Back to Journals » Nature and Science of Sleep » Volume 15
A Prediction Nomogram for Severe Obstructive Sleep Apnea in Snoring Patients: A Retrospective Study
Authors Teng G, Zhang R, Zhou J, Wang Y, Zhang N
Received 30 January 2023
Accepted for publication 11 April 2023
Published 17 April 2023 Volume 2023:15 Pages 231—243
DOI https://doi.org/10.2147/NSS.S406384
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sarah L Appleton
Gang Teng,1 Rui Zhang,1 Jing Zhou,1 Yuanyuan Wang,2 Nianzhi Zhang2
1Graduate School, Anhui University of Chinese Medicine, Hefei, Anhui, 230012, People’s Republic of China; 2Department of Respiratory Medicine, The First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, Anhui, 230031, People’s Republic of China
Correspondence: Nianzhi Zhang, Department of Respiratory Medicine, The First Affiliated Hospital of Anhui University of Chinese Medicine, No. 117 Meishan Road, Hefei, Anhui, 230031, People’s Republic of China, Tel/Fax +86-551-62850057, Email [email protected]
Purpose: Snoring patients, as a high-risk group for OSA, are prone to the combination of severe OSA and face serious health threats. The aim of our study was to develop and validate a nomogram to predict the occurrence of severe OSA in snorers, in order to improve the diagnosis rate and treatment rate in this population.
Patients and Methods: A training cohort of 464 snoring patients treated at our institution from May 2021 to October 2022 was divided into severe OSA and non-severe OSA groups. Univariate and multivariate logistic regression were used to identify potential predictors of severe OSA, and a nomogram model was constructed. An external hospital cohort of 210 patients was utilized as an external validation cohort to test the model. Area under the receiver operating characteristic curve, calibration curve, and decision curve analyses were used to assess the discriminatory power, calibration, and clinical utility of the nomogram, respectively.
Results: Multivariate logistic regression demonstrated that body mass index, Epworth Sleepiness Scale total score, smoking history, morning dry mouth, dream recall, and hypertension were independent predictors of severe OSA. The area under the curve (AUC) of the nomogram constructed from the above six factors is 0.820 (95% CI: 0.782– 0.857). The Hosmer-Lemeshow test showed that the model had a good fit (P = 0.972). Both the calibration curve and decision curve of the nomogram demonstrated the corresponding dominance. Moreover, external validation further confirmed the reliability of the predicted nomograms (AUC=0.805, 95% CI: 0.748– 0.862).
Conclusion: A nomogram predicting the occurrence of severe OSA in snoring patients was constructed and validated with external data for the first time, and the findings all confirmed the validity of the model. This may help to improve existing clinical decision making, especially at institutions that do not yet have devices for diagnosing OSA.
Keywords: obstructive sleep apnea, snoring, risk factor, prediction model
Introduction
Obstructive sleep apnea (OSA) is a common chronic sleep-related disorder characterized by recurrent airway obstruction.1 The disease is associated with decreased oxygen saturation, sleep disruption, and excessive daytime sleepiness, and can associated with hypertension, cerebrovascular disease, cognitive dysfunction, type 2 diabetes, and other multisystem damage.2 Typical symptoms of OSA include sleep snoring, apnea, decreased sleep quality, daytime sleepiness or sleepiness, increased nighttime urination, and other neuropsychiatric symptoms, including poor concentration, memory loss, irritability, anxiety or depression.3 In 2007, the WHO estimated that more than 100 million people worldwide were affected by OSA. However, in 2019, studies suggested that more than 936 million people worldwide are already affected by OSA, with China being the highest (more than 170 million), followed by the United States, Brazil and India.4 Awareness of OSA in the general population is significantly low, and diagnostic and therapeutic approaches are often nonexistent or inappropriate. Even in developed countries, most cases of OSA remain undiagnosed and untreated.5
Snorers have a high prevalence of OSA, but some of them are also simple snorers.6 Unfortunately, the majority of studies have failed to distinguish patients with simple snoring from those with combined OSA in an objective manner.7 And the risk of severe OSA is significantly higher than that of mild and moderate OSA, which may be related to its greater susceptibility to serious complications and vehicle accidents.8 A study by Gunes et al9 revealed structural changes and malformations in the corpus callosum of the brain in patients with severe OSA, but this change was not found in the snoring-only group and in the mild and moderate OSA groups. Lin et al found that among asthma patients with combined mild, moderate, and severe OSA, those with combined severe OSA had lower lung function parameters, such as peak expiratory flow, forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and FEV1/FVC, than those with mild or moderate OSA.10 Pedreño et al demonstrated that severe OSA impairs the latency of auditory event-related potentials because of the increased latency of the P300 component of tone burst stimuli in patients with severe OSA compared to patients with non-OSA or mild and moderate OSA, which implies a decreased speed of sound information processing in patients with severe OSA.11 Therefore, it is advisable to be more sensitive to patients with suspected severe OSA during clinical treatment, and early diagnosis and intervention can minimize their disability and mortality rates.
