Back to Journals » International Journal of Nanomedicine » Volume 19
A Pralidoxime Nanocomplex Formulation Targeting Transferrin Receptors for Reactivation of Brain Acetylcholinesterase After Exposure of Mice to an Anticholinesterase Organophosphate
Authors Pirollo KF, Moghe M, Guan M, Rait AS, Wang A, Kim SS, Chang EH , Harford JB
Received 7 October 2023
Accepted for publication 25 December 2023
Published 12 January 2024 Volume 2024:19 Pages 307—326
DOI https://doi.org/10.2147/IJN.S443498
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Kathleen F Pirollo,1 Manish Moghe,1 Miaoyin Guan,1 Antonina S Rait,1 Aibing Wang,1 Sang-Soo Kim,1,2 Esther H Chang,1 Joe B Harford2
1Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, 20057, USA; 2SynerGene Therapeutics, Inc., Potomac, MD, 20854, USA
Correspondence: Esther H Chang, Lombardi Comprehensive Cancer Center, Georgetown University, 3970 Reservoir Road N.W., Research Building E420, Washington, DC, 20057, USA, Tel +1 202 687 8418, Email [email protected]
Introduction: Organophosphates are among the deadliest of known chemicals based on their ability to inactivate acetylcholinesterase in neuromuscular junctions and synapses of the central and peripheral nervous systems. The consequent accumulation of acetylcholine can produce severe acute toxicities and death. Oxime antidotes act by reactivating acetylcholinesterase with the only such reactivator approved for use in the United States being 2-pyridine aldoxime methyl chloride (a.k.a., pralidoxime or 2-PAM). However, this compound does not cross the blood–brain barrier readily and so is limited in its ability to reactivate acetylcholinesterase in the brain.
Methods: We have developed a novel formulation of 2-PAM by encapsulating it within a nanocomplex designed to cross the blood–brain barrier via transferrin receptor-mediated transcytosis. This nanocomplex (termed scL-2PAM) has been subjected to head-to-head comparisons with unencapsulated 2-PAM in mice exposed to paraoxon, an organophosphate with anticholinesterase activity.
Results and Discussion: In mice exposed to a sublethal dose of paraoxon, scL-2PAM reduced the extent and duration of cholinergic symptoms more effectively than did unencapsulated 2-PAM. The scL-2PAM formulation was also more effective than unencapsulated 2-PAM in rescuing mice from death after exposure to otherwise-lethal levels of paraoxon. Improved survival rates in paraoxon-exposed mice were accompanied by a higher degree of reactivation of brain acetylcholinesterase.
Conclusion: Our data indicate that scL-2PAM is superior to the currently used form of 2-PAM in terms of both mitigating paraoxon toxicity in mice and reactivating acetylcholinesterase in their brains.
Keywords: lipid nanoparticle, nanodelivery, organophosphate, paraoxon, blood–brain barrier, transcytosis
Introduction
Organophosphates (OPs) are among the most toxic of chemicals1,2 with a history of use as chemical weapons.3 The extreme toxicity of OPs has led to a curtailment in their worldwide use as pesticides in agriculture, but intentional OP self-poisoning particularly in poorer rural settings has emerged as a significant global health problem.4 While reliable estimates of unintentional occupational and/or environmental exposures are difficult to obtain,5,6 it is clear that acute or repetitive sublethal OP exposures can have deleterious health consequences including long-term brain damage.2,7,8
Although toxicities vary among individual chemical compounds in the OP category, they all share a common mechanism of toxicity based in their ability to inhibit acetylcholinesterase (AChE, EC 3.1.1.7), the enzyme primarily responsible for clearing acetylcholine (ACh) from cholinergic synapses and neuromuscular junctions.4,9,10 When AChE is inhibited by OPs, the consequent accumulation of neurotransmitter results in excitotoxicity termed “cholinergic crisis” that can produce hypersecretion, tremors, convulsions, seizures, respiratory distress and death.11 Death due to respiratory failure appears to involve both ACh-triggered spasm of the diaphragm and inhibition of the brain’s respiratory control center.4,12,13 Both early death due to severe exposures to OPs14 and ventilatory effects of lower doses15 appear to be mediated by the central nervous system (CNS).
Inactivation of AChE by OPs involves nucleophilic attack on a critical serine in the active site that initially produces a reversibly inhibited enzyme. Subsequent dealkylation (termed “aging”) results in irreversible AChE inactivation. The time required for aging varies depending on the OP involved,11,16 but this interval between the initial inhibition and aging provides a window of opportunity within which AChE can be reactivated by nucleophiles capable of displacing the OP from the enzyme’s active-site.11,17,18 This ability to resurrect OP-inactivated AChE is the basis for the use of oxime antidotes like pralidoxime (a.k.a., 2-PAM) that was first synthesized more than 60 years ago.19,20 The current standard therapy for OP intoxication consists of 2-PAM together with atropine, a muscarinic ACh receptor antagonist, plus a benzodiazepine anticonvulsant (eg, diazepam).9,21 Although current countermeasures can be effective in preventing OP-caused death if administered timely, these countermeasures do not completely eliminate all long-term neurological sequelae of OP exposures22 that appear to be linked, at least in part, to OP-triggered neuroinflammation.23,24
It has long been recognized that current OP antidotes (including but not limited to 2-PAM) have significant limitations and that additional research aimed at developing improved countermeasures is warranted.12,25 Despite substantial effort in the quest for improved AChE reactivators, 2-PAM from the 1950s is still considered to be the “gold standard”,26,27 and it remains the only such drug approved for use in the United States. A major limitation of 2-PAM and other positively charged oximes is their inability to traverse efficiently the blood–brain barrier (BBB), and so considerable effort has been directed at development of BBB-penetrant reactivators that would enable reactivation of AChE within the CNS.27–30 In addition to exploring new chemical compounds as potential reactivators, attempts to improve reactivation of AChE in the brain have included creation of new formulations of older reactivators eg, encapsulation of 2-PAM within solid lipid nanoparticles.31
The brain acquires iron for metabolism by moving diferric transferrin from the blood to the brain via endothelial cell transcytosis mediated by the transferrin receptor (TfR). The hijacking of this physiological process has long been recognized as having potential to deliver molecules into the CNS that would otherwise be excluded by the BBB.32,33 We have developed a versatile nanodelivery system called scL (for single-chain Liposome) that consists of a liposome with a targeting moiety (termed TfRscFv) that is a single-chain monoclonal antibody fragment recognizing TfR. We demonstrated the utility of this delivery system in treating intracranial brain tumors34,35 and in curbing neuroinflammation triggered by bacterial lipopolysaccharide.36 The scL nanocomplex has been shown to act like a “Trojan horse” for payload delivery into the brain using nanocomplexes carrying fluorescently tagged oligonucleotides for direct visualization.35,36 This delivery of a fluorescent payload into the brain after intravenous administration of the scL nanocomplexes was dependent upon the TfRscFv targeting moiety. Similarly, the TfRscFv was shown to be crucial in delivery of an siRNA payload across the BBB with this siRNA subsequently detected within isolated neurons, astrocytes and microglial cells.36
In the current study, we leverage our experience in delivery of therapeutics to the brain by encapsulating 2-PAM within scL nanocomplexes. To assess this novel formulation of 2-PAM (termed scL-2PAM), we have utilized paraoxon as a challenge agent in mice. Paraoxon is the active metabolite of the pesticide parathion. Although less toxic than some other OPs, paraoxon is quite deadly as shown below, and it shares with other OPs their characteristic mechanism of toxicity (ie, anticholinesterase activity).10 Paraoxon-inactivated AChE can be reactivated prior to undergoing aging, and paraoxon has been widely used as a tool in development of OP countermeasures.37 In head-to-head comparisons, we show here that the scL nanocomplex formulation of 2-PAM is superior to unencapsulated 2-PAM (a.k.a. “free 2-PAM”) in preventing death of mice exposed to otherwise-lethal levels of paraoxon. The scL-2PAM formulation has also been demonstrated to be superior to free 2-PAM in ameliorating paraoxon-triggered cholinergic crisis and in reactivating and/or preserving active AChE in the brains of paraoxon-exposed mice.
