Back to Journals » OncoTargets and Therapy » Volume 11
A patient with classic biphasic pulmonary blastoma harboring CD74–ROS1 fusion responds to crizotinib
Authors Meng Z, Chen P, Zang F, Liu Y, Xu X , Su Y, Chen J, Lin L, Zhang L , Zhang T
Received 25 August 2017
Accepted for publication 16 November 2017
Published 28 December 2017 Volume 2018:11 Pages 157—161
DOI https://doi.org/10.2147/OTT.S150001
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Yao Dai
Zhaoting Meng,1,* Peng Chen,1,* Fenglin Zang,1 Ying Liu,1 Xiaoyan Xu,1 Yudong Su,1 Jinliang Chen,1 Li Lin,1 Lu Zhang,2 Tengfei Zhang2
1Department of Thoracic Medical Oncology, Lung Cancer Diagnosis and Treatment Center, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, 2Burning Rock Biotech, Guangzhou, China
*These authors contributed equally to this work
Abstract: Pulmonary blastoma (PB) is a rare aggressive lung malignancy with a poor prognosis. Surgical resection is the treatment of choice for localized disease, and there are no standard treatment guidelines for metastatic PB. Due to its rareness, its molecular profile has not been elucidated. We present the first case of classic biphasic pulmonary blastoma (CBPB) with CD74–ROS1 rearrangement in a 44-year-old Asian female with stage IV disease diagnosed using capture-based ultra-deep targeted sequencing. It has been reported that ROS1 rearranged lung adenocarcinoma and squamous cell carcinoma are sensitive to crizotinib, an ALK/MET/ROS1 multitargeted tyrosine kinase inhibitor. However, its efficacy has not been reported in CBPB patients harboring ROS1 rearrangement. This CBPB patient was given crizotinib and she achieved partial response after 1 month of treatment. We report the first clinical evidence of efficacy shown by crizotinib for targeting CD74–ROS1 fusion in CBPB.
Keywords: classic biphasic pulmonary blastoma, ROS1 rearrangement, crizotinib, targeted DNA sequencing
Introduction
Pulmonary blastoma (PB), comprising approximately 0.25%–0.5% of all lung malignancies, is a rare aggressive primary lung tumor and portends a poor diagnosis reflected by a 5-year survival rate of 16%.1 Since its first identification, Barnard described it as “lung embryoma” based on the histologic resemblance of the tumor to fetal lung tissue.2 There are only about a few hundreds of cases reported worldwide. PB has been subdivided into three categories based on the tissue components. Classic biphasic pulmonary blastoma (CBPB) is characterized by its biphasic feature of both epithelial and mesenchymal malignant components. Well-differentiated fetal adenocarcinoma is a monophasic PB comprising epithelial malignant component only, which was recategorized as a variant of adenocarcinoma in 2004 by the World Health Organization. Pleuropulmonary blastoma is a childhood tumor showing features of mesenchymal malignant components only.3 Pleuropulmonary blastoma is a distinct entity that occurs in children aged <15 years; well-differentiated fetal adenocarcinoma and CBPB mainly occur in adults, with a median age of 43, and predominantly in smokers.
CBPB is characterized by a biphasic feature of both epithelial and mesenchymal components, resembling the fetal lung tissue.4 It typically presents with cough, hemoptysis, dyspnea or chest pain due to tumor impinging the bronchi or pleura. Average age at diagnosis is 40 years. Surgical excision is the optimal treatment choice for localized disease, and there is no standard treatment available for patients with metastatic disease. Prognosis for CBPB is poor, with two-thirds of patients dying within 2 years and the 5-year survival is only 16%. CBPB prognosis is determined by the size of the tumor and metastasis status at the time of diagnosis.
ROS1, first discovered as the oncogene product of an avian sarcoma RNA tumor virus, encodes an orphan receptor tyrosine kinase, which belongs to the insulin receptor family with downstream signaling via MAPK, PI3K-mTOR and JAK-STAT pathways.5 Its activation by chromosomal rearrangement was observed in a variety of cancers, including non-small cell lung cancer (NSCLC).6 Nowadays, there has been an ever-surmounting body of clinical data supporting the efficacy of crizotinib, an ALK/MET/ROS multitargeting tyrosine kinase inhibitor, in adenocarcinoma and limited cases of squamous cell carcinoma patients harboring ROS1 fusion.7,8 Thus, the US Food and Drug Administration approved crizotinib as a treatment for patients with ROS1-positive metastatic NSCLC in March 2016. However, its efficacy has not been reported in patients with PB.