Currently, manual overnight Polysomnography (PSG) is the gold standard for confirming the diagnosis of OSA and determining its severity, but manual overnight PSG suffers from high equipment and environmental requirements, complex analytical techniques, and high costs, making it difficult to meet the clinical needs of screening and diagnosis of the large OSA population.12 With the continuous updating of monitoring equipment and accumulation of clinical practice, the results of a large number of studies have shown that portable monitoring (PM) has a high sensitivity and specificity in the diagnosis of OSA under the premise of rational application, and plays an essential role in the current OSA diagnosis and treatment.13 For patients with a high likelihood of OSA and no serious comorbidities, PM is not inferior to laboratory PSG.14
The discomfort of wearing and the cumbersome process of both PSG and PM have caused a lot of inconvenience to physicians and patients. Therefore, it is urgent to find easy ways to identify OSA, especially patients with severe OSA should be identified in time to significantly reduce the danger of complications caused by the disease, reduce the burden on families, and save medical resources. Based on this, in the present study, we attempted to explore the potential risk factors for the occurrence of severe OSA in snoring patients and to construct a clinically feasible nomogram to improve the diagnosis of severe OSA, followed by timely intervention to reduce the harm caused by it as much as possible.
Materials and Methods
Study Design and Population
In this retrospective study, patients who referred to the Department of Respiratory Medicine at the First Affiliated Hospital of Anhui University of Chinese Medicine for snoring between May 2021 and October 2022 and underwent overnight PM served as the training cohort for this study. Data for the validation cohort of 210 cases were obtained from the Affiliated Hospital of Hangzhou Normal University. Patients were excluded based on the following criteria: (a) age below 18 years; (b) combination of severe underlying disease (eg, acute heart failure, respiratory failure, or patients requiring oxygen for other conditions); (c) incomplete clinical information; (d) previous diagnosis of OSA; (e) sleep monitoring results of central sleep apnea.
Sleep Evaluation
All participants are required to use an Australian ApneaLink Air Class III portable sleep monitor for overnight sleep breathing monitoring, and the main contents recorded included: chest and abdominal movements, oral and nasal airflow, snoring, oxygen saturation, heart rate and body position. According to the American Academy of Sleep Medicine (AASM) 2012 guidelines, sleep apnea is defined as a complete cessation of airflow during sleep, or a reduction in airflow of more than 90% for ≥10 seconds. Hypoventilation was defined as a ≥30% decrease in nasal and oral airflow during sleep from baseline levels for ≥10 s, accompanied by a ≥3% decrease in oxygen saturation or microarousals.15 The degree of the condition was judged according to the sleep apnea hypoventilation index (AHI): simple snoring (AHI < 5 events/h), mild OSA (5 events/h ≤ AHI ≤ 15 events/h), moderate OSA (15 events/h < AHI ≤ 30 events/h), and severe (AHI > 30 events/h).
Data Collection
Data collected from patients who met the inclusion criteria included general information (sex, age, height, weight, history of smoking, history of alcohol consumption, where BMI was calculated by dividing weight by the square of height), clinical manifestations common to OSA (dizziness, chest tightness, dry mouth in the morning, dream recall), and comorbid underlying disease conditions (hypertension, diabetes, gastroesophageal reflux disease (GERD)). Among them, dream recall refers to being able to recall dreams at least 5 mornings a week.16,17 In addition, patients were asked to complete the Epworth Sleepiness Scale, which scores patients on the likelihood of falling asleep in eight different situations. A score of 0 (never drowsy), 1 (mildly likely), 2 (moderately likely), and 3 (very likely), with a total score of 0 to 24, meaning no daytime sleepiness to extreme sleepiness, and a score of >10 indicating excessive daytime sleepiness.18
Development and Assessment of the Nomogram
Single-factor regression analysis was employed to screen the influencing factors for the occurrence of severe OSA in snoring patients (P < 0.05 was considered significant), and then multi-factor logistic regression analysis was performed to analyze the screened influencing factors, and finally the independent influencing factors (P < 0.05) were selected to develop a clinical prediction model and create the corresponding nomogram. The Hosmer-Lemeshow test was conducted to determine the fit of the model (P >0.05 indicates a good scoring model), and the AUC and consistency index (C-index) were used to evaluate the discrimination of the model, and the calibration curve and DCA were applied to assess the calibration and the net clinical benefit of the model, respectively.