Materials and Methods
Cationic lipid 1.2-dioleoyl-3-trimethylammonium propane (DOTAP) and neutral lipid dioleolylphosphatidyl ethanolamine (DOPE) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Paraoxon (diethyl p-nitrophenyl phosphate) was obtained from Santa Cruz Biotechnology (Dallas, TX, USA). The oxime 2-PAM (1-methylpyridinium-2-aldoxime chloride) was purchased from TCI Chemicals (Tokyo, Japan), and atropine sulfate (1 mg/mL) was from American Regent, INC (Shirley, NY, USA). Sterile endotoxin-free LAL reagent water was purchased from Lonza (Walkersville, MD, USA). Sucrose and Triton X-100 were obtained from Sigma Aldrich (St. Louis, MO, USA). The colorimetric AChE assay kit (ab138871) and the AChE inhibitor donepezil hydrochloride (ab120763) were from Abcam (Cambridge, MA, USA). The Pierce BCA protein assay kit was purchased from ThermoFisher (Waltham, MA, USA).
Preparation of Nanocomplexes
The cationic liposomes that are the basis of the scL nanocomplex are comprised of a mixture of DOTAP and DOPE in a 1:1 molar ratio. The oxime 2-PAM was incorporated into the liposomes at the time of their preparation with a molar ratio of lipids to 2-PAM of 1:8. Liposomes encapsulating 2-PAM (termed Lip-2PAM) were prepared by the ethanol injection method as previously described38 with a minor modification. Briefly, the two constituent lipids were solubilized in ethanol (25 mg/mL) and mixed at a 1:1 molar ratio after which 2-PAM dissolved in ethanol was added to the lipids, and the lipids plus 2-PAM injected into rapidly stirring LAL water pre-warmed to 65°C. The full scL-2PAM nanocomplex was prepared as previously described for encapsulating temozolomide (TMZ), another small-molecule payload.34 Briefly, the targeting moiety (recombinant TfRscFv) is added to liposomal 2PAM with mixing by inversion. The association of the TfRscFv with the liposomes occurs without covalent interactions. Analogous self-assembling TfR-targeting nanocomplexes have been employed over the past two decades to deliver a variety of payload types including plasmid DNA for gene therapy, siRNA to modulate gene expression and small therapeutic chemical entities.35,39,40 Once the scL-2PAM nanocomplexes are formed, their size (expressed as number-average diameter), polydispersity index, and zeta potential were determined by dynamic light scattering at 25°C using the Malvern Zetasizer Pro (Worcestershire, UK).
Assessment of Encapsulation Efficiency
The percent encapsulation of the input 2-PAM into the scL-2PAM nanocomplex was determined by separation of any unencapsulated 2-PAM from the scL-2PAM via filtration using a Vivaspin 500 centrifugal concentrator (GE Healthcare, UK) with 5000 Dalton molecular weight cut-off. This device retains the scL nanocomplex while unencapsulated 2-PAM passes through the filter. After preparation, the scL-2PAM nanocomplex was diluted with LAL water to a 2-PAM concentration of approximately 1 mM, and 200 ul of this material loaded onto the centrifugal concentrator and centrifuged at 14,000 g for 15 minutes at room temperature. The material passing through the device was collected and the filter washed with another 200 ul of LAL water with centrifugation as before. 2-PAM in the filtrate plus wash was deemed to be unencapsulated. Input 2-PAM and unencapsulated 2-PAM in the pass-through plus wash were determined by measurement of absorption at 293 nm. We have shown that the contributions to the absorption at 293 nm of the other components of scL-2PAM are negligible compared to that of 2-PAM under these conditions. Absorption at 293 nm was also employed to confirm that scL-2PAM and unencapsulated 2-PAM were equimolar in oxime when compared head-to-head.
Lyophilization and Reconstitution of Nanocomplexes
For mouse injections, 5% sucrose (final concentration) as an excipient was used for both free 2-PAM and the scL-2PAM nanocomplexes. After the preparation of scL-2PAM (in 5% sucrose), samples (2.1 mg 2-PAM/mL based on weight of 2-PAM) were flash-frozen in liquid nitrogen, and lyophilized to dryness (three to four days) using a Labconco Freeze Dryer System (Kansas City, MO, USA). Upon removal from the lyophilizer, the tubes were sealed with parafilm and routinely stored with desiccant at 4°C. For scL-2PAM stability studies, tubes of lyophilized scL-2PAM were stored (with desiccant) at either room temperature, 4°C, or −20°C for various times prior to reconstitution. To reconstitute the lyophilized scL-2PAM, sterile, endotoxin-free LAL water equivalent to the original volume was added to the lyophilized scL-2PAM. The tube was inverted 10 times to solvate the lyophilized material, and then rotated by inversion at 30 RPM for 10 minutes at room temperature on the Benchmark RotoBot™ Programmable Rotator (Benchmark Scientific, Inc, Sayreville, NJ, USA). Prior to injection and based on the body weight of individual mice, the reconstituted scL-2PAM was diluted with a sterile, endotoxin-free 5% sucrose solution to yield the required dose of 2-PAM in an injected volume of 0.25 mL. Free 2-PAM was dissolved at 100 mM in sterile, endotoxin-free 5% sucrose and further diluted in the 5% sucrose solution for injection of 0.25 mL to yield the desired dose of 2-PAM based on the body weight of individual mice. Injected doses of both scL-2PAM and free 2-PAM are expressed in terms of mg of oxime per kg body weight.
Preparation of Paraoxon
When fifty individual OP chemicals were compared as inhibitors of human AChE, a large range of values were observed in the bimolecular inhibition rate constant ki against human AChE.10 The ki for paraoxon-ethyl was reported to be 33.0 (expressed as 105 M−1min−1) ranking 25th of the fifty OPs compared. Paraoxon is an oily liquid and to obtain the working solutions of paraoxon used in our experiments, the commercially obtained material (Santa Cruz Biotechnology, Dallas, TX, USA; 1.27 g/mL) was serially diluted in extra virgin olive oil. The paraoxon and olive oil were mixed by vortex at high speed for 1 minute then rotated at 10 RPM at room temperature on the aforementioned rotator. The working stock solutions were 2.5, 1.5 or 0.25 mg/mL, and these stock solutions were used to expose mice to the desired level of paraoxon expressed either as mg/kg body weight or in terms of a multiple of the LD50 for paraoxon in the BALB/c mice used in this study.
Animal Studies
All animal experiments were approved by the Georgetown University Institutional Animal Care and Use Committee and by the Animal Care & Use Review Office (ACURO) of the US Department of Defense. All experiments were performed in the AAALAC-accredited, USDA-registered animal facility of Georgetown University adhering to the Guide for the Care and Use of Laboratory Animals as required by the Office of Laboratory Animal Welfare (OLAW). Six-week-old, female BALB/c AnNHsd mice (Harlan/Envigo, Indianapolis, IN, USA) were used in these studies. Upon arrival, the mice were allowed to acclimate to their cage environment for at least 3 days before use. Mice were housed at 20–22°C and 30–70% relative humidity at five per cage with corncob bedding in a 12-hour light/dark cycle and food/water ad libitum. The LD50 for paraoxon (the dose that yielded 50% survival in a group of mice) was determined by intraperitoneal (IP) injection of increasing doses of paraoxon into groups of mice in the same weight range (± 0.5 g) with at least 4 mice/group. For studies to assess the ability of free 2-PAM or scL-2PAM to rescue mice from the cholinergic effects of paraoxon, the mice were injected IP on the right side with the paraoxon at the indicated level. At ~1 minute after paraoxon administration, mice were injected IP on the upper left side with the oxime countermeasure (ie, either scL-2PAM or free 2-PAM). If atropine was included, the atropine was injected IP immediately after the oxime on the lower left side. Based upon the weight of individual mice, atropine was diluted in sterile, endotoxin-free 5% sucrose solution to yield a dose of 1.1 mg/kg given in an injection volume of 0.15 mL. For studies comparing the routes of administration in addition to the IP administration described above, the oximes were administered to groups of mice either intravenously (IV) or subcutaneously (SQ). For IV administration, tails of mice were dilated in warm water, and either free 2-PAM or scL-2PAM administered via the lateral vein of the tail. When administered SQ, the oximes preparations were injected under the loose skin between the shoulder blades on the dorsal side of the mouse. Irrespective of the route by which the oxime formulations were administered, paraoxon was always administered IP on the right side, and atropine when included was always administered IP on the left side of the abdomen. Survival was assessed 60 minutes after the injection of paraoxon. Longer periods of observation confirmed that 60 minutes was an appropriate time at which to score mice for survival. Reflective of the varying numbers of mice and groups in a given day’s experiment, survival results were pooled and presented as weighted means ± standard deviations (SigmaPlot, Systat Software, Chicago, IL, USA). Statistical significance was calculated using the Rank Sum Test (SigmaPlot).