In this case report, we present the first CBPB case harboring CD74–ROS1 fusion, which responded to crizotinib. To the best of our knowledge, this is the first clinical evidence of efficacy shown by crizotinib targeting CD74–ROS1 fusion in CBPB.
Case presentation
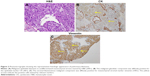
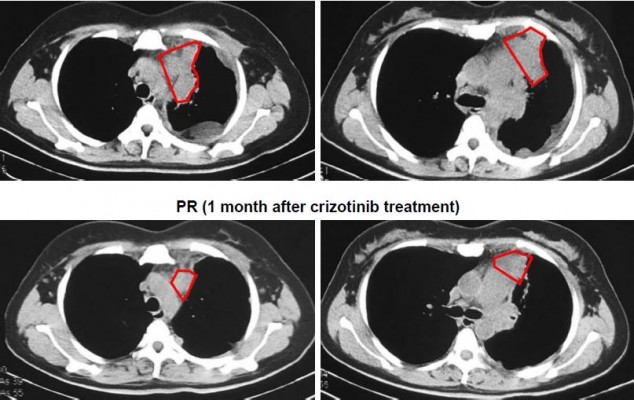
A 44-year-old Asian woman with no history of smoking presented to the outpatient clinic with intermittent cough, bloody sputum and left chest pain that lasted for 1 month. Chest computed tomography scan revealed a mass with its largest diameter measuring 5.8 cm, located in the left upper lobe and extending into the chest wall with pleural invasion and rib metastases (Figure 1). Computed tomography-guided biopsy followed by hematoxylin–eosin staining revealed a mixture of malignant epithelial and mesenchymal components. The mesenchymal component consisted of round and shuttle cells with atypia nuclear and visible karyokinesis; in contrast, the epithelial component consisted of tubular and glandular structures (Figure 2A). Immunohistochemistry showed the tubular epithelial component to be positive for epithelial marker cytokeratin and negative for vimentin, while the mesenchymal blastematous malignant component showed reverse expression (negative cytokeratin, diffusely positive for vimentin), as shown in Figure 2B and 2C. The morphologic appearance combined with the immune profile of the tumor was diagnostic of CBPB. The patient was diagnosed with stage IV cT3N1M1b CBPB according to the clinical Tumor–Node–Metastasis staging system seventh edition.
Capture-based ultra-deep targeted sequencing was performed on tissue biopsy using a panel consisting of all exons and critical introns of 295 cancer-related genes, spanning 2.02 MB of human genome. Our data revealed the presence of CD74–ROS1 rearrangement, joining intron 7 of CD74 with intron 33 of ROS1, with an allelic fraction of 43.6% (Figure 3). No other crizotinib-targeted molecular alteration was observed. The patient was given treatment with crizotinib (250 mg, twice daily), and she achieved partial response 1 month after treatment initiation. She achieved reduction in pleural effusion and 34.4% shrinkage of tumor size, accompanied by alleviation of symptoms such as coughing, thoracalgia and dyspnea (Figure 1). However, she finally developed progressive disease with enlarged left lung lesion and increased left pleural effusion, achieving a progression-free survival of 3 months. At progressive disease, sequencing of liquid biopsy revealed the newly acquired ROS1 site mutation G2032R, which was reported as the most pronounced resistant mutation of crizotinib.9
Patient consent
This study was approved by the Institutional Review Board at the Tianjin Medical University Cancer Institute and Hospital. The patient provided written informed consent and gave permission for the use of biopsies and publication of case details.
Discussion
ROS1 rearrangements were found in a variety of human cancers beside NSCLC, including gastric adenocarcinoma, colorectal cancer, melanoma, cholangiosarcoma, glioblastoma, ovarian cancer, angiosarcoma, inflammatory myofibroblastic tumor and epithelioid hemangioendothelioma.6,10–13 In this study, we reported the first clinical CBPB case with CD74–ROS1 fusion identified by capture-based ultra-deep targeted sequencing. This finding has provided us the possibility that ROS1 fusion may also function as a driver mutation in other rare cancer types beside the most mainstream type of NSCLC.
PB is a rare aggressive form of neoplasm, comprising 0.25%–0.5% of all primary lung malignancies with a poor prognosis.1 Standard targeted therapy guidelines are not available for CPBP. Surgery is the treatment of choice for localized disease. A mean survival of 33 months was reported in 66 resected cases, compared to 2 months in 17 unresected patients.14 Both chemotherapy and radiotherapy have been utilized in advanced patients with varying results. Larsen and Sorensen reported a 16% overall response rate to chemotherapy in a cohort of 43 CBPB patients.14 No chemotherapy agent has been shown to be superior to others, while most studies have shown lack of response to radiotherapy.15 Surgery is the preferred treatment, often with postoperative adjuvant chemotherapy and/or radiotherapy.16 No treatment guidelines have been established for metastatic CBPB.