Statistical Analysis
The descriptive analyses were performed using SPSS version 23.0 for Windows (IBM, Chicago, IL, USA). Information on numerical variables conforming to a normal distribution was expressed using the mean ± standard deviation and compared using the student’ t-test. Skewed distributions were then expressed as median (M) and interquartile range (P25-P75) using the nonparametric Mann–Whitney U. Count data were expressed as ratios (%) using chi-square tests. The nomograms, fit, discrimination, calibration, and clinical decision curves of the model were then statistically analyzed using the packages “rms”, “pROC”, “ggplot2”, and “rmda” in the R version 4.2.1 software (http://www.r-project.org/). All statistics were tested using a two-sided test, and differences were considered statistically significant at P<0.05.
Results
General Characteristics
The clinical data of the 515 patients in the training cohort were collected from the First Affiliated Hospital of Anhui University of Chinese Medicine. A total of 51 did not meet the inclusion criteria because 7 were younger than 18 years old, 14 had severe combined underlying diseases (mainly acute heart failure, end-stage malignancy, liver failure, etc.), 16 had incomplete clinical data (missing information on age, height, weight, ESS questionnaire.), 6 were previously diagnosed with OSA, and 8 others were finally diagnosed with CSA. Therefore, 464 patients were included in the final training cohort (Figure 1). The mean BMI in the training group was 27.3 (24.4, 29.7) kg/m2 and the mean ESS was 5.0 (3.0, 9.0) points. 42% of the patients had a history of smoking. The proportion of those presenting with morning dry mouth and dream recall was 29.1% and 18.5%, respectively. The percentage of those with combined hypertension was 43.8% (Table 1). The 210 patients in the external validation group were obtained from the Affiliated Hospital of Hangzhou Normal University. Their mean BMI was 26.4 (24.2, 28.4) kg/m2 and mean ESS was 4.5 (1.0, 6.0) points. The smokers accounted for 41.4%. The rates of people with symptoms of dry mouth in the morning and dream recall at night were 58.6% and 33.8%, respectively. Combined hypertension was 41.0% (Table 1).
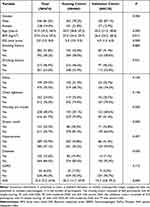 |
Table 1 Baseline Clinical Features of the Study Participants [M (P25, P75)/N (%)] |
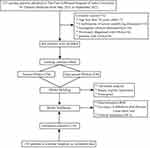 |
Figure 1 Process diagram for patient selection. Abbreviations: OSA, obstructive sleep apnea; ROC, receiver operating characteristic curve; DCA, decision curve analysis. |
According to the AHI, 234 (50.4%) of the training group were included in the severe OSA group and 230 (49.6%) were included in the non-severe OSA group. 60 (26.1%) of the non-severe OSA group had simple snoring, 75 (32.6%) had mild OSA, and 95 (41.3%) had moderate OSA. Compared with the non-severe OSA group, the severe OSA group was characterized by a larger proportion of males, higher BMI and ESS scores, a higher existence of a history of smoking and alcohol consumption, a greater tendency to have dry mouth in the morning and dream recall, and often combined with a higher prevalence of hypertension (P<0.05).However, there were no significant differences between the two groups in terms of age, dizziness, chest stuffiness, diabetes and GERD, and a total of 14 relevant factors were incorporated (Table 2).
