Back to Journals » Cancer Management and Research » Volume 15
A Novel Nomogram for Identifying the Patients at Risk for Rapid Progression of Advanced Hormone-Sensitive Prostate Cancer
Authors Wu M , Pan C, He Y, Yang B
Received 9 June 2023
Accepted for publication 7 September 2023
Published 18 September 2023 Volume 2023:15 Pages 1015—1024
DOI https://doi.org/10.2147/CMAR.S425181
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eşkazan
Mingshuang Wu,* Chenxi Pan,* Yi He, Bo Yang
Department of Urology, The Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yi He; Bo Yang, The Second Affiliated Hospital of Dalian Medical University, 467, Zhongshan Road, Shahekou District, Dalian, 116044, People’s Republic of China, Tel/Fax +86 411 84671291, Email [email protected]; [email protected]
Purpose: The goal of this study was to assess the prognostic impact of the lower urinary tract symptoms (LUTS) in advanced prostate cancer (PCa) patients before progression to castration-resistant prostate cancer (CRPC).
Methods: A retrospective analysis of the follow-up data for 152 CRPC patients was performed. Severe LUTS symptom was defined as an International Prostate Symptoms Score (IPSS) ≥ 20 at baseline. Cox regression analysis was conducted to assess CRPC prognostic factors. Nomogram model was created and assessed using the concordance index (C-index), calibration curves, receiver operating characteristic (ROC) curves, and decision curve analyses (DCA).
Results: The median CRPC free survival of patients with severe LUTS was 20.5 months, significantly longer than that (7.5 months) of less symptomatic patients. Furthermore, severe LUTS, the hemoglobin, albumin, lymphocyte, and platelet (HALP) score, and Gleason sum were determined to be independent prognostic markers and combined to establish a nomogram, which performed well in the customized prediction of CRPC progression at 6th, 12th, 18th and 24th month. The C-index (0.794 and 0.816 for the training and validation cohorts, respectively), calibration curve, and ROC curve all validated the prediction accuracy. DCA curve showed that it could be effective in helping doctors make judgments. The Nomogram-related risk score separated the patients into two groups with notable progression differences.
Conclusion: Severe LUTS was significantly associated with decreased risk for rapid progression to CRPC. The developed nomogram could help identify patients who are at a high risk of rapid CRPC progression and provide tailored follow-up and therapeutic advice.
Keywords: LUTS, HALP, castration-resistant prostate cancer, nomogram
Introduction
Prostate cancer (PCa) is currently one of the leading causes of cancer-specific death in males, accounting for approximately 350,000 fatalities globally each year.1 Androgen deprivation therapy (ADT), the cornerstone of care for people with locally or metastatic hormone-sensitive prostate cancer (HSPC), slows the growth of PCa by lowering the level of circulating testosterone. But most patients who received ADT eventually developed to castration-resistant prostate cancer (CRPC), which has a median survival period of just 14 months.2,3 Hussain et al’s theory states that if the CRPC stage appeared within the first seven months of ADT, the risk of death would increase four times.4 In order to execute an early follow-up plan to rapidly detect progression and optimize treatment regimens, such as chemotherapy, immunotherapy and so on, it is vital to clarify the signs that potentially predict progression to CRPC in patients with locally advanced PCa and metastatic PCa.5–10
Lower urinary tract symptoms (LUTS), which include issues with urine storage and voiding, are quite prevalent in men and are thought to affect 80% of men over the age of 60.11 One of the most frequent reasons for patient visits to urologists is LUTS, which are always considered to be a possible symptom of PCa. Thus, a link between LUTS and PCa is almost inevitable given the high prevalence of LUTS in elderly men. In our clinical setting, we discovered that patients with advanced PCa who originally sought care for LUTS symptoms had a better prognosis than those who sought care for other conditions including elevated PSA, bone pain, etc. Although several studies about LUTS and PCa have been published, they mainly focused on effect of LUTS on PCa screening,12 describing the LUTS‐related quality of life in patients with PCa.13 Ours is the first study to investigate the effect of LUTS on the progression from HSPC to CRPC for identifying HSPC patients with a rapid progression.