Assessment of Cholinergic Crisis
The intensity of the cholinergic crisis induced by the paraoxon exposure was assigned based on signs and symptoms using a modified Racine scale: Racine score=0 (walking, bright and alert movements); Racine score=1 (lethargic movements, startled, immobile); Racine score=2 (mild tremoring, head bobbing); Racine score=3 (tremors, head bobbing, twitching, extensions of body muscles); Racine score=4 (early seizure-like activity, partial body clonus); Racine score=5 (sustained seizure-like activity, loss of posture, myoclonic jerks, behavior consistent with status epilepticus); Racine score=6 (intense seizure-like behavior, repetitive jumping or bouncing, wild running, tonic seizures); Racine score=7 (death). Mice behavior was monitored continuously for an hour from the paraoxon exposure for signs and symptoms associated with cholinergic crisis and a Racine score assigned at 30 minutes, 45 minutes and 60 minutes. For 3-hour and 6-hour timepoints, scores were assigned based on 15 minutes of observation around each of these times.
Enzyme Assays
To obtain samples for analysis of AChE activity levels in the brain, surviving animals were euthanized with an overdose of isoflurane 60 minutes after exposure to paraoxon. The brain was removed immediately, washed in ice-cold PBS, minced with surgical scissors, and flash frozen in liquid nitrogen. Subsequently, the frozen brain tissue was pulverized using a Bessman tissue pulverizer (Fisher Scientific, Waltham, MA, USA). During this process, the pulverizer, spatulas, tubes were kept on dry ice and/or in liquid nitrogen to prevent the tissue from thawing. The pulverized brain tissue, which remained frozen throughout the process, was stored at −80°C. Brains were removed immediately upon death from mice that succumbed prior to 60 minutes after paraoxon exposure, and these brains were processed for enzyme assays in the same way. To prepare brain lysates for use in the AChE assay, the samples of the pulverized frozen brain tissue were weighed without thawing and then added to ice-cold 0.1% Triton X-100 in AChE assay kit buffer (20 uL/mg of tissue). The Triton X-100 in the lysis buffer disrupts cellular membranes and renders soluble any membrane-bound AChE. The tissue was homogenized using a pellet pestle mixer (Kimble cordless motor 749,540–000, Fisher Scientific) for 10 seconds while the sample was maintained on wet ice. Each brain sample was subjected to three 10-second rounds of homogenization with a 30-second pause between each round. Subsequently, the brain homogenates were centrifuged at 17,000 g for 30 minutes at 4°C. Portions of the supernatant (lysate) were frozen in liquid nitrogen and stored at −80°C until use in the AChE assay. For these enzyme assays, the lysates were thawed on wet ice then diluted 2:5 (for mice that had been exposed to paraoxon) or 1:40 (for naïve mice with higher activity levels) with AChE assay kit buffer. The AChE activity assay was performed in duplicate using the colorimetric AChE assay kit (Abcam 138,871) as per the supplier’s protocol. This assay employs the Ellman method41 using the reduction of 5.5-dithio-bis-(2-nitrobenzoic acid) (DTNB) to quantify the thiocholine produced over time from the hydrolysis of the substrate, acetylthiocholine, by cholinesterases in the lysates. The absorption at 405 nm was measured using a Wallac 1420 Victor2 Microplate Reader (Perkin Elmer, Waltham MA, USA) to quantify the 5-thio-2-nitrobenzoate as a measure of the amount of thiocholine produced. This assay does not differentiate between AChE and butyrylcholinesterase (BChE; 3.1.1.8) both of which can hydrolyze ACh and cleave the substrate in the assay kit. Inclusion of an AChE-specific inhibitor (donepezil hydrochloride) was used to determine AChE-specific activity.42 Two portions of each sample were assayed, one with and one without donepezil hydrochloride (0.1 uM) present. The difference between the readout of the total cholinesterase (without inhibitor) and that with inhibitor present represents the AChE (donepezil-inhibitable) enzyme activity. To correct for the background color of the DTNB, the absorbance of the samples was obtained before the addition of the substrate. Absorbance readings at room temperature were collected for 10~15 minutes after the addition of DTNB, after which the substrate was added, and the absorption at 405 nm monitored for 10~15 minutes. The standard AChE enzyme included in the AChE assay kit was used to create a standard curve for each experiment using enzyme concentrations of 0, 10, 50, and 150 mU/mL. The enzyme activities per mL of the samples were calculated based upon this standard curve and are expressed as mU/mg protein with protein concentration determined using the BCA protein assay (Pierce BCA Protein Assay, ThermoFisher, Waltham, MA, USA). Results are presented as mean ± standard deviation or standard error of the mean as indicated. Statistical significance was assessed using the SigmaPlot (Systat Software, Chicago, IL, USA).
Results
Paraoxon Toxicity and Inhibition of Brain Acetylcholinesterase
To assess whether scL-2PAM represents an improved countermeasure against OP toxicity, we used a mouse model wherein BALB/c mice were exposed to paraoxon, the active metabolite of the insecticide parathion.37,43 Although individual OPs differ in their chemistries and potencies, paraoxon shares with all toxic OPs the anticholinesterase activity that can trigger cholinergic crisis and death depending on exposure levels. A survival study was performed in female BALB/c mice to determine a median lethal dose (LD50) for paraoxon after IP administration. In this experiment, all mice survived the paraoxon exposure at the dose of 2.0 mg/kg, but all mice succumbed to paraoxon poisoning at the dose of 3.5 mg/kg (Figure 1A). An LD50 of 2.5~2.6 mg/kg was observed in this experiment with the paraoxon mortality curve being relatively sharp (ie, going from 100% survival to 100% fatality over a relatively small paraoxon dose range). In subsequent experiments, a confirmation of the LD50 was routinely included using a smaller number of mice.
 |
Figure 1 The effect of exposing mice to increasing levels of paraoxon. (A) Determination of an LD50 for paraoxon (structure shown in the upper right). Paraoxon at the indicated level was injected intraperitoneally (IP) and the percentage of mice surviving recorded. An LD50 of approximately 2.5–2.6 mg/kg was observed here. Numbers by the data points indicate the number of mice represented by that data point. (B) Total cholinesterase activity was determined in lysates of whole brains derived from paraoxon-exposed mice (n=3). The apparent IC50 for paraoxon as an inhibitor of brain cholinesterase activity was ~0.8 mg/kg. The inset shows that approximately three-quarters of the total cholinesterase activity measurable in whole brain lysates from naïve mice was inhibited by donepezil. Both AChE and BChE contribute to total cholinesterase activity in the assay since both hydrolyze the substrate acetylthiocholine. AChE is selectively inhibited by donepezil.42. |
We also evaluated the effect of paraoxon exposures on the total cholinesterase activity in lysates of whole brains that were removed from surviving mice at one-hour post-exposure or immediately upon the death of a mouse. Exposure to paraoxon decreased the total cholinesterase activity in a dose-dependent manner with the half-maximal inhibitory concentration (IC50) for paraoxon of ~0.8 mg/kg (Figure 1B). The inset in Figure 1B shows that approximately three-quarters of the total cholinesterase is inhibited by donepezil, the specific inhibitor of the canonical AChE.42 The donepezil-refractory cholinesterase activity is presumed to be primarily BChE, an enzyme that is also inhibited by OPs. It is of note that the IC50 for paraoxon of ~0.8 mg/kg was considerably lower than the LD50 observed for paraoxon (~2.5 mg/kg). Comparing Figure 1A and B, one can see that at a paraoxon exposure of 2.0 mg/kg, ~90% of the total cholinesterase activity in the whole brain lysates was inhibited, while 100% of the mice were still alive. Although cholinergic symptoms begin to appear when AChE is ~50% inhibited, death from paraoxon poisoning requires >90% inhibition of the enzyme.44 A corollary of this observation is that the rescue of mice from otherwise-lethal exposures to paraoxon might be achievable if even a rather modest portion of the total AChE is reactivated or preserved. This notion is confirmed by the data presented below.