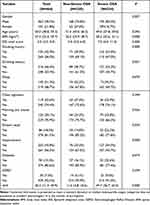 |
Table 2 General Characteristics of Patients in the Training Cohort |
Screening Predictors for the Nomogram Construction
Predictors that were statistically significant in the univariate analysis were subjected to multiple covariance testing to avoid interference of covariance between variables. The results demonstrated the absence of covariance between the variables (Tolerance > 0.1, VIF < 5). These predictors were then entered into a binary logistic regression analysis, and the results suggested that BMI (P=0.000, odds ratio [OR]=1.158, 95% confidence interval [CI]: 1.093–1.226), ESS total score (P=0.000, OR=1.268, 95% CI: 1.192–1.349), Smoking (P=0.000, OR=1.268, 95% CI: 1.192–1.349), Morning dry mouth (P=0.049, OR=1.633, 95% CI: 1.002–2.663), Dream recall (P=0.020, OR=2.228, 95% CI: 1.426–3.482), and Hypertension (P=0.000, OR=2.394, 95% CI: 1.533–3.740) (Table 3) were independent predictors of severe OSA. Consequently, a nomogram with six predictors was developed (Figure 2). For each patient, a high total score means that snorers are at high risk of suffering from severe OSA.
 |
Table 3 Covariance Diagnosis and Binary Logistic Regression Analysis of Screening Predictors |
Assessment of the Discrimination, Accuracy and Clinical Utility of the Nomogram
In the training cohort, the AUC is 0.820 (Figure 3A) and the calibration curve is close to the ideal diagonal (Figure 4A). The Hosmer-Lemeshow test also showed a good fit of the model (P=0.972). Besides, the DCA indicated that the model has superior overall net benefits (Figure 5A).
 |
Figure 3 ROC curves. (A) Training cohort. (B) Validation cohort. ROC=receiver operating characteristic; AUC=area under the ROC curve. |
 |
Figure 4 Calibration curves predicting the probability of severe OSA in snoring patients. (A) Training cohort. (B) Validation cohort. |
 |
Figure 5 Decision curve analysis for predicting the occurrence of severe OSA in snoring patients. (A) Training cohort. (B) Validation cohort. |
Additionally, external validation of the nomogram using 210 patients from other hospitals. The nomogram also presented good discrimination (AUC: 0.805) (Figure 3B) and calibration in the external validation set (Figure 4B). DCA likewise demonstrated that when decisions are made through nomograms, there is still a net benefit to be gained (Figure 5B).
Discussion
Our study utilized the clinical data collected to develop the first model capable of predicting the development of severe OSA in snoring patients. This study revealed that BMI, ESS total score, smoking history, morning dry mouth, dream recall, and hypertension were risk factors for the incidence of severe OSA in snoring patients. The results of our study will help to assess the risk of severe OSA in snoring patients and help clinicians to identify such high-risk patients early. Especially in areas where medical care is scarce, patients can be referred for definitive diagnosis and timely treatment.
As we know, obesity has become a global public health problem, with approximately 46% of adults and 15% of children in China being obese or overweight.19 Obesity leads to increased upper airway resistance, heavier respiratory system, and reduced respiratory center drive which predisposes to OSA.20 In a study of 251 patients diagnosed with OSA by PSG, Rezaie21 found that the severe OSA population showed higher BMI, increased respiratory impairment scores, older age, and lower oxygen saturation compared to patients with mild to severe OSA. A prospective cohort study by Peppard,22 involving 690 participants showed that a 10% weight gain predicted an increase in AHI of approximately 32% (95% CI: 20–45%) and a 10% weight loss predicted a 26% (95% CI: 18–34%) decrease in AHI relative to those with more stable weight. In our study BMI was significantly higher in the severe OSA group than in the non-severe OSA group, and BMI, as an independent risk factor for severe OSA, would increase the fractional risk of developing severe OSA by 1.158 times for each unit of increase.