Nomogram use has been compared favorably to the conventional cancer staging systems, and as a result, it has been suggested as a substitute or even as a new benchmark.14 In order to develop a model for predicting the advancement of CRPC and assisting clinicians in recommending personalized therapeutic guidance, the follow-up information of advanced HSPC was retrospectively analyzed in conjunction with clinico-pathological data.
Materials and Methods
Patient Recruitment and Clinico-Pathological Variables Collection
One hundred and fifty-two PCa patients with complete follow-up information were included in this retrospective analysis from November 2016 to November 2022 in the Second Hospital of Dalian Medical University. The protocol was accepted by the ethical committee of the Second Hospital of Dalian Medical University complying with the Declaration of Helsinki (approval number: 2023064), all patients submitted written informed consent prior to the research. The inclusion criteria were as follows: (1) Patients with prostate adenocarcinoma newly confirmed by pathology; (2) patients received LHRH agonists coupled with anti-androgen as initial and only therapy before progression; (3) patients with adequate imaging evidence were diagnosed with locally advanced or metastatic disease; and (4) patients with complete clinico-pathological variables and follow-up data. Exclusion criteria were as follows: (1) PCa patients with other pathological types; (2) patients had received ADT; and (3) patients with other blood systems or solid tumors. Clinico-pathological variables at the time of PCa diagnosis, such as age, PSA levels, clinical TNM stage, Gleason sum and platelet, lymphocyte, haemoglobin, and serum albumin values, were collected and given in Table 1. LUTS symptoms were assessed at baseline using the International Prostate Symptoms Score (IPSS). The seven symptom questions have a severity scale of 0 to 5, and the total IPSS ranges between 0 and 35. Total IPSS values of 20–35 indicate severe urinary symptoms. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score was calculated as hemoglobin (g/L) × albumin (g/L) levels × lymphocyte count (/L)/platelet count (/L). Patients progressed to CPRC met the following criteria: 1) serum testosterone level <50 ng/dl, or 1.7 nmol/L; 2) PSA progression: PSA value >2.0 ng/mL, interval 1 week, three times over the baseline level >50%. All patients were followed up to the advancement of CRPC.
 |
Table 1 Clinico-Pathologic Characteristics of Study Participants |
Establish a Nomogram for Predicting the CRPC Progression
In this study, 152 patients were randomly assigned to training set of 108 samples (~7/10), and internal validation set of 44 samples (~3/10). The R package “survival” was used to integrate data on survival time, survival status, and the clinico-pathological characteristics. The multivariate Cox regression analysis was used to determine the prognostic significance of these characteristics in the training set. On the basis of multivariate Cox proportional hazards analysis, nomogram predicting rates of disease progression at 6, 12, 18, and 24 months were created using the “RMS” software. The nomogram presents graphical data for these factors, and from the points linked with each risk factor, the prognosis risk of an individual patient may be computed. During the validation of the nomogram, the total points of each patient in the validation cohort were calculated according to the established nomogram. The concordance index (C-index) and the receiver operating characteristic (ROC) curve were used to evaluate the discriminating ability of the nomogram. The calibration plots and decision curve analysis (DCA) were used to assess the predictive power and clinical utility of the nomogram.
Survival Analysis of Patients with High-Risk and Low-Risk Score Based on Nomogram
The best risk score cut-off value was determined for the entire cohort using the R package “maxstat” (Maximally selected rank statistics with multiple p-value approximations, version: 0.7–25). The minimum and maximum numbers of samples in each category were set at more than 25% and less than 75%, respectively. Based on available information, patients were divided into high-risk and low-risk groups. The prognosis difference between the two groups was examined using the Survfit function of the R software package, and the significance of the prognostic difference between the several groups of samples was assessed using the Log rank test.