Production and Characterization of Nanocomplexes
Based on our prior use of scL nanocomplexes in brain cancer treatment,34,35 we hypothesized that an scL formulation of 2-PAM should be able to ferry an oxime AChE reactivator across the BBB where it could restore enzyme activity in the brain. We believed that by virtue of its activity in the CNS, scL-2PAM might prove to be superior to unencapsulated 2-PAM as a countermeasure, since OP-induced toxicity involves the brain’s respiratory control center.14,15 We have previously reported that when mixed in defined ratios under appropriate conditions, cationic liposomes carrying a small-molecule payload with TfRscFv used as the targeting moiety self-assemble to form a nanocomplex.34 The nanocomplex that we call scL-2PAM is schematically represented in Figure 2. The scL delivery vehicle has been demonstrated to move fluorescently tagged oligonucleotides from blood to brain in a manner dependent upon its TfRscFv targeting moiety as assessed by direct visualization of the nanocomplex’s fluorescent payload.35,36 An siRNA payload carried by intravenously administered scL nanocomplexes has been directly detected and quantified within isolated mouse brain cells.36
 |
Figure 2 Schematic representation of scL-2PAM. The immuno-lipid nanoparticle termed scL-2PAM was designed to carry its 2-PAM payload across the brain capillary endothelial cells that form the BBB via transcytosis mediated by the TfR. The scL (for single chain Liposome) nanocomplexes have been described,35,36 and the ability of these nanocomplexes to cross the BBB in a fashion dependent upon the targeting moiety of the nanocomplex has been documented.36 In this schematic representation of scL-2PAM, the oxime payload in the complex is depicted using a space-filling calotte model. The chemical structure of 2-PAM is also shown. The targeting moiety for the scL nanocomplex (represented in the schematic by the diamond shaped objects) is a recombinant single-chain antibody fragment that recognizes the TfRs on both human and murine cells. Under appropriate conditions, liposomal 2-PAM and the targeting moiety (TfRscFv) self-assemble to form the TfR-targeting nanocomplex. |
We have characterized the physical properties of the scL-2PAM preparation using a Malvern Nanosizer. The size analysis by dynamic light scattering of freshly prepared scL-2PAM routinely yields a single peak with a number-average diameter of 100–300 nm. The polydispersity index (PDI) of this preparation was routinely under 0.4 indicating a relatively homogenous population of scL-2PAM nanocomplexes. The net charge of scL-2PAM preparations indicated by their measured zeta potential was found to be in the range of +30 mV to +50 mV. The positive surface charge of the scL-2PAM iLNPs has the effect of inhibiting aggregation of nanocomplexes. Using a method based on passage through a filter with a molecular weight cut-off of 5000 Daltons, we determined that ~50% of the input 2-PAM was encapsulated into the scL-2PAM. Although we have seen encapsulation efficiencies approaching 100% for scL nanocomplexes carrying plasmid DNA payloads, an encapsulation efficiency for 2-PAM of ~50% is comparable to what we have seen for nanocomplexes carrying TMZ, a chemotherapeutic agent used for treatment of patients with brain tumors.34 It is of note that 2-PAM, like TMZ, is an approved drug currently used in its unencapsulated form. We do not anticipate that having a 50:50 mix of unencapsulated 2-PAM and scL-2PAM in a preparation will be problematic should we seek regulatory approval for the scL-2PAM as a new and improved OP countermeasure so long as the preparations are consistent from lot to lot. We have observed a relatively narrow range of encapsulation efficiencies around 50% when this parameter has been assessed in different preparations of scL-2PAM.
Reactivating Acetylcholinesterase to Relieve Cholinergic Crisis
Various cholinergic signs and symptoms that develop after exposure to OPs can be assigned a score based on their severity as depicted in Figure 3A. The Racine scale was originally conceived as a tool in epilepsy research to describe seizure intensities,45 but it has been adapted for use in describing the severity of chemically induced signs and symptoms leading to seizures and death in rats46 and mice.47 At the lower end of the Racine scale, mice with weak OP exposure become lethargic (Racine score=1). Increasing exposure to paraoxon results in tremors and convulsions, including those that resemble mice undergoing grand-mal seizures (Racine score=6) before dying (Racine score=7). It is very clear that when mice are exposed to increasing levels of paraoxon, they experience cholinergic signs and symptoms of increasing severity as would be expected (Figure 3A). With paraoxon exposure at the LD50, the exposed mice were in severe cholinergic crisis within 30 minutes, and these symptoms persisted for at least 6 hours absent intervention (Figure 3B). At one minute after the paraoxon exposures, groups of mice were administered (via IP injection) either unencapsulated 2-PAM or our scL-2PAM formulation at the dose of 25 mg/kg (in terms of the oxime). As shown in Figure 3B, cholinergic symptoms were markedly reduced by 30 minutes in mice treated with scL-2PAM but not in mice given free 2-PAM. By 45 minutes, the mice administered scL-2PAM were at Racine score=2 while those administered free 2-PAM only attained Racine score=2 at 6 hours after antidote administration. In contrast, the mice treated with scL-2PAM were fully recovered at 6 hours (Racine=0). Collectively, these data indicate that scL-2PAM is superior to free 2-PAM in lessening both the severity and duration of cholinergic symptoms triggered by paraoxon exposures.
Based on the known mechanisms of OPs and of oximes (ie, AChE inhibition and AChE reactivation, respectively), our hypothesis that a brain-penetrating formulation of 2-PAM (scL-2PAM) would be more effective than free 2-PAM that does not readily cross the BBB appears to be supported by above data. The improved ability to reduce cholinergic crisis after OP exposure is presumed to reflect an improved ability of the nanocomplex formulation of 2-PAM to reactivate AChE in the CNS. To confirm this basis for the observed superiority of scL-2PAM, we assessed whether scL-2PAM was more effective at AChE reactivation than free 2-PAM following an OP exposure. Mice were treated with a dose of paraoxon that was equivalent to 0.85x LD50 where all mice survive the OP exposure (Figure 1A), despite extensive loss of brain AChE activity assessed at one-hour post-exposure (Figure 1B). As shown in Figure 4, AChE assays confirmed that paraoxon at 0.85x LD50 decreased AChE activities in brain lysates by approximately 92% compared to activities seen in brain lysates from naïve mice (ie, those not exposed to paraoxon). At one hour after paraoxon exposure, some mice received no antidote, while other groups of mice were given injections of either free 2-PAM or scL-2PAM (both at 25 mg/kg in terms of the oxime). One hour after antidote administration (ie, at 2 hours post-exposure to paraoxon), brains were removed and AChE activity measured in whole brain lysates. Only scL-2PAM treatment resulted in a significant increase of AChE with brain lysates from mice receiving no antidote or free 2-PAM remaining low for at least 2 hours. The differences between mice receiving no antidote or free 2-PAM were not significant. AChE activity assays at a single time have difficulty distinguishing between an oxime reactivating brain AChE or merely preserving the enzyme activity (ie, preventing inactivation). Here, we first confirmed that brain enzyme activity is low at 1 hour before rising over the next hour following scL-2PAM administration. This experiment demonstrates scL-2PAM-mediated reactivation of AChE in the brains of mice exposed to paraoxon with no comparable increase in AChE activity seen with free 2-PAM. As noted in describing the data in Figure 1, it appears that the difference between life and death after paraoxon exposure hinges on whether a rather modest percentage of the AChE in the brain remains active. Collectively, our data indicate that scL-2PAM is more effective than free 2-PAM as a countermeasure in ameliorating both the severity and duration of cholinergic symptoms in mice exposed to sublethal levels of paraoxon, and this superiority appears to be reflective of the enhanced ability of scL-2PAM to reactivate AChE in the CNS. The neuropharmacology observed here is certainly consistent with scL-2PAM delivering its reactivator payload across the BBB via TfR-mediated transcytosis of the brain’s capillary endothelial cells. We are currently developing and validating a method based on mass spectrometry to quantify directly the amount of 2-PAM in brain tissue.
