Back to Journals » International Journal of General Medicine » Volume 16
A Novel Wound Therapy Modality: Autologous Wound Edge Dotted Full-Thickness Skin Grafting Improving Diabetic Foot Ulcer Healing
Authors Huang J, Sun J, Wang Q, Mo J, Nong Y, Zhai Z, Huang X, Mo J , Lu W
Received 24 June 2023
Accepted for publication 22 August 2023
Published 28 August 2023 Volume 2023:16 Pages 3815—3827
DOI https://doi.org/10.2147/IJGM.S427401
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Luca Testarelli
Jianhao Huang,1,2,* Jingxia Sun,2,* Qiu Wang,2,* Jianming Mo,2,* Yuechou Nong,2 Zhenwei Zhai,2 Xiuxian Huang,2 Jiacheng Mo,3 Wensheng Lu2
1The Department of Endocrinology and Metabolism, Jinan University, Guangzhou, Guangdong, 510632, People’s Republic of China; 2The Department of Endocrinology and Metabolism, Guangxi Academy of Medical Sciences and the People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, 530021, People’s Republic of China; 3Information Network Center of Guangxi Academy of Medical Sciences and the People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, 530021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wensheng Lu, Email [email protected]
Aim: To explore the therapeutic efficacy of autologous wound edge-dotted full-thickness skin grafting in improving diabetic foot ulcer healing.
Methods: Sixty-three patients were divided into three groups: conventional wound therapy (CWT) (n = 23), platelet-rich plasma (PRP) (n = 20), and graft (n = 20). All participants were followed up for 12 weeks. The therapeutic efficacy of the three different wound treatment modalities was analyzed.
Results: After follow-up, 37 (58.7%) patients showed complete wound re-epithelialization, of which 10 (43.5%) occurred in the CWT group, 14 (70.0%) in the PRP group, and 13 (65.0%) in the graft group. Multivariate Cox analysis showed that the independent predictive factors for ulcer healing were different treatment modalities (graft: HR = 3.214, 95% CI=1.300– 7.945, P < 0.05; platelet-rich plasma: HR = 3.075, 95% CI=1.320– 7.161, P < 0.01), ABI (HR = 9.917, 95% CI=2.675– 36.760, P < 0.01), and TcPO2 (HR = 1.040; 95% CI=1.005– 1.076; P < 0.05). Stratified analysis showed that higher ABI in graft group or PRP group had higher wound healing rate (graft group: HR = 3.748, 95% CI=1.210– 11.607, P < 0.05; PRP group: HR = 5.029, 95% CI=1.743– 14.509, P < 0.05); higher TcPO2 in the graft group had higher wound healing rate (HR = 15.805, 95% CI=4.414– 56.594, P < 0.01). Additionally, the wound healing time (P < 0.0167) and cumulative healing rate (P < 0.05) in both the PRP group and graft group were more advantageous. The graft group promotes wound re-epithelialization earlier and faster than in the CWT group and PRP group (P < 0.05). Meanwhile, the graft group had lower medical costs (P < 0.0167).
Conclusion: Autologous wound edge dotted full-thickness skin grafting has a higher cost-performance ratio than traditional diabetic foot ulcer wound care and is worthy of further clinical application.
Keywords: diabetic foot ulcers, autologous wound edge dotted full-thickness skin graft, platelet-rich plasma
Introduction
Diabetes mellitus (DM) is a common chronic metabolic disease associated with various complications including diabetic foot ulcers (DFUs). DFUs is a frequent and potentially life-threatening complication that refers to foot infections, ulcers, or deep tissue destruction caused by aberrant nerves and varying degrees of vascular lesions in the distal lower limbs.1 It is estimated that DFUs occurs in approximately 25% of diabetic patients,2 causing a high rate of amputation, extensive healthcare expenditure, and reduced quality of life.3 Thus, the new treatment modality to improve and promote wound healing is extremely urgent and worthwhile to further explore.