As the most common complication of OSA patients, daytime sleepiness is the tendency of patients to experience involuntary weakness and sleepiness during the day, which can cause deterioration of mental and cognitive functions in severe cases, thus reducing the ability to learn and work, quality of life, and even increasing the risk of work and traffic accidents.23 While, The Epworth Sleepiness Scale (ESS) is one of the commonly used methods to screen for OSA. It is a comprehensive assessment of a patient’s degree of sleepiness in different daytime settings to predict the likelihood of OSA. The ESS is widely used in clinical practice because of its simplicity and ease of use, and an ESS score of >10 is used as a criterion to determine the likelihood of OSA.24 It can be said that the more severe the OSA, the higher the prevalence of excessive sleepiness.25 According to Shao,26 the ESS score was significantly higher in the severe OSA group (8.75±4.82) than in the mild and moderate groups (6.13±4.27 and 6.75±3.46, respectively; P<0.001). This is generally consistent with the results of our current study. Therefore, it is reasonable to assume that the ESS score of OSA patients correlates well with the severity of the disease and can be used as its predictor. This may be related to the fact that OSA is characterized by frequent apnea and hypoventilation during nighttime sleep and the resulting increase in the number of patient awakenings or microarousals, which affects sleep quality. Therefore, the ESS score, which assesses the degree of daytime sleepiness, can visualize the quality of sleep and thus the severity of OSA.27
As early as 1988, Bloom28 found that smokers were more likely to snore. In addition to active smoking, passive smoking and previous smoking are associated with snoring.29 And snoring is considered to be a common symptom and preclinical form of OSA. A series of studies is continuing to confirm the strong correlation between smoking and OSA severity. The severity of smoking status and nicotine dependence were found to be higher in patients with more severe OSA.30 Kim31 found that moderate to severe OSA was more common in smokers. In addition, the duration of smoking was significantly associated with the severity of OSA.Bielicki32 assessed the effect of smoking status on AHI through 3613 patients with OSA, similarly suggesting that smokers had higher AHI, lower MSaO2, and higher ESS scores compared to nonsmokers. And a recent study by Yosunkaya33 again suggested that patients with severe OSA are often accompanied by smoking and that AHI is positively correlated with the amount of smoking. In addition, smoking is likewise associated with lower oxygen saturation and reduced deep sleep at night in patients with OSA. Our study likewise showed that smoking was an independent predictor of the occurrence of severe OSA in snoring patients and was included in the prediction model. Patients with a history of smoking have a correspondingly higher score on the nomogram, correlating to a higher probability of developing severe OSA. This may be related to the effects of smoking on OSA through various mechanisms, including sleep architecture, upper airway neuromuscular function, arousal mechanisms, and enhanced upper airway inflammation.34
Dry mouth in the morning was also an independent risk factor for severe OSA and was similarly included in our prediction model. Dry mouth in the morning is the main discomfort symptom when patients visit the clinic, mainly caused by prolonged open-mouth breathing, which can be well distinguished from dry mouth caused by other diseases such as diabetes, uremia and dry syndrome. Studies have shown that the incidence of dry mouth upon waking is fully two times higher in OSA patients than in primary snorers, and increases linearly in mild, moderate and severe OSA, respectively.35 The higher the AHI, the more pronounced the symptoms corresponding to dry mouth. Not only that, but as OSA becomes more severe, the patient’s saliva volume decreases significantly and the saliva flow rate gradually slows down, but the acidity of the saliva increases instead.36 Therefore, dry mouth symptoms should also be considered as an important indicator for OSA screening and diagnosis.37
Dreams are a special state of consciousness characterized by sensory, cognitive and emotional experiences that arise during sleep and represent the mental-psychological activity during sleep.38 The International Classification of Sleep Disorders, 3rd edition, also does not classify dream recall separately, and there is still a lack of unified and perfect diagnostic criteria.39 However, patients with the main complaint of dream recall are often accompanied by symptoms such as post-waking fatigue and daytime sleepiness, which seriously affect normal work and study. Patients presenting with sleep apnea are more likely to report dreams, which may have negative content compared to normal individuals. And this is more pronounced in patients with severe OSA.40 OSA itself leads to sleep fragmentation features as well as prolonged light sleep and repetitive awakenings, which may increase the chances of patients becoming aware of dreams and increase dream recall, leading to excessive dreaming in patients with OSA.41 And hypoxia and sleep fragmentation may be associated with short-term memory cognitive dysfunction, which would tend to worsen dream recall.42
OSA is a long-standing disease in which early and repeated oxidative stress injury and pro-inflammatory release lead to systemic and local inflammation, sympathetic nervous system excitation, activation of the renin-angiotensin system, and aldosterone hypersecretion. The consequences are endothelial dysfunction, arterial constriction, arterial stiffness, and water and sodium retention, which are also high-risk causative factors for hypertension.43 OSA is comorbid in more than 30% of hypertensive patients, and its prevalence is as high as 80% in patients with resistant hypertension.44 In a cross-sectional, prospective study of 205 patients, Dashzeveg45 found that sOSA (30 < AHI < 60, severe group) and vsOSA (AHI > 60, very severe group) were predominantly composed of obese young men and mostly combined with hypertensive disease, and concluded that the severity of OSA was positively correlated with hypertension and BMI. It has also been shown that the magnitude of hypertension is linearly correlated with the severity of OSA, but confounding factors of obesity are not.46 The proportion of severe OSA with comorbid hypertension in our study was as much as 54.3%, significantly higher than the 33% in the non-severe group (P < 0.000).Similarly, studies have indicated that diastolic hypertension is considered a strong predictor of severe OSA in the sleep clinic population.47 And as a very influential predictor in this model, having hypertension was 2.394 folds higher than the fractional risk of occurring severe OSA without hypertension.