Rescuing Mice from Otherwise-Lethal Exposures to Paraoxon
Based on the above data demonstrating the superiority of scL-2PAM to reactivate AChE and to relieve cholinergic crisis, we postulated that scL-2PAM could improve survival of mice exposed to otherwise-lethal levels of paraoxon. We therefore performed head-to-head comparisons of scL-2PAM and free 2-PAM for their ability to rescue mice exposed to paraoxon at 5 mg/kg (~2x LD50), a dose that kills 100% of the mice without intervention (Figure 1A). At one minute after the injection of paraoxon, mice were treated with either free 2-PAM or scL-2PAM both at dose of 12.5 mg/kg, and survival assessed at 60 minutes (Figure 5A). Note that mice routinely die within 60 minutes of these paraoxon exposures, and mice surviving beyond 60 minutes eventually recover. All of the mice (n=10) administered free 2-PAM died as a result of their paraoxon exposure, whereas all of the mice receiving scL-2PAM (n=9) survived. This dramatic all-or-none result indicates that, free 2-PAM was relatively ineffective as a life-saving countermeasure under these conditions and that scL-2PAM was clearly superior in rescuing the mice. When the paraoxon dose was increased to 10 mg/kg (~4x LD50), a survival benefit was still observed with scL-2PAM compared to free 2-PAM, and this effect was dependent on dose of the antidote (Figure 5B). At all doses of the countermeasures tested (12.5, 25, and 37.5 mg/kg in oxime), scL-2PAM displayed superiority over free 2-PAM in rescuing mice from the otherwise-lethal paraoxon exposures. Not surprisingly, with paraoxon at 10 mg/kg (~4x LD50), the scL-2PAM dose of 12.5 mg/kg was not as effective in rescuing the mice as had been seen in the mice were exposed to the lower level of paraoxon (5 mg/kg or ~2x LD50). However, when scL-2PAM dose was increased to 25 mg/kg, survival was improved to 40%. In contrast, free 2-PAM at 25 mg/kg was incapable of rescuing these mice. With a still higher dose (37.5 mg/kg) of the countermeasures, survival of ~90% was observed when scL-2PAM was administered, whereas free 2-PAM resulted only ~50% survival. In summary, these data demonstrate improved survival rates in mice exposed to otherwise-lethal levels of paraoxon when the mice receive scL-2-PAM rather than free 2-PAM as a countermeasure. Not unexpectedly, the efficacies of the countermeasures in rescuing mice vary according to the paraoxon exposure level with higher countermeasure doses required when the OP exposure level is higher.
Based on these results, we varied both paraoxon exposure and scL-2PAM doses and monitored survival as the unambiguous end-point (Figure 6A). The paraoxon doses were increased to levels as high as 15 mg/kg (~6x LD50) and scL-2PAM was given at 12.5, 25, or 37.5 mg/kg. As anticipated, the doses of scL-2PAM required to achieve survival increased as the paraoxon exposure levels were elevated. These data are in line with scL-2PAM shifting the apparent LD50 of paraoxon to higher values. Indeed, when the apparent LD50 for paraoxon from Figure 6A is plotted as a function of the dose of scL-2PAM, the apparent LD50 for paraoxon at 37.5mg/kg was elevated ~6-over that seen in mice absent as an antidote (Figure 6B). It should be noted that at all three doses of scL-2PAM employed, the apparent LD10 for paraoxon is shifted to a level that exceeds the LD90 value seen with no antidote present (see upper and lower dashed lines in Figure 6A). This criterion is recognized as being indicative of an efficacious treatment against OP toxicity.48
Rescuing Mice Using Oxime Formulations Plus Atropine
Oxime reactivators are routinely used in conjunction with atropine, a competitive antagonist of muscarinic ACh receptors.9,21 In the experiments described thus far, we have compared scL-2PAM and free 2-PAM without atropine present. We have observed that atropine alone does not improve survival rates after lethal paraoxon exposures. Nonetheless, addition of atropine to either oxime formulation yielded survival rates higher than those seen with that oxime formulations as a monotherapy. Based on this observation and the results described above, we standardized our mouse model experiments to compare head-to-head with the two oxime formulations (ie, free 2-PAM and scL-2PAM) when given in conjunction with atropine. In most subsequent experiments, mice were exposed to paraoxon at 4x LD50 (~10mg/kg) and one minute later, mice were given either scL-2PAM or free 2-PAM at 25 mg/kg together plus atropine at 1.1 mg/kg (both oxime formulations and atropine administered IP). This dose of atropine was selected based on it being the approximate mouse equivalent of the atropine dose contained in the three DuoDote® autoinjectors issued to warfighters anticipating a possible OP exposure. Under these standardized conditions, ~85% of the mice receiving the scL-2PAM plus atropine survived whereas in mice receiving free 2-PAM plus atropine, a survival rate of only ~25% was observed (Figure 7A). Administration of atropine alone at 1.1 mg/kg was totally ineffective at rescuing mice (ie, there were no survivors among mice exposed to paraoxon at ~4x LD50 that received only atropine). The pooled experiments represented in Figure 7A confirmed a superiority of scL-2PAM over free 2-PAM that was highly statistically significant.
Maintenance of Biological Activity Upon Nanocomplex Lyophilization and Storage
A current product on the market (Protopam® Chloride for injection) is provided as a lyophilized product for reconstitution just prior to administration of OP-exposed individuals. Similarly, our scL-based investigational anticancer agents (SGT-53 and SGT-94) are also prepared in lyophilized form for reconstitution just prior to their use in cancer patients. If scL-2PAM is ever to be marketed as a new and improved formulation of 2-PAM, we anticipate that it will also be offered as a lyophilized product for reconstitution before use. Accordingly, we sought to determine if scL-2PAM retained its physical properties and biological activity after lyophilization and reconstitution. Because life and death are unambiguous, we relied upon assessment of survival rates after otherwise-lethal exposures to paraoxon to assess the activity of scL-2PAM after lyophilization and reconstitution. We used lyophilization and reconstitution protocols comparable to those that had been employed for our oncology products, SGT-53 and SGT-94. Initially, lyophilized scL-2PAM was stored for one week at +4°C in a desiccator prior to reconstitution. After reconstitution, the scL-2PAM that had been lyophilized was found to be similar in size (a single peak with number-average diameter of ~120 nm, PDI of ~0.3) and surface charge (zeta potential of +35 mV) to freshly prepared material (Figure 8). The more important comparison of fresh and reconstituted scL-2PAM involved assessment of the retention of the ability to rescue mice from otherwise-lethal exposures to paraoxon. Survival rates were determined in a head-to-head comparison of freshly prepared scL-2PAM with reconstituted scL-2PAM (Figure 7B) using our standardized conditions described in Figure 7A. Equivalent survival rates (~75-80%) were observed with both freshly prepared and lyophilized and reconstituted scL-2PAM (Figure 7B), and these survival rates were very comparable to those observed earlier with freshly prepared scL-2PAM (Figure 7A). It is noteworthy that we have now stored SGT-53, our scL-based investigational oncology product, in a lyophilized form under refrigeration (~+2°C to +8°C) for more than 2 years without loss of its biological activity. Anticipating the field uses of scL-2PAM as an antidote for OP exposures, we explored its retention of biological activity of lyophilized scL-2PAM that had been stored for various lengths of time (ie, from 3 to 30 months) under different storage conditions (ie, at room temperature, in a freezer, or in a refrigerator). An experiment in mice using the standardized experimental condition described in Figure 7A demonstrates that the ability to rescue mice from otherwise-lethal exposures to paraoxon was retained under all three storage conditions for periods of up to at least 30 months (Figure 9).
 |
Figure 9 Comparison of lyophilized scL-2PAM stored under different conditions for various times for efficacy in rescuing mice from otherwise-lethal paraoxon exposures. Mice were exposed to paraoxon and scL-2PAM as described in Figure 7A. The scL-2PAM used in each assessment had been lyophilized and stored for the indicated periods (from 3 to 30 months) prior to reconstitution and assessment. Storage of lyophilized scL-2PAM was either at room temperature (~25°C), in a refrigerator (+2°C to +8°C) or in a freezer (−20°C). Shown are the survival rates in groups of mice (number of mice per group indicated for each bar). For comparison, the upper dashed line and error bars are the values obtained using freshly prepared scL-2PAM from Figure 7A. The lower dashed line represents the values for survival rate for mice receiving free 2-PAM from Figure 7A. |
Routes of Nanocomplex Administration
We were also interested in comparing different administration routes [either intraperitoneal (IP), intravenous (IV), or subcutaneous (SQ) administration] of scL-2PAM. Here too, we compared the efficacy of scL-2PAM and free 2-PAM under our standardized conditions (described in Figure 7A) for their ability to rescue mice from paraoxon when the oxime formulations were given either IP, IV, or SQ. We observed that scL-2PAM administration resulted in ~85% survival with all three routes of administration with significantly lower survival rates seen when the countermeasures provided were free 2-PAM (Figure 10). Both survival rates were very comparable to that previously seen in Figure 7A, which involved IP administration of the oxime formulations. Atropine was in all cases administered IP, so the only variable in this experiment was the route of administration of the oxime formulations.
