregular branched vessels, irregular distribution of vessels, irregular vascular diameter, increased brittleness of tissue, calcification and dilated glandular orifices was significantly higher in the endometrial cancer group (p < 0.001). The rate of localized endometrial thickening was higher in the benign endometrial lesion group than in the endometrial carcinoma group (p < 0.001), while the rate of endometrial polyps (single polyps) showed no significant difference (Table 3).
 |
Table 3 Descriptive Analysis and Chi-Square Test for Hysteroscopy-Obtained Morphological Parameters |
Binary logistic regression analysis showed that the independent risk factors for endometrial carcinoma were abnormal endometrial color, irregular branched vessels, irregular distribution of vessels, irregular vascular diameter, and increased brittleness of tissue (Table 4). Based on the final model, we generated a nomogram by assigning a weighted score to each of the factors associated with endometrial carcinoma (shown in Figure 2). The total score of the nomogram was calculated as: 50.62242 (irregular branched vessels) + 74.96612 (irregular distribution of vessels) + 67.49865 (irregular vascular diameters) + 63.43083 (calcification) + 100 (increased brittleness of tissue) + 40.87858 (widespread endometrial thickening) + 46.90257 (irregular endometrial color). Risk of endometrial carcinoma was calculated as:  .
.
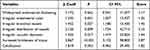 |
Table 4 Stepwise Binomial Logistic Regression Analysis of Main Variables and Their Scores |
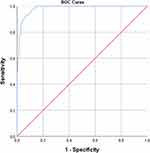
We performed a receiver operator characteristic curve (ROC curve) analysis of this prediction model, which showed that the area under the curve was 0.984 (95% CI: 0.974–0.995), and the Jordan index was 0.89 with a sensitivity of 96.7%, a specificity of 92.3%, a PPV of 77.3%, and an NPV of 99.0% (shown in Figure 3).
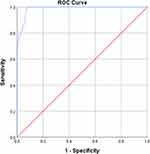
The model was externally validated. The receiver operating characteristic curve from the validation cohort showed that the area under the curve was 0.976 (95% CI: 0.950–1.000), indicating that the model had a good fit (shown in Figure 4).
The calibration plots (apparent and bias-corrected) overlapped with the ideal lines in the training and validation cohorts, showing a good agreement between the nomogram predictions with the actual observations (shown in Figure 5).
Discussion
Hysteroscopic biopsy has become the gold standard for the diagnosis of endometrial cancer. However, endometrial carcinoma shows evident morphological heterogeneity, which often leads to empirical diagnosis. Therefore, objective criteria are needed to standardize the diagnosis.
In this study, we reviewed relative literature and filtered out a number of morphological features that are closely related to endometrial cancer, including endometrial thickening (widespread or localized), polyposis (multiple or single), irregular branched vessels, irregular distribution of vessels, irregular vascular diameters (dilation, stenosis or interruption), abnormal endometrial color, increased brittleness of tissue (worse fragility), dilated glandular orifices, and calcification, etc.12,13,18,20 Subsequently, through binary logistic regression analysis, we determined predictive factors for endometrial cancer, including widespread endometrial thickening, abnormal endometrial color, irregular branched vessels, irregular distribution of vessels, irregular vascular diameters, increased brittleness of tissue, and calcification. The diagnosis was made based on the cutoff value obtained from the ROC curve and the total characteristic score of all significant indexes. The cutoff had high sensitivity and specificity, indicating its high value in the diagnosis of endometrial carcinoma. The negative predictive value of 99.0% indicated the low rate of missed diagnosis by this model. However, the positive predictive value of 74.7% was low, indicating a certain rate of misdiagnosis. In this retrospective study, tissue fragility, with a higher contribution to the model’s predictive power, was judged through pictures and videos. The operations, such as exploration before hysteroscopy and dilation of uterus, can damage the intrauterine tissue, resulting in wrong judgment by the researcher. Reducing intrauterine manipulations before hysteroscopy, researchers can judge tissue fragility in a real setting, which may increase the positive rate of the prediction model.
Lanieri et al have created a scoring system for hysteroscopic diagnosis of endometrial hyperplasia and adenocarcinoma, using eight variables: atypical vessels, widespread and irregular endometrial thickening, dilated glandular orifices, crumbling of the endometrial neoplasm, multiple endometrial polyps, irregular aspect of the polyp, growth of cerebroid and arborescent aspects, and irregular endometrial color.20 The system achieved a sensitivity of 95.4%, a specificity of 98.2%, a PPV of 85.7% and an NPV of 99.5%, all basically consistent with those in our study. Some of these variables were not used in our study. In our study, we observed that malignant tumors had rich blood vessels. The positive rates of three manifestations of atypical blood vessels (irregular branched vessels, irregular distribution of vessels, and irregular vascular diameter) were significantly higher than those in the benign lesion group. The values of three atypical vessels were assigned to the prediction model of our study. It was found that the rates of multiple polyps and dilated glandular orifices were significantly higher in the endometrial carcinoma group, but both features were also observed in the benign lesion group. Therefore, both were not scored in the logistic regression analysis and incorporated into the model. Our model showed the ability to distinguish benign lesions from multiple polyps but with less angiogenesis.