In the current study, we evaluated predictors of the onset of severe OSA in snoring patients and developed a risk prediction model. We also validated the model’s discrimination, accuracy, and net clinical benefit using external data, and the results all highlighted the significant advantages of the model. The visualization of the nomogram constructed will not only facilitate the generalization of this model, but will also simplify the screening of severe OSA, which is expected to improve the current low diagnosis rate of severe OSA. However, there are some limitations to our study. First, even though the sample size of the training and validation sets met the requirements of the study, it is still necessary to further expand the sample size and enhance the persuasiveness of the model in subsequent in-depth studies in order to improve the accuracy of the model. Second, in this included population, we observed a higher prevalence of patients with severe OSA compared with other groups, which may affect the accuracy of the nomogram. Finally, the selection of external validation data can avoid data heterogeneity to some extent, but both cohort populations belong to East China, which may have some regional limitations.
Conclusions
In conclusion, in this study, we found that BMI, ESS total score, smoking history, morning dry mouth, dream recall, and hypertension were independent risk factors for the incidence of severe OSA in snoring patients. Based on these predictors, we built a predictive nomogram for early prediction in this population. The external validation results similarly confirmed the relative high performance of the model. For each patient, a higher total score reflects a greater risk of presenting with severe OSA in snoring patients. The visualization of predictors and the personalized model provide clinicians with a simple and intuitive tool for early detection and identification of snoring patients at risk of serious OSA, which is extremely important for clinical improvement of the diagnosis of severe OSA for early intervention and treatment to reduce serious cardiovascular and cerebrovascular complications and traffic accidents that may result from severe OSA.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Ethical Statement
The study protocol was reviewed and approved by the Medical Ethics Committee of the First Affiliated Hospital of Anhui University of Chinese Medicine, and the procedures followed were in accordance with the Declaration of Helsinki. The requirement of informed consent was waived because patient information was extracted from the electronic medical records of the sleep center and the patient’s identity was anonymous. At the same time, all researchers maintain confidentiality of patient data in accordance with the Declaration of Helsinki.
Acknowledgments
We are grateful to the Affiliated Hospital of Hangzhou Normal University for providing the external validation data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; have drafted or written, or substantially revised or critically reviewed the article; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors have no conflicts of interest to disclose.
References
1. Veasey SC, Rosen IM. Obstructive sleep apnea in adults. N Engl J Med. 2019;380(15):1442–1449. doi:10.1056/NEJMcp1816152
2. Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323(14):1389–1400. doi:10.1001/jama.2020.3514
3. Trzepizur W, Blanchard M, Ganem T, et al. Sleep apnea-specific hypoxic burden, symptom subtypes, and risk of cardiovascular events and all-cause mortality. Am J Respir Crit Care Med. 2022;205(1):108–117. doi:10.1164/rccm.202105-1274OC
4. Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respirat Med. 2019;7(8):687–698. doi:10.1016/S2213-2600(19
5. Zhang Y, Ren R, Lei F, et al. Worldwide and regional prevalence rates of co-occurrence of insomnia and insomnia symptoms with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2019;45:1–17. doi:10.1016/j.smrv.2019.01.004
6. Deary V, Ellis JG, Wilson JA, Coulter C, Barclay NL. Simple snoring: not quite so simple after all? Sleep Med Rev. 2014;18(6):453–462. doi:10.1016/j.smrv.2014.04.006
