Back to Journals » Infection and Drug Resistance » Volume 15
A Novel Nomogram for Predicting Risk Factors and Outcomes in Bloodstream Infections Caused by Klebsiella pneumoniae
Authors Chen Y, Ying S , Jiang L, Dong S, Dai J, Jin X, Yu W, Qiu Y
Received 27 November 2021
Accepted for publication 16 March 2022
Published 29 March 2022 Volume 2022:15 Pages 1317—1328
DOI https://doi.org/10.2147/IDR.S349236
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yingsha Chen,1,2 Shuaibing Ying,1 Lushun Jiang,1 Shaohua Dong,1 Jinyao Dai,1 Xuehang Jin,1 Wei Yu,1 Yunqing Qiu1
1State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 2Infectious Diseases, the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
Correspondence: Wei Yu; Yunqing Qiu, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China, Tel +86-571-87236606, Fax +86-571-87236606, Email [email protected]; [email protected]
Background: Our study aimed to explore the risk factors in bloodstream infections Klebsiella pneumoniae (BSI-KP) patients and establish nomograms to predict the probability of BSI-CRKP and the prognosis of BSI-KP.
Methods: A total of 252 BSI-KP patients were enrolled from a tertiary teaching hospital between January 1, 2015, and May 31, 2020. Risk factors associated with BSI-CRKP and factors associated with the 30-day mortality were identified using LASSO analysis, univariate and multivariate analysis.
Results: There were 121 (48.0%) patients with carbapenem-resistant K. pneumoniae (CRKP) and 131 (52.0%) patients with carbapenem-susceptible K. pneumoniae (CSKP). The multivariate logistic regression analysis demonstrated that gastric tube indwelling before BSI (OR=2.442, P=0.043) and more types of antibiotics use before BSI (OR=1.305, P=0.009) were independent risk factors for BSI-CRKP. And previous transplantations, prior ICU stay, gastric tube indwelling before BSI, more types of antibiotics use before BSI, lower Hb and cholinesterase were associated with CRKP-BSI. The C-index of models indicated its good accuracy (C-index 0.816, 95% CI 0.763– 0.868). In patients with BSI-CRKP, further logistic regression analysis revealed urinary catheterization (OR=0.298, P=0.017) was found to be an independent risk factor for 30-day mortality, while ceftazidime/avibactam use (OR=8.438, P=0.003) was an independent favorable prognostic factor. The nomogram predicated CRKP, ICU hospitalization, more types of antibiotics use, tigecycline, PLT, urinary catheterization were associated with 30-day mortality in patients with BSI-KP. The discriminative ability of the predictive model, as assessed by C-index, was 0.813 (95% CI: 0.780– 0.867).
Conclusion: Previous transplantations, prior ICU stay, gastric tube indwelling before BSI, more types of antibiotics use before BSI, lower Hb and cholinesterase represent significant risk factors for the development of BSI-CRKP. Our nomogram predicated thrombocytopenia was a sign for poor prognosis. Tigecycline resulted in higher mortality for patients with BSI-KP. Rational use of nomograms may help clinicians make better Clinical decisions when treating BSI-KP patients.
Keywords: carbapenem-resistant Klebsiella pneumoniae, prognostic model
Introduction
Klebsiella pneumoniae (KP) has emerged as a major clinical and public health threat, owing to increasing prevalence of healthcare-associated infections caused by multidrug-resistant strains with extended-spectrum β-lactamases or carbapenemases.1 The proportion of KP increased from 6.6% during 2001–2004 to 9.9% during 2013–2016.2 SENTRY Antimicrobial Surveillance Program data showed BSI-KP accounting for 7.7%.3 Recent studies demonstrated carbapenem-resistant Klebsiella pneumoniae (CRKP) is associated with a prolonged hospital stay, increased medical costs and higher mortality for patients.4
Hospital-acquired infections, intensive care unit (ICU) stay, illness severity, and inappropriate antibiotic Treatment have been identified as risk factors contributing to increased mortality rates in patients with BSI-CRKP.5,6 However, current studies only briefly introduced several risk factors for mortality rates in patients with BSI-CRKP. In addition, these factors were partly varied or contradictory. Therefore, a personalized prediction model is needed for patients with BSI-CRKP. The nomogram, as an integrated model, has been widely used as a predictive method for clinicians. In this retrospective analysis, we aimed to establish nomograms to predict the probability of BSI-CRKP and the prognosis of BSI-KP, in order to improve infection control and clinical treatment.