In another study by Dueholm et al,13 a scoring system was established for assessing the risk of endometrial carcinoma based on endometrial surface contour changes, evidence of necrosis, and vessel pattern, achieving a sensitivity of 89% and a specificity of 92%. In their study, the morphological features were divided into three major categories, and each category was further subdivided. For example, abnormal blood vessels were subdivided, as we did in the present study. However, their system was used only for predicting endometrial cancer in women with postmenopausal bleeding women, not all the women with non-menopause.
In the present study, initial analysis showed that age was associated with endometrial carcinoma probability. The ROC curve analysis of age showed that the probability increased in patients over 51 years old. However, once age is included in the regression analysis, its weight was so large as to disbalance the model. As a potential independent factor for endometrial carcinoma, it was excluded from the model.
In this study, the patients were only assigned to two groups. Considering the small proportion of patients with normal endometrium or atypical endometrial hyperplasia, we did not set up related groups. Therefore, we did not evaluate the power of the model to distinguishing normal endometrium or atypical endometrial hyperplasia. To be more accurate, new models should be designed via incorporating these two groups or non-morphological clinical data, such as age, abnormal uterine bleeding and BMI.
Recently, research has focused on the genetic characteristics of tumors in order to make early diagnosis, determine prognosis and personalized medicine. It has been found that genetic mutation and epigenetic modification are the basis of endometrial carcinoma. Bartosch et al reviewed the progress of epigenetics in endometrial carcinoma in recent years, and found that epigenetic changes related to DNA methylation, histone modifications, chromatin remodeling and noncoding RNAs played an important role in the occurrence and development of endometrial carcinoma.21 Piergentili et al found that noncoding RNAs (ncRNAs) are deregulated in endometrial carcinoma, which is helpful to determine the subtypes of endometrial carcinoma. With further research, ncRNAs are expected to become biomarkers to improve risk stratification for endometrial carcinoma patients and an important tool to formulate personalized medicine.22 Novel minimally invasive approaches for the early detection of EC, including blood, uterine lavage, and cervicovaginal fluid, show promise, and novel genomic biomarkers detected in biofluid samples show the potential for early screening for endometrial cancer.23 In the future, we consider adding the biomarkers related to the epigenetics of endometrial carcinoma into the predictive model to further enhance the ability of the model for early diagnosis of endometrial carcinoma.
Conclusion
Our model has high sensitivity and specificity in diagnosing endometrial carcinoma, with a low rate of missed diagnosis. Junior clinicians perform hysteroscopic surgery under the guidance of senior clinicians. Using this predictive model can better guide clinicians to biopsy suspicious lesions in the uterine cavity, help clinicians to preliminarily judge whether there is endometrial carcinoma in morphology, and greatly reduce the missed diagnosis of patients with endometrial carcinoma. The model should also be optimized with more data and validated in the clinical environment. In the future, we will incorporate the detection of new biomarkers related to endometrial carcinoma into the predictive model, which will further improve the early screening efficiency of the predictive model.
Synopsis
This prediction model improves the accuracy of diagnosis of endometrial cancer, and overcomes clinicians’ subjectivity in judging hysteroscopic morphology.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Statement
Our study complies with the Declaration of Helsinki.
Acknowledgments
The authors gratefully acknowledge the help of the staff at Department of Obstetrics and Gynecology of the Second Affiliated Hospital of Nanjing Medical University, the Affiliated Sir Run Run Hospital of Nanjing Medical University and Pukou Branch of Jiangsu People’s Hospital for collecting hysteroscopic images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the 789 Excellent Talents Training Program of The Second Affiliated Hospital of Nanjing Medical University [789ZYRC202070208] and the Special Fund for Health Science and Technology Development in Nanjing [YKK21260].