7. Taylor C, Kline CE, Rice TB, Duan C, Newman AB, Barinas-Mitchell E. Snoring severity is associated with carotid vascular remodeling in young adults with overweight and obesity. Sleep Health. 2021;7(2):161–167. doi:10.1016/j.sleh.2020.12.004
8. Bosi M, Incerti Parenti S, Sanna A, Plazzi G, De Vito A, Alessandri-Bonetti G. Non-continuous positive airway pressure treatment options in obstructive sleep apnoea: a pathophysiological perspective. Sleep Med Rev. 2021;60:101521. doi:10.1016/j.smrv.2021.101521
9. Gunes A, Sigirli D, Ercan I, Ozdemir ST, Durmus Y, Yildiz T. Evaluation of the corpus callosum shape in patients with obstructive sleep apnea. Sleep Breath. 2022;26(3):1201–1207. doi:10.1007/s11325-021-02502-0
10. Lin J-L, Feng X-K, Zhang D-M, Sun H-Y. Clinical features and risk factors in patients with asthma complicated with obstructive sleep apnea-hypopnea syndrome: a hospital-based study. Sleep Breath. 2021;25(1):339–345. doi:10.1007/s11325-020-02127-9
11. Pedreño RM, Matsumura E, Silva LAF, et al. Influence of obstructive sleep apnea on auditory event-related potentials. Sleep Breath. 2022;26(1):315–323. doi:10.1007/s11325-021-02406-z
12. Kukwa W, Migacz E, Lis T, Ishman SL. The effect of in-lab polysomnography and home sleep polygraphy on sleep position. Sleep Breath. 2021;25(1):251–255. doi:10.1007/s11325-020-02099-w
13. Cooksey JA, Balachandran JS. Portable monitoring for the diagnosis of OSA. Chest. 2016;149(4):1074–1081. doi:10.1378/chest.15-1076
14. Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307–349. doi:10.5664/jcsm.6470
15. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of sleep medicine. J Clin Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.2172
16. Blain S, de la Chapelle A, Caclin A, Bidet-Caulet A, Ruby P. Dream recall frequency is associated with attention rather than with working memory abilities. J Sleep Res. 2022;31(5):e13557. doi:10.1111/jsr.13557
17. Vallat R, Nicolas A, Ruby P. Brain functional connectivity upon awakening from sleep predicts interindividual differences in dream recall frequency. Sleep. 2020;43(12). doi:10.1093/sleep/zsaa116
18. Budhiraja R, Quan SF. Sleepiness in obstructive sleep apnea using hypopneas defined by a 3% oxygen desaturation or arousal but not by 4% or greater oxygen desaturation. Sleep Breath. 2022;26(3):1135–1139. doi:10.1007/s11325-021-02494-x
19. Wang Y, Wang L, Qu W. New national data show alarming increase in obesity and noncommunicable chronic diseases in China. Eur J Clin Nutr. 2017;71(1):149–150. doi:10.1038/ejcn.2016.171
20. Goyal A, Pakhare A, Tiwari IR, Khurana A, Chaudhary P. Diagnosing obstructive sleep apnea patients with isolated nocturnal hypoventilation and defining obesity hypoventilation syndrome using new European respiratory society classification criteria: an Indian perspective. Sleep Med. 2020;66:85–91. doi:10.1016/j.sleep.2019.08.009
21. Rezaie L, Maazinezhad S, Fogelberg DJ, Khazaie H, Sadeghi-Bahmani D, Brand S. Compared to Individuals with Mild to Moderate Obstructive Sleep Apnea (OSA), Individuals with Severe OSA Had Higher BMI and respiratory-disturbance scores. Life. 2021;11(5). doi:10.3390/life11050368
22. Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi:10.1001/jama.284.23.3015
23. Javaheri S, Javaheri S. Update on persistent excessive daytime sleepiness in OSA. Chest. 2020;158(2):776–786. doi:10.1016/j.chest.2020.02.036
24. Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth sleepiness scale. Chest. 1993;103(1):30–36. doi:10.1378/chest.103.1.30
25. Bjorvatn B, Lehmann S, Gulati S, Aurlien H, Pallesen S, Saxvig IW. Prevalence of excessive sleepiness is higher whereas insomnia is lower with greater severity of obstructive sleep apnea. Sleep Breath. 2015;19(4):1387–1393. doi:10.1007/s11325-015-1155-5
26. Shao C, Jiang JB, Wu HC, Wu SB, Yu BY, Tang YD. Clinical assessment and polysomnographic study of sleep apnea in a Chinese population of snorers. J Zhejiang Univ Sci B. 2015;16(3):215–223. doi:10.1631/jzus.B1400236