The rapid development of next-generation sequencing technologies has greatly revolutionized our ability to characterize cancers. Remarkable advances in targeted therapy have been made and have benefited many molecularly defined mainstream subsets of NSCLC patients. However, the evidence to effectively target gene alterations in clinical PB, which is rare, has been poorly reported. Carboplatin, paclitaxel and bevacizumab were reported to be effective in a pulmonary patient with distant metastasis, but failed after four courses of chemotherapy.17 Sorafenib showed efficacy in a rare case of renal metastasis of biphasic PB, and the tumor size decreased sufficiently.18
ROS1 rearrangements, occurring in 1% of patients with NSCLC, have been reported to be sensitive to crizotinib.19 To the best of our knowledge, this is the first report of CD74–ROS1 rearrangement identified in a CBPB patient, who responded to crizotinib, providing a novel option for treating CBPB patients. We also found that CD74–ROS1, a driver gene in other subtypes of NSCLC, can act as a driver gene in CBPB.
Acknowledgments
We owe thanks to the patient and her family. We thank the staff at Tianjin Medical University Cancer Institute and Hospital. We also thank Jingjing Li and Yan Wang from Burning Rock Biotech for valuable discussion and support. This work was supported by funding from the Natural Science Foundation of Tianjin Grant No 13JCYBJC23600.
Disclosure
The authors report no conflicts of interest in this work.
References
Francis D, Jacobsen M. Pulmonary blastoma. Curr Top Pathol Ergebnisse Der Pathologie. 1983;73:265–294. | ||
Barnard WG. Embryoma of lungs. Thorax. 1952;7(4):299–301. | ||
Koss MN, Hochholzer L, O’Leary T. Pulmonary blastomas. Cancer. 1991;67(9):2368–2381. | ||
Robert J, Pache JC, Seium Y, de Perrot M, Spiliopoulos A. Pulmonary blastoma: report of five cases and identification of clinical features suggestive of the disease. Eur J Cardiothorac Surg. 2002;22(5):708–711. | ||
Davies KD, Le AT, Theodoro MF, et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res. 2012;18(17):4570–4579. | ||
Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190–1203. | ||
Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963–1971. | ||
Gibelin C, Avrillon V, De La Fouchardiere A, Mc Leer-Florin A, Lantuejoul S, Fayette J. Clinical relevance of ROS1 rearrangements detection in advanced squamous cell carcinomas. Lung Cancer. 2016;102:42–43. | ||
Awad MM, Katayama R, McTigue M, et al. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med. 2013;368(25):2395–2401. | ||
Lee J, Lee SE, Kang SY, et al. Identification of ROS1 rearrangement in gastric adenocarcinoma. Cancer. 2013;119(9):1627–1635. | ||
Aisner DL, Nguyen TT, Paskulin DD, et al. ROS1 and ALK fusions in colorectal cancer, with evidence of intratumoral heterogeneity for molecular drivers. Mol Cancer Res. 2014;12(1):111–118. | ||
Gu TL, Deng X, Huang F, et al. Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PLoS One. 2011;6(1):e15640. | ||
Birch AH, Arcand SL, Oros KK, et al. Chromosome 3 anomalies investigated by genome wide SNP analysis of benign, low malignant potential and low grade ovarian serous tumours. PLoS One. 2011;6(12):e28250. | ||
Larsen H, Sorensen JB. Pulmonary blastoma: a review with special emphasis on prognosis and treatment. Cancer Treat Rev. 1996;22(3):145–160. | ||
Kliem V, Bugge M, Leimenstoll K, Maschek H. Pulmonary blastoma a rare tumour. Clin Investig. 1992;70(10):927–931. | ||
Cutler CS, Michel RP, Yassa M, Langleben A. Pulmonary blastoma: case report of a patient with a 7-year remission and review of chemotherapy experience in the world literature. Cancer. 1998;82(3):462–467. | ||
Sakata S, Saeki S, Hirooka S, Hirosako S, Ichiyasu H, Kohrogi H. A case of biphasic pulmonary blastoma treated with carboplatin and paclitaxel plus bevacizumab. Case Rep Oncol Med. 2015;2015:842621. | ||
Mulamalla K, Truskinovsky AM, Dudek AZ. Pulmonary blastoma with renal metastasis responds to sorafenib. J Thorac Oncol. 2007;2(4):344–347. | ||
Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–870. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.