Methods
Study Cohort and Route
This retrospective cohort study was carried out at The First Affiliated Hospital, Zhejiang University of Medicine, a 5000-bed National-level hospital in Eastern China. The study focused on patients with BSI-KP (including CRKP and carbapenem-susceptible K. pneumoniae (CSKP) between January 1, 2015, and May 31, 2020. The inclusion criteria were as follows: (i) patients older than 16 years and less than 90 years, (ii) patients confirmed by blood culture positive for KP and met the diagnostic criteria for BSI. Only the first episode of bloodstream infection was included if there were repeated episodes of BSI-KP during the study period. Patient demographics, clinical and microbiological data, Laboratory tests, antimicrobial treatment and outcome, past history and underlying disease, and other relevant information were obtained from the hospitals electronic medical record system. Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) scores were used to assess the severity of the disease.
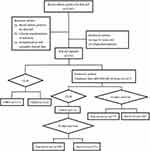
The predefined route of this study is demonstrated in Figure 1. 30-day mortality was used as treatment outcome. The primary end-point was all-cause death, that is, crude death. In view of the current situation in China, many families are reluctant to let sick people die outside. Those who died or gave up treatment due to their worsening condition and were discharged automatically during hospitalization were defined as death cases. The risk factors for BSI-CRKP and 30-day mortality were further analyzed.
Definitions
The onset of BSI-KP was defined as the collection date of a positive blood culture. Species confirmation and sensitivity testing were performed using standard biochemical methods, via a VITEK 2 automated system (bioMe´rieux, Marcy l’Etoile, France). Carbapenem resistance was defined as isolates resistant to imipenem, meropenem or ertapenem according to Clinical and Laboratory Standards Institute (CLSI) interpretation.7 Tigecycline susceptibility was determined using the US Food and Drug Administration (FDA) breakpoints.8
Corticosteroid therapy was defined as the administration of at least 20 mg of a prednisone equivalent for a period of ≥7 days. Antimicrobial drug exposure was defined as the use of antibiotics for more than 48h within the 30 days prior to onset of BSI. All antibiotics in the treatment regimen were administered for over a period of 48 hours. The dosage of tigecycline used to treat BSI-KP was divided into regular dose (initial loading dose of 100 mg followed by 50 mg every 12 hours) and high dose (100 mg every 12 hours) throughout the study period. Severity of illness was assessed using APACHE II and SOFA scoring system.
Statistical Analysis
For continuous variables, results were presented as mean ± standard deviation or median (IQR) as appropriate. Categorical variables were presented by numbers with percentages. Continuous variables were assessed for normality test and analyzed using t-test or Mann–Whitney U-test. For categorical variables, chi-square test or two-tailed Fisher’s exact test was employed appropriately. The strength of associations was assessed in terms of the odds ratio (OR) and 95% confidence interval (CI).
Multivariate analysis was performed after further screening for variables with a P-value of <0.05 in the univariate analysis. LASSO analysis, univariate and multivariate analysis were used to identify independent risk factors. The LASSO regression analyses were performed to screen candidate predictors before obtaining a final prediction model. The final multiple regression model is constructed from the set of candidate variables by gradually removing predictor variables based on p-values.9 The nomogram for predicting BSI-CRKP probability and prognosis of BSI-KP were established basing on the regression model by employing the R package. Model discrimination was assessed with calibration curves and C-index basing on Bootstrap. All statistical tests were two-tailed, and a P-value <0.05 indicated statistically significant. Data were performed using SPSS Version 22.0 and RStudio.
Results
Incidence of BSI-KP
A total of 252 non-duplicate BSI-KP were included in this study. There were 121 CRKP and 131 CSKP. The number of BSI-KP, especially for CRKP, showed a clear upward trend during 2017 to 2018, reaching the highest in 2018 (Figure 2A).
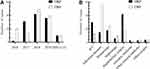 |
Figure 2 The number of BSI-KP cases. (A) The number of CRKP cases and CSKP from 2016 to May 2020; (B) The departments distribution of patients with BSI-KP. |
The average age was 52.7 years old, without significant difference between CRKP and CSKP patients. There were 174 patients (69.0%) were male. The incidence of BSI-CRKP in the department of hepatobiliary surgery and ICU was higher than other departments (Figure 2B). However, the incidence of BSI-CSKP in the department of infectious diseases and hematology was higher than other departments.