Current clinical therapies for DFUs involve multifaceted interventions, including wound repair, restoration of blood supply, neurotrophic treatment, and rational use of antibiotics. Diabetic wounds, which are chronic wounds, are difficult to heal owing to multiple risk factors such as ischemia, infection, and foreign bodies; therefore, rapid wound healing to restore skin barrier integrity is essential for the treatment of DFUs.4 The main treatments for DFUs include conventional wound therapeutic (CWT) (dressing replacement), negative pressure wound therapy (NPWT), platelet-rich plasma gel (PRP), biological agents, skin grafts, and stem cell therapy.5 CWT refers to after conventional NPWT, the wounds were only continually covered with dressings changed several times a week until complete re-epithelialization of the wounds. NPWT is a kind of wound therapy modality commonly used through wound negative pressure pump. PRP is an autologous blood-derived product containing numerous angiogenic growth factors that have been widely used to promote delayed wound healing.6 Skin grafts, including artificial skin, autografts, and allografts, are promising alternatives for promoting diabetic wound healing and have broad application prospects. Recently, inspired by the skin harvesting method in dermatology, we were the first to develop and evaluate autologous wound edge-dotted full-thickness skin grafting for treating DFUs. Our previous study showed that compared with CWT, autologous wound edge dotted full-thickness skin grafts could shorten wound healing time and is a reliable, safe, and cost-effective treatment modality.7
Therefore, to identify more effective therapies, our study aimed to compare the safety, effectiveness, and cost-effectiveness of CWT, PRP, and graft groups.
Methods
Subjects
Eighty inpatients with DFUs were recruited for this study between October 2019 and October 2022 from our hospital (Figure 1). Of the 80 patients screened for DFUs, 63 met the following inclusion criteria. The inclusion criteria were as follows: (1) adults aged 18 years or older; (2) diabetes diagnosed in accordance with the 1999 World Health Organization (WHO);8 (3) diagnosis of DFU based on the International Working Group on Diabetic Foot (IWGDF); all the ulcers were newly formed wounds, including recurrent ulcers located near the ankle. (4) complete healing of the wound after debridement and vacuum-assisted closure (VAC) therapy; and (5) granulation tissue formation that could achieve a healthy granular bed. The exclusion criteria were as follows: (1) non-diabetic foot ulcers included venous ulcers,9 tophus ulcers, and bedsores; (2) long-term use of glucocorticoids or immunosuppressants; and (3) loss to follow-up or incomplete follow-up information. Before performing any procedures related to the study, all patients provided written informed permission. The enrolled patients were randomly divided into three groups after 14 days of NPWT for all wounds: CWT group (n = 23), PRP group (n = 20), and graft group (n = 20). The Ethics Committee of the People’s Hospital of the Guangxi Zhuang Autonomous Region gave its approval to this study, which was carried out in conformity with the Declaration of Helsinki. All participants volunteered to participate in the study and provided written, informed consent.
 |
Figure 1 The flowchart of the study design and patient recruitment. |
Treatment Method
Routine Treatment
All enrolled subjects underwent a medical history survey, physical examination, laboratory tests, and imaging examinations and were treated with standardized and individualized regimens. First, blood glucose was monitored using a portable glucometer (Johnson and Johnson, Ltd., New Jersey, USA), and glucose-lowering therapies to control blood sugar to 4.4 ~ 10 mmol/L were based on the results of self-monitored capillary blood glucose. Second, empiric antibiotic therapy before susceptibility results and sensitive antibiotic therapy based on the results of the drug sensitivity test were selected to control the infection for seven days. Third, comorbidities (such as hypertension or dyslipidemia) and diabetes-related complications were treated according to relevant guidelines. Fourth, nutritional support was provided to malnourished patients. In brief, routine treatment for all patients involved individualized glucose-lowering therapy, antibiotic therapy, nutritional support, ultrasonic debridement, and symptomatic treatment.