Statistical Analysis
The ideal cutoff values for age and HALP score were determined using X-tile software v3.6.1 (Yale University).15 For continuous variables, the medians and interquartile ranges were evaluated; frequencies and proportions are given for categorical variables. The corresponding hazard ratios (HRs) and 95% confidence intervals (CIs) were determined using multivariate Cox regression analysis of “survival” R package. For DCA, the “ggDCA” R package was utilized. ROC analysis was performed using the R software package pROC to determine the area under the curve (AUC) (version 1.17.0.1). In particular, we gathered the patients’ follow-up duration and risk score and performed ROC analysis using the ROC function of pROC at 6, 12, 18, and 24 months. For all analyses, a P value of <0.05 was considered statistically significant.
Results
Clinico-Pathologic Characteristics of Patients
The clinicopathological characteristics of all patients are summarized in Table 1. Based on the results obtained using X-tile software, the following cutoff values were observed: 70 for age and 25–34 for HALP. Among the 152 PCa patients, at the time of diagnosis, 108 (71.05%) and 19 (12.50%) patients were diagnosed as M1a/b and M1c stage. One hundred and one (66.45%) patients had lymph node metastasis. Seventy-four (48.68%), 57 (37.50%) and 21 (13.82%) patients were diagnosed as T4, T3 and T2 stages, respectively. Among the Gleason sum values, 28 (18.42%), 58 (38.16%), 54 (35.53%) and 12 (7.89%) patients had a score of 7, 8, 9 and 10, respectively. The median CRPC-free survival of entire cohort was 12.5 months (range: 2–65 months). On the other hand, the median CRPC-free survival of patients with severe LUTS was 20.5 months, significantly longer than that (7.5 months) of less symptomatic patients.
Construction of a Combined Nomogram for Individualized Prediction
The clinico-pathologic factors, including age, PSA levels, clinical TNM stage, Gleason sum, HALP score and severe LUTS were used for multivariate Cox regression analyses (Table 2). The results demonstrated that severe LUTS, HALP and Gleason sum were independent risk factors for CRPC progression.
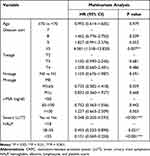 |
Table 2 Multivariate Analyses of Factors Associated with CRPC Progression |
By the multivariate Cox regression analysis, a nomogram integrated with the severe LUTS, HALP and Gleason sum was established (Figure 1A). The C-index was 0.794 (95% CI, 0.759–0.829), indicating a good consistency. The calibration plots (Figure 1B) showed that the nomogram performed well in the individualized prediction of progression to CRPC. DCA curve revealed that the nomogram provided obvious net benefit at 6, 12, 18 and 24 month to the none or all strategy, which represented patients correctly treated (Figure 2A). As shown in the ROC curve, the 6-month, 12-month, 18-month and 24-month AUC of the nomogram were 0.921 (95% CI, 0.870–0.971), 0.897 (95% CI, 0.833–0.961), 0.897 (95% CI, 0.836–0.957) and 0.857 (95% CI, 0.788–0.926), respectively (Figure 2B).
Validation of Predictive Accuracy of the Nomogram
In the validation cohort, the C-index of the nomogram for predicting CRPC progression was 0.816 (95% CI, 0.757–0.875), the calibration curve showed good agreement between prediction and observation in the probability of 6-month, 12-month, 18-month and 24-month survival (Figure 3A). DCA curve also showed favorable net advantages for assisting with clinical judgments (Figure 3B). Anymore, the AUC for predicting 6-month, 12-month, 18-month and 24-month survival were 0.915 (95% CI, 0.829–1.0), 0.909 (95% CI, 0.815–1.0), 0.937 (95% CI, 0.870–1.0) and 0.948 (95% CI, 0.890–1.0), respectively (Figure 4A). Each patient in this investigation was converted into a single risk score using nomogram modeling. The best risk score cutoff value was calculated as 0.33. Based on this information, patients were split into high and low risk groups, with 36.84% (n = 45) in the high risk group and 63.16% (n = 95) in the low risk group. Between the two groups, there was a statistically significant difference in prognosis (Figure 4B).