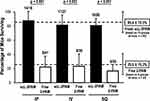 |
Figure 10 Comparison of different routes of administration on ability of scL-2PAM to rescue mice from otherwise-lethal paraoxon exposures. Paraoxon at 4x LD50 was administered to groups of mice. At one minute after the paraoxon exposures, atropine at 1.1 mg/kg was administered IP along with either free 2-PAM or scL-2PAM given either intraperitoneally (IP), intravenously (IV) or subcutaneously (SQ). Bars represent weighted mean survival rates with error bars representing the weighted standard deviations. Numbers above each bar represent the survivors and total mice used for survival assessment. For comparison, the upper dashed line and error bars are the values obtained with freshly prepared scL-2PAM from Figure 7A. The lower dashed line represents the survival rate for mice receiving free 2-PAM from Figure 7A. The percentage of mice surviving after receiving scL-2PAM were comparable for all routes of its administration and significantly higher than the corresponding survival rate in mice receiving free 2-PAM (p<0.001). |
Acetylcholinesterase Activities in Brain Reflect Survival
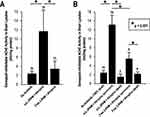
The nanocomplex scL-2PAM was designed to ferry its 2-PAM payload across the BBB to yield higher levels of AChE reactivation in the CNS than can be achieved using free 2-PAM. Having demonstrated that scL-2PAM can indeed reactivate brain AChE (Figure 4), we sought to correlate the levels of AChE activity in the brain with the life-or-death outcomes of paraoxon exposures. Mice were treated with paraoxon and the countermeasures as described in Figure 7A and the brains of surviving mice removed at one hour and processed for enzyme assays. Brains of mice that succumbed during the first hour after paraoxon exposure were removed immediately upon death and similarly processed. Cholinesterase in whole brain lysates was assessed with and without donepezil to determine the AChE activities present in the brains.
AChE activities in whole brain lysates from animals receiving paraoxon at 4x LD50 (no antidote) are very low (~2 mU/mg protein) with nearly all of the enzyme present in the naïve mouse brain having been inhibited by this otherwise-lethal paraoxon exposure (Figure 11A). The average AChE activity seen in mice that had received scL-2PAM following paraoxon exposures was significantly higher than those seen in mice that had received free 2-PAM (Figure 11A). In this experiment, we noted larger than usual error bars in this comparison where we did not distinguish mice who lived from those that died. When we segregated the AChE activity values according to whether a given brain lysate originated from a survivor or from a dead mouse, the enzyme activities were seen to be considerably higher in survivors than in dead mice (Figure 11B). Survivors in both treatment groups had higher AChE activity in their brains than seen in the brains of mice that succumbed. It appears that when the AChE activity in a given brain lysate is above ~4 mU/mg protein, that mouse is found to be among the survivors, whereas mice with lysate enzyme activities below ~4 mU/mg protein will have succumbed to the paraoxon poisoning. As had been shown previously (Figure 4), the AChE activities seen in whole brain lysates from surviving mice were a relatively small fraction of the AChE levels in lysates from naïve mice (ie, mice without paraoxon exposure). Although these data do not definitively show that the observed improvement in survival is causally linked to higher AChE activities in the brain, the correlation between AChE activities and survival supports the hypothesis that maintaining or reactivating only a rather modest fraction of the AChE in the brain may be sufficient for that mouse to survive otherwise-lethal exposures to paraoxon.
Discussion
The BBB protects the brain from blood-borne pathogens and harmful xenobiotics, but the BBB also blocks many therapeutic agents intended to treat neurological conditions from entering the CNS.49,50 Numerous and varied strategies have been employed to circumvent the BBB including invasive approaches such as deliberate disruption of the BBB or direct intraventricular/intrathecal infusions. Although BBB disruption may allow drugs to enter the brain parenchyma, this approach carries risks associated with compromising the critical protective function of the BBB. Much effort has been expended to seek less invasive strategies for transporting therapeutic agents to the brain. A detailed discussion of these diverse strategies is beyond the scope of this paper, but there have been two excellent recent reviews covering this topic.51,52
One example of a drug that does not readily cross the BBB is 2-PAM, the currently used oxime for AChE reactivation.53,54 It is interesting that there are some disparities in the descriptions of the ability of 2-PAM to enter the CNS.55 Some researchers choose to assert categorically that 2-PAM does not cross the BBB, while other authors describe penetration of the CNS by 2-PAM as being “minimal”, “limited” or “insufficient”. These differences likely hinge on differing sensitivities for measuring 2-PAM in brain tissue. Most studies infer BBB crossing (or lack thereof) from pharmacological effects in the brain (ie, impact on AChE activities).56,57 In our study, we observed that levels of AChE activities in whole brain lysates from mice treated with scL2-PAM were clearly elevated compared to those seen in mice receiving free 2-PAM. Particularly in surviving mice (which were in the majority in the scL-2PAM treatment group and in the minority in the free-2PAM treatment group), AChE activities were significantly higher (Figure 11B). The fact that the mice given free 2-PAM had higher brain AChE activity than seen in animals receiving no antidotes suggests that BBB penetration by free 2-PAM in these paraoxon-exposed mice is above zero. Nonetheless, the ability of the free oxime to act as a reactivator of AChE in the CNS under these conditions is indeed limited, and there is certainly room for improvement in the efficacy of therapeutic countermeasures to address the CNS sequelae of OP exposures.
To improve CNS delivery of oximes (including 2-PAM) and to improve reactivation of AChE in the CNS, the use of diverse drug delivery systems based on nanoparticles has been attempted.29,30 We have been developing a platform technology based on scL nanocomplexes that hijack TfR-mediated transcytosis normally used by brain capillary endothelial cells to move iron transporter transferrin (an ~80kDa serum protein) across the BBB to supply iron for critical metabolic processes.32,58 TfRs are upregulated on brain capillary endothelial cells,59 and the physiological process of TfR-mediated transcytosis has long been recognized as a potential means to transport therapeutics into the brain that would otherwise be excluded by the BBB.33 Transport of therapeutic payloads across the BBB using nanoparticles that target TfR has been recently reviewed.60 Our scL nanodelivery system is highly versatile in terms of the variety of payloads accommodated including plasmid DNAs, siRNAs, and small-molecule drugs. Using fluorescently labeled oligonucleotides, we have direct visual evidence that scL nanocomplexes carry payloads deep into the brains of mice using TfR-mediated transcytosis of the BBB.36 Perhaps most relevant to the current discussion is a nanocomplex termed scL-TMZ that encapsulates TMZ for treatment of brain tumors. TMZ is a small-molecule drug of about the same size as 2-PAM, and, like 2-PAM, TMZ does not readily cross the intact BBB. We have shown that the efficacy of scL-TMZ is superior to that of unencapsulated TMZ in treating intracranial glioblastoma in mice.34
Other research groups have also sought to use TfR-targeted nanoparticles to deliver AChE reactivators into the brain. The AChE reactivator HI-6 oxime was conjugated to mesoporous silica nanoparticles that were modified with transferrin (Tf), the natural ligand for TfR.61 These Tf-modified silica nanoparticles were shown to restore cerebral AChE after soman poisoning of mice. Another AChE reactivator (obidoxime) has also been packaged in a liposome that was conjugated with an oligonucleotide aptamer that binds to TfR as a targeting moiety.62 These authors concluded that the TfR-aptamer-targeted formulation was more effective in reactivating brain AChE consistent with this formulation crossing the BBB using TfR-mediated transcytosis. Recently, another group has also reported the use of antibody-targeted liposomes to carry payloads including 2-PAM across the BBB.63 In this case, liposomes were targeted via a single-chain antibody designated scFv46.1, which had been selected from a phage display library for its ability to traverse the BBB.64 Although identification of the antigen recognized by scFv46.1 has not been published, it appears to be distinct from both the receptors for transferrin or insulin that are both known to mediate brain endothelial cell transcytosis.33 None of these other TfR-targeting nanoparticles carrying any payload have been tested in human safety trials. In our case, two investigational agents employing the scL delivery system have completed successful Phase I trials in oncology65,66 wherein good safety profiles have been observed. The prior use in humans of investigational agents incorporating scL nanocomplexes mitigates to a degree what might be termed the “regulatory risk” associated with taking scL-2PAM forward in development as a new OP countermeasure.
Conclusion
The results described in this first publication on scL-2PAM indicate that this new formulation of an old AChE reactivator is superior to the currently used form of 2-PAM by every criteria thus far assessed (ie, relief of overt cholinergic signs and symptoms, survival after otherwise-lethal doses of paraoxon, and reactivation of AChE activity in the brain). To date, only data using paraoxon as a challenge agent are available, so testing scL-2PAM against more potent OPs is clearly warranted. While our data are consistent with enhanced delivery of 2-PAM into the brain, we intend to directly quantify 2-PAM in brain tissue using a mass spectrometry method now under development. In addition to its ability to mitigate cholinergic crisis and improve survival, scL-2PAM may also prove useful in preventing long-term brain damage in survivors of OP exposures, and we certainly intend to explore this hypothesis. Our choice of 2-PAM as the oxime payload was based largely on the fact that it is the only AChE reactivator currently approved in the United States. Considerable research effort is focused on design and testing of other chemical compounds for their ability to reactivate AChE.10,25,67 The in vivo efficacy of any countermeasures that emerge may also be enhanced by their encapsulation within the versatile TfR-targeted scL delivery system described here that could increase their access to OP-inhibited AChE in the brain.