The Wounds Debridement
Regular wound debridement was performed to remove the necrotized tissues and secretions. After that, the wound was debrided thoroughly according to ultrasonic instrumentation (the ultrasonic frequency was set at 50kHz) and 0.9% sodium chloride (NaCl) injection was used to clean the deep wound and irregular sinus tracts, which made the area of wound debridement reach 1–2 mm away from the wound edge.10
Negative Pressure Wound Therapy (NPWT)
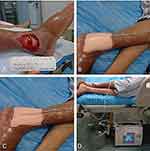
As shown in Figure 2, we initially trimmed the disposable negative pressure drainage materials based on the assessment of the shape and size of the wound (Figure 2A). A negative pressure drainage tube (PU-C, Shandong Chuangkang Biotechnology Co., Ltd.) was embedded in the dressing, which further covered the wound (Figure 2B). Next, the wound, dressing, and drainage tube were completely sealed with semi-permeability to make the wound airtight, and the drainage tube was connected to an intelligent negative pressure pump (ZN100, Shandong Chuangkang Biotechnology Co., Ltd) (Figure 2C). Finally, based on the size, depth, and infection of the wound, the negative pressure was set to a range of −80 to −125 mmHg.11 The mode was conducted for 5 min and suspended for 2 min, and dressing and drainage tubes were replaced every 5–7 days. All wounds were treated with NPWT for 14 days (Figure 2D). After 14 days of NPWT treatment, the wound granulation tissue had grown well and was essentially parallel to the epidermis, according to the IWGDF guidelines, no further negative pressure treatment was required. Then, according to the doctor’s professional suggestions and the patients’ personal wishes, these enrolled patients divided into three groups: conventional wound therapy (CWT), platelet-rich plasma (PRP), and graft. A special reminder is that all wounds were covered with the same specification alginate dressings (Biatain Alginate, 3710, Advanced Medical Ltd) and foam dressings (Biatain Adhesive, 3420, Advanced Medical Ltd).
Conventional Wound Therapeutic (CWT) Group
After 14 days of NPWT, the wounds in the CWT group were cleaned with 0.9% NaCl solution and then only continually covered with alginate dressings and foam dressings every other day change for 14 days. Subsequently, the dressings were changed twice a week until complete re-epithelialization of the wounds.
Platelet-Rich Plasma (PRP) Group
For PRP preparation, antecubital venous blood samples were collected using EDTA tubes, and the volume of blood was dependent on the size of the wound area. PRP was isolated and purified by centrifugation as previously described.12 Blood samples were first centrifuged for 4 min at 313 × g and red blood cells (RBCs) were discarded. After centrifugation at 1252 rpm for 6 min, the supernatant was discarded. Platelets were then thoroughly mixed with 1mL calcium gluconate and 1000 IU thrombin to obtain platelet-rich gels (PRGs). Furthermore, PRGs evenly covered the surface of the wounds, which were dressed with sterile gauze (Figure 3).
Graft Group
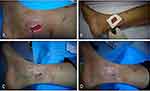
About 6-mm-diameter full thickness skin samples including the epidermis, dermis, and a small amount of subcutaneous tissue, were directly taken from the edge of wounds with skin samples (Skin Biopsy Punches, Huaian Zhonglin Dongsheng Medical Equipment Co. Ltd)13 (Figure 4). The number of skin samples was determined based on the wound size. The sampled skin was evenly distributed on the wound surface with a spacing of 2 cm and covered with lipid hydrocolloid silver-sulfate dressings (Urgotul Ag/Silver, LABORATOIRES URGO, France). An additional 7 days of NPWT was performed to allow skin samples to adhere to the wound surface to ensure the survival of skin graft (Figure 5).
 |
Figure 4 Skin extractor for the autologous wound edge dotted full-thickness skin grafting. |
Data Collection
Detailed information regarding the demographic information, clinical data, and laboratory measurements were anonymously collected. Demographic information regarding age, sex, body mass index (BMI), duration of diabetes, history of smoking, alcoholism, hypertension, dyslipidemia, ischemic heart disease (IHD), foot ulcers, and minor amputations were recorded. Clinical data included complications, such as diabetic retinopathy, lower extremity venous insufficiency, wound secretion culture, and the University of Texas Diabetic Wound Classification (UTDWC).14 Laboratory measurements of hemoglobin, albumin, glycosylated hemoglobin (HbA1c), estimated glomerular filtration rate (eGFR), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and transcutaneous partial pressure of oxygen (TcPO2) were collected.