Discussion
In this study, we assessed the prognosis role of severe LUTS (IPSS ≥20) in HSPC patient, who received LHRH agonists coupled with anti-androgen as initial and only therapy before progression. It was observed to be significantly associated with the HSPC prognosis. Furthermore, we established a nomogram model, comprising severe LUTS, HALP score and Gleason sum, hypothesized that its predictive significance could help clinicians to identify high-risk HSPC patients in a timely manner as well as to provide reasonable treatment options.
The clinical staging of newly diagnosed HSPC patients in China differs from western developed countries. A multi-center Chinese study showed that only 1/3 of the newly diagnosed HSPC patients are clinically localized, most patients are in the locally advanced or metastatic stage at the diagnosis.16 Due to its heterogeneity, HSPC has a complex disease spectrum, ranging from clinically indolent subtypes to aggressive ones. The progress span to CRPC varies significantly among patients. Multiple randomized controlled clinical phase-III trials, such as CHAARTED17 and LATITUDE,18 reported that ADT plus docetaxel or abiraterone acetate in the early hormone-sensitive phase would result in longer overall survival in patients with high-volume or high-risk HSPC. An early and suitable follow-up and treatment approach can be put in place when HSPC patients are determined to have either a long- or short-term progression to CRPC before initial treatment. Predicting and identifying HSPC patients at risk for quick development to CRPC is therefore crucial.
One of the most frequent reasons for patient visits to urologists is LUTS. The onset of PCa has not traditionally been linked to these symptoms. In our clinical setting, we discovered that patients with advanced PCa who originally sought care for LUTS symptoms had a better prognosis than those who sought care for other conditions including elevated PSA, bone pain, etc. As seemed to be expected, our findings showed that severe LUTS was significantly associated with decreased risk for rapid progression to CRPC. It remains unclear why a severe LUTS is associated with a better prognosis of HSPC. The onset of LUTS in patients with PCa is the parallel increase in prostate volume due to benign prostatic hypertrophy.19 Interestingly, recent studies showed that men with low testosterone had significantly larger prostate volume than those in the normal testosterone group.20,21 Several works of literature to assess the relationship between serum testosterone and PCa have been reported, the authors concluded that the relationships among serum testosterone levels, the incidence, aggressiveness, and oncological outcomes showed conflicting results and has been controversial.22,23 Accordingly, our findings could support the hypothesis that a severe LUTS may represent a relatively low baseline testosterone, which is related to a good prognosis of HSPC.
HALP score has been identified as a significant predictive factor in patients with various malignancies and is thought to be an easily computed index of systemic inflammation and nutritional status.24,25 In men with PCa and benign prostatic hyperplasia, Kaya et al investigated the diagnostic value of the preoperative HALP score.26 However, it is unknown whether the HALP score can predict CRPC progression in HSPC patients who receive ADT as their first and only treatment. According to our findings, the prognosis of HSPC was substantially correlated with HALP score. Consistently, Gleason sum and HALP were found to be particular risk factors for progression-free survival in metastatic PCa patients who underwent cytoreductive radical prostatectomy by Guo et al.27
The nomogram has been used extensively by oncologists to produce prognostic information for specific patients owing to its intuitive design and statistical probability.28,29 With the aid of multivariate Cox regression analysis, an unique nomogram was created in this work by combining the severe LUTS, HALP score, and Gleason sum. The combined nomogram worked well as an all-encompassing scoring system for predicting the progression of CRPC, the calibration curve and C-index (0.794, 95% CI: 0.759–0.829 and 0.816, 95% CI: 0.757–0.875 for the training and validation cohorts, respectively) provided support for this prediction. DCA curve also demonstrated positive net benefits in guiding clinical decisions. The ROC curve revealed that the nomogram in this study demonstrated accurate discrimination in both training and validation cohort, especially for 6-month progression (0.921, 95% CI: 0.870–0.971 and 0.915, 95% CI: 0.829–1.0), when is important for identifying HSPC patients with worse prognosis.4
Although more recent techniques such as fluid biopsy and genetic testing appear to improve the precision of PCa prognostic predication, they have limitations such as a high cost and labor-intensive analysis.30–32 Additionally, the bulk of them are still through clinical trials. In clinical practice, a prostate biopsy can be used to determine the Gleason sum, and laboratory investigations can be used to calculate the HALP when a patient with prostate cancer visits a doctor. The total points of all the variables in the nomogram could therefore aid us in making informed choices regarding adjuvant therapy and follow-up plans because it is a quick, precise, and affordable risk assessment.