Risk Factors Associated with BSI-CRKP
Univariate logistic analysis revealed ICU hospitalization, pulmonary disease, cardiac, previous transplantations, previous surgery, previous hospitalization, previous corticosteroid or immunosuppressant use, previous ICU stay, prior antibiotic use, prior tigecycline use or a nonsurgical invasive procedure before BSI were associated to CRKP (Supplementary Table 1). Moreover, patients with BSI-CRKP were more likely to have higher neutrophil proportion, lower Hb, lower platelet (PLT), lower Cholinesterase or serum total bilirubin (TBIL) before BSI within 2 days. There was no significant difference between CRKP and CSKP in terms of APACHE II and SOFA scores. Variables were further screened by LASSO regression to eliminate variables with no clinical significance, and seven factors were incorporated into the model. The multivariate logistic regression analysis demonstrated that BSI-CRKP was independently associated with gastric tube indwelling before BSI (OR=2.442, P=0.043) and more types of antibiotics use before BSI (OR=1.305, P=0.009). To visualize the logistic regression model, a nomogram predicting the probability of BSI-CRKP was constructed (Figure 3), including previous transplantations, prior ICU stay, gastric tube indwelling, more types of antibiotics use before BSI, Hb and Cholinesterase. The C-index of models indicated its good accuracy (C-index 0.816, 95% CI 0.763–0.868). Calibration curve using the bootstrap method (1000 times) was plotted (Figure 4), which showed a good agreement between the predicted model and the actual observations.
Risk Factors for 30-Day Mortality in Patients with BSI-CRKP
A total of 113 patients were included in this analysis (Supplementary Table 2). The rate of 30-day mortality among BSI-CRKP patients was 51.3%. Previous transplantations, past corticosteroid or immunosuppressant use, urinary catheterization, arterial catheterization, and use of more than 2 antibiotics were risk factors for 30-day mortality among BSI-CRKP patients. Further logistic regression multivariate analysis revealed urinary catheterization (OR=0.298, P=0.017) was found to be an independent risk factor for 30-day mortality in patients with BSI-CRKP, while the ceftazidime/avibactam use (OR=8.438, P=0.003) was an independent favorable prognostic factor.
The Nomogram to Predict the 30-Day Survival in Patients with BSI-KP
Table 1 showed the main characteristics of the BSI-KP survivor and non-survivor subgroups. Patients treated for less than 48 hours after infection were excluded. Among all variables evaluated, univariate logistic regression analysis showed that more than 30 factors were significantly correlated with 30-day mortality for BSI-KP. In addition, BSI-KP non-survivors generally had lower Hb, lower platelet, lower cholinesterase, higher international normalized ratio (INR) and TBIL before BSI within 2 days.
 |  |  |
Table 1 Analysis of Risk Factors for 30-Day Mortality in 237 Patients with BSI-KP |
Further LASSO regression analysis was used to screen predicting features of 30-day survival in patients with BSI-KP. A multivariate predictive model was established, including CRKP, ICU hospitalization, types of antibiotics, tigecycline, PLT, urinary catheterization (Figure 5). The 30-day mortality could be measured by the sum of the scores for each predictor variable through the nomogram. It is worth mentioning that the weight of ICU hospitalization is maximum.
The discriminative ability of the predictive model, as assessed by C-index, was 0.813 (95% CI: 0.780–0.867). There was a strong consistency between the predicted mortality rate and the actual mortality rate (Figure 6A). Meanwhile, 81 patients were used for further model external validation (Figure 6B). The C-index based on the validation set was 0.700 (0.550–0.832). The shape of the curve on the calibration plots indicated that the external validation model was well-calibrated.
Discussion
CRKP has the potential to extensively disseminate and has a high fatality rate, presenting a great challenge to public health around the world.1 Several risk factors had been linked to BSI-CRKP and mortality.5,6 Data from 252 BSI-KP patients were evaluated, demonstrating previous transplantations, prior ICU stay, gastric tube indwelling before BSI, more types of antibiotics use before BSI, lower Hb and Cholinesterase were associated with CRKP-BSI, most of which were consistent with previous Research.5,10 Antimicrobial use prior to BSI is known as an important factor in drug-resistant infections.11,12 However, some studies showed no association between CRKP infection and prior antibiotic use.6 In our study, we found that the frequent antibiotic changes before BSI-KP was likely to promote the occurrence of carbapenem-resistant Klebsiella pneumoniae.
It was interesting to note that decreased cholinesterase was a risk factor for BSI-CRKP, which has been rarely reported. As is well known, the acetylcholinesterase plays a crucial role in the restoration of cholinergic neurons after activation by hydrolyzing the neurotransmitter acetylcholine. Sunita et al, mentioned that overuse of acetylcholinesterase inhibitors led to resistance in organisms via mutations in the corresponding binding site in their study.13 However, this fundamental question awaits further investigation.