Acknowledgments
The authors wish to thank Chris Poki Leung and Francis Cheung for their assistance with statistical analysis of the data presented here. The research described herein was supported in part by the Defense Threat Reduction Agency of the US Department of Defense through grant HDTRA1-13-1-0049 and contract HDTRA1-20-C-0045 to SynerGene Therapeutics, Inc. The content is solely the responsibility of the authors and does not necessarily reflect the official view of the Defense Threat Reduction Agency.
Disclosure
E.H.C. and K.F.P. are two of the inventors of the described scL technology, for which several patents owned by Georgetown University have been issued. The patents have been licensed to SynerGene Therapeutics, Inc. for commercial development. K.F.P. serves as Principal Investigator for research at Georgetown University that is supported by SynerGene Therapeutics, Inc. E.H.C. owns an equity interest in SynerGene Therapeutics, Inc., and E.H.C. and A.R. serve as paid scientific consultants to SynerGene Therapeutics, Inc. S.S.K. is salaried employee of SynerGene Therapeutics, Inc. M.M. is a graduate student and M.G. an undergraduate student who were supported via a research agreement between Georgetown University and SynerGene Therapeutics, Inc. A.W. is a former paid employee of SynerGene Therapeutics, Inc. J.B.H. serves as salaried President & CEO of SynerGene Therapeutics, Inc. and owns stock in same. The authors report no other conflicts of interest in this work.
References
1. Costa LG. Organophosphorus compounds at 80: some old and new issues. Toxicol Sci. 2018;162(1):24–35. doi:10.1093/toxsci/kfx266
2. Naughton SX, Terry AV. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology. 2018;408:101–112. doi:10.1016/j.tox.2018.08.011
3. Aroniadou-Anderjaska V, Apland JP, Figueiredo TH, De Araujo Furtado M, Braga MF. Acetylcholinesterase inhibitors (nerve agents) as weapons of mass destruction: history, mechanisms of action, and medical countermeasures. Neuropharmacology. 2020;181:108298. doi:10.1016/j.neuropharm.2020.108298
4. Eddleston M. Novel clinical toxicology and pharmacology of organophosphorus insecticide self-poisoning. Annu Rev Pharmacol Toxicol. 2019;59:341–360. doi:10.1146/annurev-pharmtox-010818-021842
5. Litchfield MH. Estimates of acute pesticide poisoning in agricultural workers in less developed countries. Toxicolog Rev. 2005;24(4):271–278. doi:10.2165/00139709-200524040-00006
6. Boedeker W, Watts M, Clausing P, Marquez E. The global distribution of acute unintentional pesticide poisoning: estimations based on a systematic review. BMC Public Health. 2020;20(1):1–19. doi:10.1186/s12889-020-09939-0
7. Figueiredo TH, Apland JP, Braga MFM, Marini AM. Acute and long-term consequences of exposure to organophosphate nerve agents in humans. Epilepsia. 2018;59(Suppl 2):92–99. doi:10.1111/epi.14500
8. Savage EP, Keefe TJ, Mounce LM, Heaton RK, Lewis JA, Burcar PJ. Chronic neurological sequelae of acute organophosphate pesticide poisoning. Arch Environm Health. 1988;43(1):38–45. doi:10.1080/00039896.1988.9934372
9. Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Advan Clin Chem. 2004;38:151–216. doi:10.1016/s0065-2423(04)38006-6
10. Worek F, Thiermann H, Wille T. Organophosphorus compounds and oximes: a critical review. Arch Toxicol. 2020;94(7):2275–2292. doi:10.1007/s00204-020-02797-0
11. Hrvat NM, Kovarik Z. Counteracting poisoning with chemical warfare nerve agents. Arh Hig Rada Toksikol. 2020;71(4):266–284. doi:10.2478/aiht-2020-71-3459
12. Watson A, Opresko D, Young RA, Hauschild V, King J, Bakshi K. Organophosphate Nerve Agents. In: Gupta RC, editor. Handbook of Toxicology of Chemical Warfare Agents. London: Academic Press; 2009.
13. Carey JL, Dunn C, Gaspari RJ. Central respiratory failure during acute organophosphate poisoning. Respir Physiol Neurobiol. 2013;189(2):403–410. doi:10.1016/j.resp.2013.07.022
14. Bird SB, Gaspari RJ, Dickson EW. Early death due to severe organophosphate poisoning is a centrally mediated process. Acad Emerg Med. 2003;10(4):295–298. doi:10.1197/aemj.10.4.295
15. Houze P, Pronzola L, Kayouka M, Villa A, Debray M, Baud FJ. Ventilatory effects of low-dose paraoxon result from central muscarinic effects. Toxicol Appl Pharmacol. 2008;233(2):186–192. doi:10.1016/j.taap.2008.08.006
16. Worek F, Szinicz L, Thiermann H. Estimation of oxime efficacy in nerve agent poisoning: a kinetic approach. Chem Biol Interact. 2005;157–158:349–352. doi:10.1016/j.cbi.2005.10.101
17. Eyer P. The role of oximes in the management of organophosphorus pesticide poisoning. Toxicolog Rev. 2003;22(3):165–190. doi:10.2165/00139709-200322030-00004
18. Dhuguru J, Zviagin E, Skouta R. FDA-approved oximes and their significance in medicinal chemistry. Pharmaceuticals. 2022;15(1):66. doi:10.3390/ph15010066
19. Childs AF, Davies DR, Green AL, Rutland JP. The reactivation by oximes and hydroxamic acids of cholinesterase inhibited by organo-phosphorus compounds. Br J Pharmacol Chemother. 1955;10(4):462–465. doi:10.1111/j.1476-5381.1955.tb00106.x
20. Wilson IB, Ginsburg B. A powerful reactivator of alkylphosphate-inhibited acetylcholinesterase. Biochim Biophys Acta. 1955;18(1):168–170. doi:10.1016/0006-3002(55)90040-8
21. Marrs TC, Maynard RL, Sidell FR. Chemical Warfare Agents: Toxicology and Treatment.
22. Jett DA. Neurological aspects of chemical terrorism. Ann Neurol. 2007;61(1):9–13. doi:10.1002/ana.21072
23. Zimmer LA, Ennis M, Shipley MT. Soman-induced seizures rapidly activate astrocytes and microglia in discrete brain regions. J Comparat Neurol. 1997;378(4):482–492. doi:10.1002/(SICI)1096-9861(19970224)378:4<482::AID-CNE4>3.0.CO;2-Z
24. Andrew PM, Lein PJ. Neuroinflammation as a therapeutic target for mitigating the long-term consequences of acute organophosphate intoxication. Front Pharmacol. 2021;12:674325. doi:10.3389/fphar.2021.674325
25. Gorecki L, Soukup O, Korabecny J. Countermeasures in organophosphorus intoxication: pitfalls and prospects. Trends Pharmacol Sci. 2022;43(7):593–606. doi:10.1016/j.tips.2022.04.008
26. Kuca K, Hrabinova M, Soukup O, Tobin G, Karasova J, Pohanka M. Pralidoxime--the gold standard of acetylcholinesterase reactivators--reactivation in vitro efficacy. Bratisl Lek Listy. 2010;111(9):502–504.