BMI was calculated as the body weight in kilograms divided by the height in meters squared (kg/m2). The ankle-brachial index (ABI) was monitored using an Ultrasonic Doppler Blood Flow Analyzer (Vista AVS; Cooper Surgical Inc. USA) and was defined as the ratio of the systolic blood pressure in the ankle to that in the arm. The eGFR for Chinese patients was calculated using the MDRD equation: 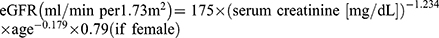 15
15
Photographs were captured with a Sony DSC-W830 digital camera, and the wound areas were quantified using Image-J software once a week until good healing of the wound healed well (defined as wound closure and complete wound re-epithelialization). The wound healing speed was calculated using the following equation: 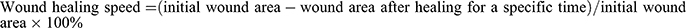 .
.
Statistical Analysis
Quantitative variables are expressed as mean ± SD or median (quartiles) according to normal or skewed distributions, respectively. Categorical variables are expressed as frequencies (%). For comparisons between the three groups, one-way analysis of variance (ANOVA) and the Kruskal–Wallis test were used. The Chi-square test or Fisher’s exact test was used for categorical variables. Kaplan-Meier analysis was performed to assess differences in the cumulative incidence of wound healing between the groups, and the Log rank test was used to identify significant differences. All relevant variables were tested using the univariate Cox proportional hazard method, and variables with P < 0.1 were further subjected to multivariate Cox proportional hazard models. Hazard ratios (HR) and corresponding 95% confidence intervals (95% CI) were calculated. The analysis was repeated after stratification by ABI or TcPO2 levels. P < 0.05 was considered statistically significant. Bonferroni’s adjustment was applied to multiple comparisons if the main effect was significant (P < 0.05). Data analyses were performed using SPSS 23.0 statistical software package (IBM Corp., Armonk, NY, USA).
Results
The Clinical Characteristics of the Participants
Patient disposition is shown in Figure 1. Of the 80 subjects, 63 were divided into three groups and completed the study: the CWT group (n = 23), PRP group (n = 20), and graft group (n = 20). The mean age was 60.17 years, with 65.08% of the participants being men. The ulcer area before treatment among the three groups was significantly different (P < 0.05). No statistically significant differences were observed in hemoglobin, albumin, HbA1c, eGFR, TC, TG, HDL-C, LDL-C, ABI, and TcPO2 among the three groups (all P-values > 0.05) (Table 1).
 |
Table 1 Comparison of Baseline Clinical Characteristics Among the Three Groups |
Favorable Predictive Factor for Diabetic Foot Ulcers Healing
Univariate and multivariate Cox regression analyses were conducted to explore factors that might influence DFU healing of diabetic foot ulcers (Table 2). Multivariate Cox analysis indicated that different treatments (PRP treatment: HR = 3.075, 95% CI=1.320–7.161, P = 0.009; graft treatment: HR = 3.214, 95% CI=1.300–7.945, P = 0.011), ABI (HR = 9.917, 95% CI=2.675–36.760, P = 0.001), and TcPO2 (R = 1.040; 95% CI=1.005–1.076; P = 0.023) were independent predictive factors for diabetic foot ulcers healing.
 |
Table 2 Cox Regression Analyses for the Predictive Factor of Diabetic Foot Ulcers Healing |
Stratified Analysis by ABI or TcPO2
The results of stratified analysis using ABI or TcPO2 are shown in Table 3. Compared to the CWT group, a higher TcPO2 (> 40 mmHg) in the graft group was associated with a higher wound healing rate (HR = 5.063, 95% CI=2.016–12.711, P = 0.001). The results were similar after adjusting for relevant confounders including sex, age, and ABI (HR = 15.805, 95% CI=2.016–12.711, P < 0.001). Furthermore, compared with the CWT group, a higher ABI (> 0.9) in the PRP group or graft group had a higher wound healing rate in both the unadjusted (PRP group: HR = 3.497, 95% CI=1.272–9.612, P = 0.015; graft group: HR = 3.009, 95% CI=1.082–8.364, P = 0.035) and adjusted models (including sex, age, and TcPO2) (PRP group: HR = 5.029, 95% CI=1.743–14.509, P = 0.003; graft group: HR = 3.748, 95% CI=1.210–11.607, P = 0.022).