Further nomogram predicated CRKP, ICU hospitalization, types of antibiotics, tigecycline, PLT, urinary catheterization were associated with 30-day mortality in patients with BSI-KP. Previous studies have demonstrated CRKP patients had shorter survival time than CSKP patients.14,15 Similar to these studies, we also found CRKP was an important risk factor for 30-day mortality rates. The main reason is due to limited antibiotic options for CRKP, resulting in poor prognosis. Prior antibiotics use has been identified as an important factor related to antibiotics resistance.11,12 Our study revealed that the number of antibiotics was closely associated with 30-day mortality. The more antibiotics used before BSI, the worse the prognosis in patients with BSI-KP. Therefore, careful attention should be paid to the frequent replacement of the antibiotics before BSI-KP.
It is of note that tigecycline regimens for patients with BSI-CRKP remains controversial. Previous meta-analysis indicated that tigecycline had no more effective than standard antimicrobial agents for serious infections.16 In addition, FDA has warned against the off-label use of tigecycline to treat nosocomial pneumonia due to increased mortality risk.17 In the present study, tigecycline resulted in higher mortality. Recently, several studies suggested high-dose treatment of tigecycline (200 mg loading dose, followed by 100 mg every 12 h) was superior to standard dosage (100 mg loading dose, followed by 50 mg every 12 h) for 30-day survival rate (OR 2.25 [0.55; 9.24]).18
However, our study did not find the same phenomenon. Thus, further research is needed to study for optimized use of tigecycline against BSI-KP.
Interestingly, our nomogram predicated thrombocytopenia was a sign of poor prognosis. Qin Wu et al reported persistent thrombocytopenia was associated with poorer outcomes in critical care patients.19 In mouse KP infection models, thrombocytopenia was observed as well.20 Furthermore, hypermucoviscous KP strains caused more obvious thrombocytopenia than classic KP strains.20 These may be related to lipopolysaccharide (LPS) released by KP.20 LPS could stimulate cell apoptosis via many pathways, such as TLR4/NF-κB and JNK/MAPK.21,22 The intrinsic apoptosis pathway was of importance to platelet survival.23,24
Although the results are meaningful, there were still several limitations in this study. Firstly, this study was retrospective form and the number of patients remained smalls. Secondly, we did not identify different virulence genes in CRKP and CSKP. In addition, it could be further optimized in the initial design of the study grouping, replacing the survivors/non-survivors with the treatment success/treatment failure. The corresponding groupings were well defined in the study of Youhua Yuan et al.25
Conclusion
This study demonstrated that previous transplantations, prior ICU stay, gastric tube indwelling before BSI, more types of antibiotics use before BSI, lower Hb and Cholinesterase represent significant risk factors for the development of BSI-CRKP. Our nomogram predicated thrombocytopenia was a sign of poor prognosis, and tigecycline resulted in higher mortality for patients with BSI-KP. CRKP, ICU hospitalization, more types of antibiotics use before BSI, tigecycline use after BSI, PLT, urinary catheterization were associated with 30-day mortality in patients with BSI-KP. Careful attention may should be paid to the frequent replacement of the antibiotics before Klebsiella pneumoniae infection, which may be beneficial to reduce the occurrence of drug resistance and mortality in severe patients. For patients with BSI-CRKP, urinary catheterization was the independent risk factor for 30-day mortality, while ceftazidime/avibactam was an independent favorable prognostic factor. The nomogram established in this study showed good consistency and discrimination in evaluating the probability of BSI-CRKP and the prognosis of patients with BSI-KP. Rational use of nomograms may help clinicians make better clinical decisions when treating BSI-KP patients.
Ethical Approval
This study was approved by the recommendations of the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University with written informed consent from all subjects (Reference Number:20210789). All subjects provided written informed consent in accordance with the Declaration of Helsinki.
Funding
This study was funded by Youth Program of National Natural Science Foundation of China (No. 81803589).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wyres KL, Lam MMC, Holt KE. Population genomics of Klebsiella pneumoniae. Nat Rev Microbiol. 2020;18(6):344–359.
2. Diekema DJ, Pfaller MA, Jones RN, et al. Trends in antimicrobial susceptibility of bacterial pathogens isolated from patients with bloodstream infections in the USA, Canada and Latin America. SENTRY Participants Group. Int J Antimicrob Agents. 2000;13(4):257–271.
3. Diekema DJ, Hsueh PR, Mendes RE, et al. The Microbiology of Bloodstream Infection: 20-Year Trends from the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother. 2019;63(7):e00355–19.
4. Pan H, Lou Y, Zeng L, et al. Infections Caused by Carbapenemase-producing Klebsiella pneumoniae: microbiological Characteristics and Risk Factors. Microb Drug Resist. 2019;25(2):287–296.