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Bourdel N, Modaffari P, Tognazza E, et al. Does experience in hysteroscopy improve accuracy and inter-observer agreement in the management of abnormal uterine bleeding? Surg Endosc. 2016;30(12):5558–5564. doi:10.1007/s00464-016-4928-4
2. Abdelazim IA, Abdelrazak KM, Elbiaa AA, et al. Accuracy of endometrial sampling compared to conventional dilatation and curettage in women with abnormal uterine bleeding. Arch Gynecol Obstet. 2015;291(5):1121–1126. doi:10.1007/s00404-014-3523-y
3. Tsonis O, Gkrozou F, Dimitriou E, et al. Comparative retrospective study on transvaginal sonography versus office hysteroscopy in the diagnosis of endometrial pathology among different subgroups. J Obstet Gynaecol Res. 2021;47(2):669–678. doi:10.1111/jog.14580
4. Clark TJ, Voit D, Gupta JK, et al. Accuracy of hysteroscopy in the diagnosis of endometrial cancer and hyperplasia: a systematic quantitative review. JAMA. 2002;288(13):1610–1621. doi:10.1001/jama.288.13.1610
5. Litta P, Merlin F, Saccardi C, et al. Role of hysteroscopy with endometrial biopsy to rule out endometrial cancer in postmenopausal women with abnormal uterine bleeding. Maturitas. 2005;50(2):117–123. doi:10.1016/j.maturitas.2004.05.003
6. Vitale SG, Capriglione S, Zito G, et al. Management of endometrial, ovarian and cervical cancer in the elderly: current approach to a challenging condition. Arch Gynecol Obstet. 2019;299(2):299–315. doi:10.1007/s00404-018-5006-z
7. De Franciscis P, Riemma G, Schiattarella A, et al. Concordance between the hysteroscopic diagnosis of endometrial hyperplasia and histopathological examination. Diagnostics. 2019;9(4):142. doi:10.3390/diagnostics9040142
8. Miyamoto T, Abiko K, Murakami R, et al. Hysteroscopic morphological pattern reflects histological grade of endometrial cancer. J Obstet Gynaecol Res. 2019;45(8):1479–1487. doi:10.1111/jog.13998
9. Su H, Pandey D, Liu LY, et al. Pattern recognition to prognosticate endometrial cancer: the science behind the art of office hysteroscopy-a retrospective study. Int J Gynecol Cancer. 2016;26(4):705–710. doi:10.1097/IGC.0000000000000676
10. Sasaki LMP, Andrade KRC, Figueiredo A, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25(5):777–785. doi:10.1016/j.jmig.2018.02.004
11. Garuti G, Mirra M, Luerti M. Hysteroscopic view in atypical endometrial hyperplasias: a correlation with pathologic findings on hysterectomy specimens. J Minim Invasive Gynecol. 2006;13(4):325–330. doi:10.1016/j.jmig.2006.03.010
12. Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138–142. doi:10.1016/j.ejogrb.2017.07.013
13. Dueholm M, Hjorth IM, Secher P, et al. Structured hysteroscopic evaluation of endometrium in women with postmenopausal bleeding. J Minim Invasive Gynecol. 2015;22(7):1215–1224. doi:10.1016/j.jmig.2015.06.018
14. Kisu I, Banno K, Kobayashi Y, et al. Flexible hysteroscopy with narrow band imaging (NBI) for endoscopic diagnosis of malignant endometrial lesions. Int J Oncol. 2011;38(3):613–618. doi:10.3892/ijo.2011.903
15. Dotto JE, Lema B, Dotto JE, et al. Classification of microhysteroscopic images and their correlation with histologic diagnoses. J Am Assoc Gynecol Laparosc. 2003;10(2):233–246. doi:10.1016/S1074-3804(05)60305-2
16. Surico D, Vigone A, Bonvini D, et al. Narrow-band imaging in diagnosis of endometrial cancer and hyperplasia: a new option? J Minim Invasive Gynecol. 2010;17(5):620–625. doi:10.1016/j.jmig.2009.10.014
17. Valli E, Zupi E, Montevecchi L. A new hysteroscopic classification of and nomenclature for endometrial lesions. J Am Assoc Gynecol Laparosc. 1995;2(3):279–283. doi:10.1016/S1074-3804(05)80109-4
18. Cicinelli E, Tinelli R, Colafiglio G, et al. Reliability of narrow-band imaging (NBI) hysteroscopy: a comparative study. Fertil Steril. 2010;94(6):2303–2307. doi:10.1016/j.fertnstert.2009.12.083
19. Tinelli R, Surico D, Leo L, et al. Accuracy and efficacy of narrow-band imaging versus white light hysteroscopy for the diagnosis of endometrial cancer and hyperplasia: a multicenter controlled study. Menopause. 2011;18(9):1026–1029. doi:10.1097/gme.0b013e31821221cd
20. Ianieri MM, Staniscia T, Pontrelli G, et al. A new hysteroscopic risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma. J Minim Invasive Gynecol. 2016;23(5):712–718. doi:10.1016/j.jmig.2016.02.017
21. Bartosch C, Lopes JM, Jerónimo C. Epigenetics in endometrial carcinogenesis - part 2: histone modifications, chromatin remodeling and noncoding RNAs. Epigenomics. 2017;9(6):873–892. doi:10.2217/epi-2016-0167
22. Piergentili R, Zaami S, Cavaliere AF, et al. Non-coding RNAs as prognostic markers for endometrial cancer. Int J Mol Sci. 2021;22(6):3151. doi:10.3390/ijms22063151
23. Shen Y, Yang W, Liu J, et al. Minimally invasive approaches for the early detection of endometrial cancer. Mol Cancer. 2023;22(1):53. doi:10.1186/s12943-023-01757-3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.