 |
Table 3 Univariate and Multivariate Cox Regression Analysis for ABI- and TcPO2-Stratified Analyses |
Evaluation of Wound Healing Rate Among Three Groups
After 12 weeks of follow-up, 37 patients (58.7%) showed complete wound re-epithelialization. Ten (43.5%), 14 (70.0%), and 13 (65.0%) patients in the CWT, PRP, and graft groups, respectively. Furthermore, the cumulative incidence of wound healing over time was significantly higher in the PRP group or graft group than that in the CWT group (P < 0.05) (Figure 6).
 |
Figure 6 Kaplan-Meier curves of the cumulative incidence of unhealing wound among each group. |
The Comparison of the Speed of Wound Healing
As expected, the wound area in each group gradually decreased over time (all P < 0.01). The wound healing rate in the graft group was higher than that in the CWT group at 4, 8, and 12 weeks (all P < 0.05). The wound healing rate in the graft group was higher than that in the PRP group at 4 and 8 weeks (all P < 0.05) (Table 4).
 |
Table 4 The Comparison of the Wound Healing Speed Among Three Groups |
Comparison of Healing Time and Total Treatment Costs Among Three Groups
Time to wound healing for CWT group, PRP group and graft group, respectively, were 75.00 (68.25, 78.50) days, 64.50 (54.75, 67.25) days and 49.00 (21.00, 62.00) days (Table 5). In addition, the total treatment costs were 657.00 (255.50, 919.80) USD, 583.60 (523.38, 721.75) USD and 394.20 (306.60, 515.49) USD, in CWT group, PRP group and graft group, respectively (Table 5). Importantly, the total treatment cost was lower in the graft group than that in the PRP group (P < 0.01) (Table 5).
 |
Table 5 Comparison of Healing Time and Total Treatment Costs Among Three Groups |
Discussion
DLEUs is a serious complication of diabetes mellitus that can cause loss of quality of life and socioeconomic burden. Although various effective therapies for DLEUs have been developed, their overall treatment efficacy remains unsatisfactory. Therefore, the creation of safer and more efficient treatments is urgently required.
In our previous study, we explored the feasibility of a new skin grafting strategy, autologous point columnar full-thickness skin graft obtained from the ulcer wound margin, which is feasible and effective for the treatment of DLEUs. In addition, PRP has been widely used for the treatment of DLEUs. Therefore, we compared the feasibility, safety, and efficacy of these two treatments in this study. According to the patients’ wishes and physicians’ recommendations, the enrolled patients could freely choose between CWT, PRP, or skin grafting. Wound healing is a complex process and is influenced by many factors, including history of smoking, history of cardiovascular diseases, disturbances in glucose and lipid metabolism, duration of diabetes, and various diabetic complications.16 In our study, we observed no statistically significant differences in demographic characteristics, biochemical parameters (glucose and lipid metabolism parameters), or comorbidities among the three groups. We found that the ulcer area before treatment among the three groups was significantly different. After the 12-week follow-up, more patients in the PRP and graft groups had complete wound re-epithelialization than those in the CWT group, whereas the cumulative incidence of wound healing over time in the PRP and graft groups was significantly higher than that in the CWT group.
DLEUs is a common and serious complication of diabetes mellitus. Elderly patients with a long duration of diabetes, poor glycemic control, malnutrition, infection, and low immunity have an extremely high risk of developing refractory DLEUs,17 which significantly increased morbidity and mortality as a result. As a result, medical treatment of patients with refractory DLEUs is highly limited, difficult, and challenging. PRP is a concentrate of platelet-rich plasma protein produced from blood plasma, and has been widely utilized in regenerative medicine to promote wound healing and accelerate regenerative processes.18 PRP is rich in multiple growth factors, cytokines, and chemokines released by platelet degranulation, including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), interleukins (ILs), monocyte chemoattractant protein-1 (MCP-1) (CCL2), and CXCL10 (IP-10), and so on.19 On the one hand, these factors can promote tissue repair, regeneration and wound healing, on the other hand, exhibit excellent antimicrobial activities and inhibit bacterial growth.20 Several clinical studies have confirmed that PRP is a safe and effective treatment for venous leg and diabetes-related foot ulcers.21 Our results revealed that PRP could accelerate diabetes-induced wound closure and promote complete wound re-epithelialization, in accordance with the findings of previous studies.