5. Shilo S, Assous MV, Lachish T, et al. Risk factors for bacteriuria with carbapenem-resistant Klebsiella pneumoniae and its impact on mortality: a case-control study. Infection. 2013;41(2):503–509.
6. Debby BD, Ganor O, Yasmin M, et al. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur J Clin Microbiol Infect Dis. 2012;31(8):1811–1817.
7. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. M100, 30th Edition. Available from: http://www.clsi.org/.
8. Food and Drug Administration. Antibacterial Susceptibility Test Interpretive Criteria. Available from: https://www.fda.gov/drugs/development-resources/antibacterial-susceptibility-test-interpretive-criteria.
9. Serenari M, Han KH, Ravaioli F, et al. A nomogram based on liver stiffness predicts postoperative complications in patients with hepatocellular carcinoma. J Hepatol. 2020;73(4):855–862.
10. Xiao T, Yu W, Niu T, et al. A retrospective, comparative analysis of risk factors and outcomes in carbapenem-susceptible and carbapenem-nonsusceptible Klebsiella pneumoniae bloodstream infections: tigecycline significantly increases the mortality. Infect Drug Resist. 2018;11:595–606.
11. Orsi GB, García-Fernández A, Giordano A, et al. Risk factors and clinical significance of ertapenem-resistant Klebsiella pneumoniae in hospitalised patients. J Hosp Infect. 2011;78(1):54–58.
12. Patel G, Huprikar S, Factor S, et al. Outcomes of Carbapenem-Resistant Klebsiella pneumoniae Infection and the Impact of Antimicrobial and Adjunctive Therapies. Infect Control Hospital Epidemiol. 2008;29(12):1099–1106.
13. Thapa S, Min L, Hui X, et al. Acetylcholinesterase: a Primary Target for Drugs and Insecticides. Mini Rev Med Chem. 2017;17(17):1665–1676.
14. Zhang H, Guo Z, Chai Y, et al. Risk Factors for and Clinical Outcomes of Carbapenem-Resistant Klebsiella pneumoniae Nosocomial Infections: a Retrospective Study in a Tertiary Hospital in Beijing, China. Infect Drug Resist. 2021;14:1393–1401.
15. Wang Q, Zhang Y, Yao X, et al. Risk factors and clinical outcomes for carbapenem-resistant Enterobacteriaceae nosocomial infections. Eur J Clin Microbiol Infect Dis. 2016;35(10):1679–1689.
16. Cai Y, Wang R, Liang B, et al. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of Infectious disease. Antimicrob Agents Chemother. 2011;55(3):1162–1172.
17. Food and Drug Administration. Drug safety announcement–tigecyclin. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm369580.htm.
18. Ni W, Han Y, Liu J, et al. Tigecycline Treatment for Carbapenem-Resistant Enterobacteriaceae Infections: a Systematic Review and Meta-Analysis. Medicine. 2016;95(11):e3126.
19. Wu Q, Ren J, Wang G, et al. The Incidence, Clinical Outcomes, and Risk Factors of Thrombocytopenia in Intra-Abdominal Infection Patients: a Retrospective Cohort Study. PLoS One. 2016;11(1):e0147482.
20. Wang Z, Ren J, Liu Q, et al. Hypermucoviscous Klebsiella pneumoniae infections induce platelet aggregation and apoptosis and inhibit maturation of megakaryocytes. Thromb Res. 2018;171:45–54.
21. Guo C, Yuan L, Wang JG, et al. Lipopolysaccharide (LPS) induces the apoptosis and inhibits osteoblast differentiation through JNK pathway in MC3T3-E1 cells. Inflammation. 2014;37(2):621–631.
22. Fan WJ, Li HP, Zhu HS, et al. NF-κB is involved in the LPS-mediated proliferation and apoptosis of MAC-T epithelial cells as part of the subacute ruminal acidosis response in cows. Biotechnol Lett. 2016;38(11):1839–1849.
23. Catani L, Fagioli ME, Tazzari PL, et al. Dendritic cells of immune thrombocytopenic purpura (ITP) show increased capacity to present apoptotic platelets to T lymphocytes. Exp Hematol. 2006;34(7):879–887.
24. Leytin V, Mykhaylov S, Starkey AF, et al. Intravenous immunoglobulin inhibits anti-glycoprotein IIb-induced platelet apoptosis in a murine model of immune thrombocytopenia. Br J Haematol. 2006;133(1):78–82.
25. Yuan Y, Wang J, Yao Z, et al. Risk Factors for Carbapenem-Resistant Klebsiella pneumoniae Bloodstream Infections and Outcomes. Infect Drug Resist. 2020;13:207–215.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.