27. Kalasz H, Nurulain SM, Veress G, et al. Mini review on blood-brain barrier penetration of pyridinium aldoximes. J Appl Toxicol. 2015;35(2):116–123. doi:10.1002/jat.3048
28. Lorke DE, Kalasz H, Petroianu GA, Tekes K. Entry of oximes into the brain: a review. Curr Med Chem. 2008;15(8):743–753. doi:10.2174/092986708783955563
29. Kobrlova T, Korabecny J, Soukup O. Current approaches to enhancing oxime reactivator delivery into the brain. Toxicology. 2019;423:75–83. doi:10.1016/j.tox.2019.05.006
30. Kuznetsova DA, Gaynanova GA, Vasilieva EA, et al. Oxime therapy for brain AChE reactivation and neuroprotection after organophosphate poisoning. Pharmaceutics. 2022;14(9):1950. doi:10.3390/pharmaceutics14091950
31. Pashirova TN, Zueva IV, Petrov KA, et al. Nanoparticle-delivered 2-PAM for rat brain protection against paraoxon central toxicity. ACS Appl Mater Interfaces. 2017;9(20):16922–16932. doi:10.1021/acsami.7b04163
32. Moos T, Morgan EH. Transferrin and transferrin receptor function in brain barrier systems. Cell Mol Neurobiol. 2000;20(1):77–95. doi:10.1023/A:1006948027674
33. Pardridge WM. Brain delivery of nanomedicines: trojan horse liposomes for plasmid DNA gene therapy of the brain. Front Med Technol. 2020;2:602236. doi:10.3389/fmedt.2020.602236
34. Kim SS, Rait A, Kim E, DeMarco J, Pirollo KF, Chang EH. Encapsulation of temozolomide in a tumor-targeting nanocomplex enhances anti-cancer efficacy and reduces toxicity in a mouse model of glioblastoma. Cancer Lett. 2015;369(1):250–258. doi:10.1016/j.canlet.2015.08.022
35. Kim SS, Rait A, Kim E, et al. A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes glioblastoma to chemotherapy and improves survival. ACS Nano. 2014;8(6):5494–5514. doi:10.1021/nn5014484
36. Kim SS, Rait A, Garrido-Sanabria ER, Pirollo KF, Harford JB, Chang EH. Nanotherapeutics for gene modulation that prevents apoptosis in the brain and fatal neuroinflammation. Mol Ther. 2018;26(1):84–94. doi:10.1016/j.ymthe.2017.10.003
37. de A Cavalcante SF, Simas AB, Kuča K. Nerve agents’ surrogates: invaluable tools for development of acetylcholinesterase reactivators. Curr. Org. Chem. 2019;23(14):1539–1559.
38. Campbell MJ. Lipofection reagents prepared by a simple ethanol injection technique. BioTechniques. 1995;18(6):1027–1032.
39. Xu L, Tang WH, Huang CC, et al. Systemic p53 gene therapy of cancer with immunolipoplexes targeted by anti-transferrin receptor scFv. Mol Med. 2001;7(10):723–734. doi:10.1007/BF03401962
40. Yu W, Pirollo KF, Yu B, et al. Enhanced transfection efficiency of a systemically delivered tumor-targeting immunolipoplex by inclusion of a pH-sensitive histidylated oligolysine peptide. Nucleic Acids Res. 2004;32(5):e48. doi:10.1093/nar/gnh049
41. Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi:10.1016/0006-2952(61)90145-9
42. Brewster JT, Dell’Acqua S, Thach DQ, Sessler JL. Classics in chemical neuroscience: donepezil. ACS Chem Neurosci. 2019;10(1):155–167. doi:10.1021/acschemneuro.8b00517
43. Garcia SJ, Abu-Qare AW, Meeker-O’Connell WA, Borton AJ, Abou-Donia MB. Methyl parathion: a review of health effects. J Toxicol Env Heal B. 2003;6(2):185–210. doi:10.1080/10937400306471
44. Moretto A. Experimental and clinical toxicology of anticholinesterase agents. Toxicol Lett. 1998;102–103:509–513. doi:10.1016/S0378-4274(98)00245-8
45. Racine RJ. Modification of seizure activity by electrical stimulation. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–294. doi:10.1016/0013-4694(72)90177-0
46. Luttjohann A, Fabene PF, van Luijtelaar G. A revised Racine’s scale for PTZ-induced seizures in rats. Physiol Behav. 2009;98(5):579–586. doi:10.1016/j.physbeh.2009.09.005
47. Van Erum J, Van Dam D, De Deyn PP. PTZ-induced seizures in mice require a revised Racine scale. Epilep Behav. 2019;95:51–55. doi:10.1016/j.yebeh.2019.02.029
48. Natoff IL, Reiff B. Quantitative studies of the effect of antagonists on the acute toxicity of organophosphates in rats. Br J Pharmacol. 1970;40(1):124–134. doi:10.1111/j.1476-5381.1970.tb10617.x
49. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi:10.1016/j.nbd.2009.07.030
50. Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barri CNS. 2020;17(1):69. doi:10.1186/s12987-020-00230-3
51. Teleanu RI, Preda MD, Niculescu AG, et al. Current strategies to enhance delivery of drugs across the blood-brain barrier. Pharmaceutics. 2022;14(5):987. doi:10.3390/pharmaceutics14050987
52. Pardridge WM. A historical review of brain drug delivery. Pharmaceutics. 2022;14(6). doi:10.3390/pharmaceutics14061283
53. Chambers JE, Dail MB, Meek EC. Oxime-mediated reactivation of organophosphate-inhibited acetylcholinesterase with emphasis on centrally-active oximes. Neuropharmacology. 2020;175:108201. doi:10.1016/j.neuropharm.2020.108201
54. Peter JV, Moran JL, Pichamuthu K, Chacko B. Adjuncts and alternatives to oxime therapy in organophosphate poisoning - Is there evidence of benefit in human poisoning? A review. Anaesth Inten Care. 2008;36(3):339–350. doi:10.1177/0310057X0803600305
55. Sakurada K, Matsubara K, Shimizu K, et al. Pralidoxime iodide (2-PAM) penetrates across the blood-brain barrier. Neurochem Res. 2003;28(9):1401–1407. doi:10.1023/A:1024960819430
56. Garcia GE, Campbell AJ, Olson J, Moorad-Doctor D, Morthole VI. Novel oximes as blood-brain barrier penetrating cholinesterase reactivators. Chem Biol Interact. 2010;187(1–3):199–206. doi:10.1016/j.cbi.2010.02.033
57. Shih TM, Skovira JW, O’Donnell JC, McDonough JH. In vivo reactivation by oximes of inhibited blood, brain and peripheral tissue cholinesterase activity following exposure to nerve agents in Guinea pigs. Chem Biol Interact. 2010;187(1–3):207–214. doi:10.1016/j.cbi.2010.03.006
58. Pardridge WM, Buciak JL, Friden PM. Selective transport of an anti-transferrin receptor antibody through the blood-brain barrier in vivo. J Pharmacol Exp Ther. 1991;259(1):66–70.
59. Descamps L, Dehouck MP, Torpier G, Cecchelli R. Receptor-mediated transcytosis of transferrin through blood-brain barrier endothelial cells. A J Physiol. 1996;270(4):H1149–1158. doi:10.1152/ajpheart.1996.270.4.H1149
60. Thomsen MS, Johnsen KB, Kucharz K, Lauritzen M, Moos T. Blood-brain barrier transport of transferrin receptor-targeted nanoparticles. Pharmaceutics. 2022;14(10):2237. doi:10.3390/pharmaceutics14102237
61. Yang J, Fan L, Wang F, et al. Rapid-releasing of HI-6 via brain-targeted mesoporous silica nanoparticles for nerve agent detoxification. Nanoscale. 2016;8(18):9537–9547. doi:10.1039/C5NR06658A
62. Zhang Y, He J, Shen L, et al. Brain-targeted delivery of obidoxime, using aptamer-modified liposomes, for detoxification of organophosphorus compounds. J Control Release. 2021;329:1117–1128. doi:10.1016/j.jconrel.2020.10.039
63. Ye Z, Gastfriend BD, Umlauf BJ, Lynn DM, Shusta EV. Antibody-targeted liposomes for enhanced targeting of the blood-brain barrier. Pharm Res. 2022;39(7):1523–1534. doi:10.1007/s11095-022-03186-1
64. Georgieva JV, Goulatis LI, Stutz CC, et al. Antibody screening using a human iPSC-based blood-brain barrier model identifies antibodies that accumulate in the CNS. FASEB J. 2020;34(9):12549–12564. doi:10.1096/fj.202000851R
65. Senzer N, Nemunaitis J, Nemunaitis D, et al. Phase I study of a systemically delivered p53 nanoparticle in advanced solid tumors. Mol Ther. 2013;21(5):1096–1103. doi:10.1038/mt.2013.32
66. Siefker-Radtke A, Zhang XQ, Guo CC, et al. A phase l study of a tumor-targeted systemic nanodelivery system, SGT-94, in genitourinary cancers. Mol Ther. 2016;24(8):1484–1491. doi:10.1038/mt.2016.118
67. Amend N, Niessen KV, Seeger T, Wille T, Worek F, Thiermann H. Diagnostics and treatment of nerve agent poisoning-current status and future developments. Ann NY Acad Sci. 2020;1479(1):13–28. doi:10.1111/nyas.14336
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.