Recently, several studies have reported that skin grafting offers significant advantages in wound healing and tissue regeneration, especially in large areas of skin defects or damage caused by burns, wounds and ulcers.22 The most common skin graft techniques include allografts, xenografts, and autologous skin transplants.23 Allografts and xenograft skin grafts may increase the risk of immune rejection and disease transmission.24 The common donor sites for autologous skin grafts are the lateral thigh, lateral femur, and medial femur. Autologous skin grafts can avoid the above risks, but are limited by the quantity and size of the donor sites, secondary injury pain, bleeding, scarring, and increased risk of infection at the donor site. Inspired by the skin biopsy punch, which is used for skin biopsies in our dermatology department, we developed a novel cutaneous wound repair, autologous point columnar full-thickness skin graft taken from the ulcer, which holds great promise for DLEUs. The reasons for this can be explained as follows. First, the structure and thickness of skin tissues from the edges of ulcers are close to the native structure of the wound ulcers. Second, the skin samples were taken from the ulcer edges in the form of microcolumns, which only slightly increased the wound area, but significantly decreased scar formation, new infections, and pain. Furthermore, given the presence of diabetic neuropathy, skin extraction from the wound edge did not increase the pain sensation. Third, platelet-rich plasma derived from skin extracts promotes wound healing by regulating nutrients, immune reactions, growth factors and inflammation.25 Furthermore, NPWT has been extensively used in the treatment of various wounds because it can enhance skin survival and accelerate wound healing through multiple mechanisms26 such as the promotion of angiogenesis and cell proliferation, modulation of inflammation and immunity, and inhibition of bacterial growth. Previous studies have shown that a split-thickness skin graft (STSG) combined with NPWT is effective and is recommended for the treatment of diabetes-related foot ulcers.27 Our previous study also indicated that a combination of NPWT and autologous full-thickness skin grafts could accelerate wound healing.11 Here, we observed a similar therapeutic effect between autologous point columnar full-thickness skin grafts and PRP grafts. The time to wound healing in the graft and PRP groups was significantly shorter than in the CWT group, and the cumulative incidence of wound healing over time was significantly higher in the graft and PRP groups than in the CWT group. Therefore, autologous wound edge dotted full-thickness skin grafting is regarded as workable, safe, and efficient.
Next, we explored several factors associated with diabetic foot ulcers healing. Cox analysis indicated that different treatments and higher ABI or TcPO2 were independent predictive factors of wound healing. ABI and TcPO2 were used to evaluate the lower extremity macrovascular status and tissue microcirculatory perfusion around the wound.28 It has been shown that ABI and TcPO2 were closely related to the outcome of wound healing in diabetic foot uclers29 and are reliable indicators for predicting the prognosis of diabetic foot ulcers, amputation risk, or even all-cause mortality.30 In the present study, multivariate Cox regression analysis indicated that the graft and PRP groups exhibited higher wound-healing abilities than the CWT group. Subsequently, in the stratified analysis by TcPO2, higher TcPO2 (> 40 mmHg) in the graft group had a higher wound healing rate than that in the CWT group, but not in the PRP group. In the stratified analysis by ABI, compared to the CWT group, a higher ABI (> 0.9) in the graft or PRP group promoted wound healing more effectively. At the same time, we also observed that lower TcPO2 (< 40 mmHg) and ABI (< 0.9) did not exhibit efficacy differences among the three groups (data not shown). This suggests that good reperfusion and improvement of blood circulation are essential for granulation tissue formation and epidermal cell regeneration in the wound tissue. In addition, the wound healing rate in the graft group was higher than those in the PRP and CWT groups. More importantly, the total treatment cost in the graft group was lower than that in the PRP group, which could reduce healthcare expenditure and the economic burden on families and society. Factors that affect the medical costs of patients may include the treatment method of ulcer wounds, wound healing time, surgical intervention caused by difficult-to-heal wounds, and length of hospital stay. Our study found that compared to the CWT groups, the wound healing time (P < 0.0167) and cumulative healing rate (P < 0.05) in both the PRP group and graft group were more advantageous. However, since ulcer wounds in our medical centers typically have a large area, the PRP group usually requires multiple PRP treatments. But the Graft group only performs one-point columnar skin grafting. This may be the reason for the difference in treatment costs. In brief, this study implied that the graft group promotes wound re-epithelialization earlier and faster than in the CWT and PRP groups (P < 0.05).