27. Chiu H-Y, Chen P-Y, Chuang L-P, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70. doi:10.1016/j.smrv.2016.10.004
28. Bloom JW, Kaltenborn WT, Quan SF. Risk factors in a general population for snoring. Importance of cigarette smoking and obesity. Chest. 1988;93(4):678–683. doi:10.1378/chest.93.4.678
29. Franklin KA, Gíslason T, Omenaas E, et al. The influence of active and passive smoking on habitual snoring. Am J Respir Crit Care Med. 2004;170(7):799–803. doi:10.1164/rccm.200404-474OC
30. Ioannidou D, Kalamaras G, Kotoulas SC, Pataka A. Smoking and obstructive sleep Apnea: is there an association between these cardiometabolic risk factors?-Gender Analysis. Medicina. 2021;57(11). doi:10.3390/medicina57111137
31. Kim KS, Kim JH, Park SY, et al. Smoking induces oropharyngeal narrowing and increases the severity of obstructive sleep apnea syndrome. J Clin Sleep Med. 2012;8(4):367–374. doi:10.5664/jcsm.2024
32. Bielicki P, Trojnar A, Sobieraj P, Wąsik M. Smoking status in relation to obstructive sleep apnea severity (OSA) and cardiovascular comorbidity in patients with newly diagnosed OSA. Adv Respir Med. 2019;87(2):103–109. doi:10.5603/ARM.a2019.0011
33. Yosunkaya S, Kutlu R, Vatansev H. Effects of smokıng on patients with obstructive sleep apnea syndrome. Clin Respir J. 2021;15(2):147–153. doi:10.1111/crj.13278
34. Pataka A, Kotoulas S, Kalamaras G, et al. Does Smoking Affect OSA? What about smoking cessation? J Clin Med. 2022;11(17). doi:10.3390/jcm11175164
35. Oksenberg A, Froom P, Melamed S. Dry mouth upon awakening in obstructive sleep apnea. J Sleep Res. 2006;15(3):317–320. doi:10.1111/j.1365-2869.2006.00527.x
36. Makeeva IM, Budina TV, Turkina AY, et al. Xerostomia and hyposalivation in patients with obstructive sleep apnoea. Clin Otolaryngol. 2021;46(4):782–787. doi:10.1111/coa.13735
37. Zhang C, Shen Y, Liping F, Ma J, Wang G-F. The role of dry mouth in screening sleep apnea. Postgrad Med J. 2021;97(1147):294–298. doi:10.1136/postgradmedj-2020-137619
38. Desseilles M, Dang-Vu TT, Sterpenich V, Schwartz S. Cognitive and emotional processes during dreaming: a neuroimaging view. Conscious Cogn. 2011;20(4):998–1008. doi:10.1016/j.concog.2010.10.005
39. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. doi:10.1378/chest.14-0970
40. Carrasco E, Santamaria J, Iranzo A, et al. Changes in dreaming induced by CPAP in severe obstructive sleep apnea syndrome patients. J Sleep Res. 2006;15(4):430–436. doi:10.1111/j.1365-2869.2006.00553.x
41. Adams N, Strauss M, Schluchter M, Redline S. Relation of measures of sleep-disordered breathing to neuropsychological functioning. Am J Respir Crit Care Med. 2001;163(7):1626–1631. doi:10.1164/ajrccm.163.7.2004014
42. Rosenlicht N, Maloney T, Feinberg I. Dream report length is more dependent on arousal level than prior REM duration. Brain Res Bull. 1994;34:2. doi:10.1016/0361-9230(94)90004-3
43. Wang Y, Li CX, Lin YN, et al. The Role of Aldosterone in OSA and OSA-Related Hypertension. Front Endocrinol (Lausanne). 2021;12:801689. doi:10.3389/fendo.2021.801689
44. Thunström E, Manhem K, Rosengren A, Peker Y. Blood pressure response to losartan and continuous positive airway pressure in hypertension and obstructive sleep apnea. Am J Respir Crit Care Med. 2016;193(3):310–320. doi:10.1164/rccm.201505-0998OC
45. Dashzeveg S, Oka Y, Purevtogtokh M, et al. Obstructive sleep apnea in a clinical population: prevalence, predictive factors, and clinical characteristics of patients referred to a sleep center in Mongolia. Int J Environ Res Public Health. 2021;18(22):12032. doi:10.3390/ijerph182212032
46. Garg Y, Kakria N, Vardhan V, Katoch CDS, Singh P. Assessing the correlation between severity of obstructive sleep apnoea and systemic hypertension. J Clin Diagn Res. 2018;12(2):OC10–OC13. doi:10.7860/jcdr/2018/30608.11173
47. Jonassen TM, Bjorvatn B, Saxvig IW, Eagan TML, Lehmann S. Clinical information predicting severe obstructive sleep apnea: a cross-sectional study of patients waiting for sleep diagnostics. Respir Med. 2022;197106860. doi:10.1016/j.rmed.2022.106860
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