To sum up, the novel wound therapy modality requires adherence to standardized wound professional assessments and individual patient wishes, as well as monitoring the wound healing process to obtain personalized negative pressure pump usage parameters and dressing changes to improve wound healing.
Limitations
Our study has several limitations. First, the sample size in our study was small, and larger sample sizes are required to confirm our results. Second, selection bias could have been introduced because this study was not designed as a randomized controlled trial. Further randomized controlled trials are required to validate these findings. Third, autologous wound edge-dotted columnar full-thickness skin grafting combined with PRP may improve the wound microenvironment and provide multiple growth factors, which in turn increases wound healing and shortens healing time. In the next step, we plan to perform two or more combined treatments to validate our idea.
Conclusions
In summary, autologous wound edge-dotted full-thickness skin grafting can effectively and safely promote wound healing in DFUs. Skin gratings have a higher wound healing rate, faster healing speed, and lower cost of treatment, which is worthy of further clinical application.
Data Sharing Statement
The original raw data used in this study is available from the corresponding author and can be provided upon reasonable request.
Ethics Approval and Consent to Participate
All the patients agreed to participate in this study and provided written informed consent. The Ethics Committee of the Guangxi Academy of Medical Sciences and the People’s Hospital of the Guangxi Zhuang Autonomous Region approved this study, which was carried out in accordance with the Declaration of Helsinki.
Acknowledgments
We thank the Diabetic Foot Disease Studio of the Department of Endocrinology and Metabolism, Information Network Center, Medical Record Information Quality Control Center of Guangxi Academy of Medical Sciences, and the People’s Hospital of Guangxi Zhuang Autonomous Region for assisting in retrieving the medical records. We thank all enrolled patients, medical staff, volunteers, and sponsors of the scientific research funds involved in this study.
Funding
This research was funded by the Natural Science Foundation of China (82160052, 81560044, 30860113) and Guangxi Zhuang Autonomous Region Health Committee Project (Z20170400).
Disclosure
The authors have no relevant financial or non-financial interests to disclose for this work.
References
1. Armstrong DG, Wrobel J, Robbins JM. Guest editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J. 2007;4(4):286–287. doi:10.1111/j.1742-481X.2007.00392.x
2. Navarro-Flores E, Gijon-Nogueron G, Cervera-Marin JA, Labajos-Manzanares MT. Assessment of foot self-care in patients with diabetes: retrospective assessment (2008–2014). Foot Ankle Spec. 2015;8(5):406–412. doi:10.1177/1938640015585963
3. Lepantalo M, Apelqvist J, Setacci C, et al. Chapter V: diabetic foot. Eur J Vasc Endovasc Surg. 2011;42(Suppl 2):S60–74. doi:10.1016/S1078-5884(11)60012-9
4. Schaper NC, van Netten JJ, Apelqvist J, et al. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(Suppl 1):e3266. doi:10.1002/dmrr.3266
5. Game FL, Apelqvist J, Attinger C, et al. Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32(Suppl 1):154–168. doi:10.1002/dmrr.2707
6. Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21(20):7794. doi:10.3390/ijms21207794
7. Mo J, Huang Y, Wang Q, et al. Autologous wound margin point columnar full-thickness skin grafting combined with negative pressure wound therapy improves wound healing in refractory diabetic foot ulcers. Int Wound J. 2023;20(5):1506–1516. doi:10.1111/iwj.14005
8. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi:10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
9. Ontario H. Skin substitutes for adults with diabetic foot ulcers and venous leg ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2021;21(7):1–165.
10. Voigt J, Wendelken M, Driver V, Alvarez OM. Low-frequency ultrasound (20-40 kHz) as an adjunctive therapy for chronic wound healing: a systematic review of the literature and meta-analysis of eight randomized controlled trials. Int J Low Extrem Wounds. 2011;10(4):190–199. doi:10.1177/1534734611424648
11. Lee KN, Ben-Nakhi M, Park EJ, Hong JP. Cyclic negative pressure wound therapy: an alternative mode to intermittent system. Int Wound J. 2015;12(6):686–692. doi:10.1111/iwj.12201
12. Pickwell KM, Siersma VD, Kars M, Holstein PE, Schaper NC. Diabetic foot disease: impact of ulcer location on ulcer healing. Diabetes Metab Res Rev. 2013;29(5):377–383. doi:10.1002/dmrr.2400
13. Powers JG, Higham C, Broussard K, Phillips TJ. Wound healing and treating wounds: chronic wound care and management. J Am Acad Dermatol. 2016;74(4):
14. Lavery LA, Armstrong DG, Harkless LB. Classification of diabetic foot wounds. J Foot Ankle Surg. 1996;35(6):528–531. doi:10.1016/s1067-2516(96)80125-6
15. Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi:10.1681/ASN.2006040368
16. Dayton KD, Lancaster LE. Part 2: effects of renal failure and its treatment on the immune system and assessment of immune system function. ANNA J. 1995;22(6):530–7, 572.
17. Haughey L, Barbul A. Nutrition and lower extremity ulcers: causality and/or treatment. Int J Low Extrem Wounds. 2017;16(4):238–243. doi:10.1177/1534734617737639
18. Alsousou J, Ali A, Willett K, Harrison P. The role of platelet-rich plasma in tissue regeneration. Platelets. 2013;24(3):173–182. doi:10.3109/09537104.2012.684730
19. Marques LF, Stessuk T, Camargo IC, Sabeh Junior N, dos Santos L, Ribeiro-Paes JT. Platelet-rich plasma (PRP): methodological aspects and clinical applications. Platelets. 2015;26(2):101–113. doi:10.3109/09537104.2014.881991
20. Mariani E, Filardo G, Canella V, et al. Platelet-rich plasma affects bacterial growth in vitro. Cytotherapy. 2014;16(9):1294–1304. doi:10.1016/j.jcyt.2014.06.003
21. Picard F, Hersant B, Bosc R, Meningaud JP. The growing evidence for the use of platelet-rich plasma on diabetic chronic wounds: a review and a proposal for a new standard care. Wound Repair Regen. 2015;23(5):638–643. doi:10.1111/wrr.12317
22. Boa O, Cloutier CB, Genest H, et al. Prospective study on the treatment of lower-extremity chronic venous and mixed ulcers using tissue-engineered skin substitute made by the self-assembly approach. Adv Skin Wound Care. 2013;26(9):400–409. doi:10.1097/01.ASW.0000433102.48268.2a
23. Alven S, Nqoro X, Aderibigbe BA. Polymer-based materials loaded with curcumin for wound healing applications. Polymers. 2020;12(10):2286. doi:10.3390/polym12102286
24. Delloye C, Cornu O, Druez V, Barbier O. Bone allografts: what they can offer and what they cannot. J Bone Joint Surg Br. 2007;89(5):574–579. doi:10.1302/0301-620X.89B5.19039
25. Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, Kusumoto K. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008;122(5):1352–1360. doi:10.1097/PRS.0b013e3181882046
26. Banwell P, Withey S, Holten I. The use of negative pressure to promote healing. Br J Plast Surg. 1998;51(1):79. doi:10.1016/s0007-1226(98)80142-2
27. Kneilling M, Breuninger H, Schippert W, Hafner HM, Moehrle M. A modified, improved, easy and fast technique for split-thickness skin grafting. Br J Dermatol. 2011;165(3):581–584. doi:10.1111/j.1365-2133.2011.10431.x
28. Jorneskog G. Why critical limb ischemia criteria are not applicable to diabetic foot and what the consequences are. Scand J Surg. 2012;101(2):114–118. doi:10.1177/145749691210100207
29. Wang Z, Hasan R, Firwana B, et al. A systematic review and meta-analysis of tests to predict wound healing in diabetic foot. J Vasc Surg. 2016;63(2 Suppl):
30. Fagher K, Katzman P, Londahl M. Transcutaneous oxygen pressure as a predictor for short-term survival in patients with type 2 diabetes and foot ulcers: a comparison with ankle-brachial index and toe blood pressure. Acta Diabetol. 2018;55(8):781–788. doi:10.1007/s00592-018-1145-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.