After dividing the patients into groups with low- and high-risk score, the group with a high-risk score developed rapidly and had a bad prognosis. For patients with low risk scores, the most common palliative therapy, ADT+ bicalutamide/flutamide, is recommended. Current clinical research suggests that patients in the high-risk score category should receive more potent medication, such as ADT coupled with chemotherapy or new endocrine therapy. Large-scale clinical trials are necessary to validate the specific treatment.
This study is limited by a small sample size, which must be increased for future validation. Second, in clinical practice, ADT combination with bicalutamide and flutamide is gradually being replaced by new endocrine medicines. In the future, individuals with several treatment approaches will be able to be enrolled and compared to determine the benefits of various treatment strategies.
In conclusion, our study demonstrated a significant positive association between severe LUTS and CRPC-free survival at 6 months, 12 months, 18 months and 24 months, suggesting that severe LUTS at admission may act as a powerful indicator of better prognosis. Further, a novel combined nomogram was established and validated for individualized risk prediction of HSPC patients. Although the prognostic ability of this nomogram has been preliminarily confirmed, it still needs further demonstration by clinical research, which will be our effort in the future.
Data Sharing Statement
The data analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
The protocol was accepted by the ethical committee of the Second Hospital of Dalian Medical University complying with the Declaration of Helsinki (approval number: 2023064), all patients submitted written informed consent prior to analysis.
Funding
This study was supported by the “1+X” program for Clinical Competency enhancement-Clinical Research Incubation Project and the Second Hospital of Dalian Medical University (2022LCYJZD02) to B.Y.; the cultivating scientific research project of the Second Hospital of Dalian Medical University (dy2yynpy202217) to Y.B.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Yao S, Zhang H, Chen Z, et al. Promotion of graphitic carbon oxidation via stimulating CO2 desorption by calcium carbonate. J Hazard Mater. 2019;363:10–15. doi:10.1016/j.jhazmat.2018.09.048
3. Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65(11):1180–1192. doi:10.1111/j.1742-1241.2011.02799.x
4. Hussain M, Goldman B, Tangen C, et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (intergroup study 0162) and 9916. J Clin Oncol. 2009;27(15):2450–2456. doi:10.1200/JCO.2008.19.9810
5. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746. doi:10.1056/NEJMoa1503747
6. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–1177. doi:10.1016/S0140-6736(15)01037-5
7. Rizzo A, Mollica V, Cimadamore A, et al. Is there a role for immunotherapy in prostate cancer? Cells. 2020;9(9):2051. doi:10.3390/cells9092051
8. Mollica V, Rizzo A, Rosellini M, et al. Bone targeting agents in patients with metastatic prostate cancer: state of the art. Cancers. 2021;13(3):546. doi:10.3390/cancers13030546
9. Rosellini M, Santoni M, Mollica V, et al. Treating prostate cancer by antibody-drug conjugates. Int J Mol Sci. 2021;22(4):1551. doi:10.3390/ijms22041551
10. Rizzo A, Santoni M, Mollica V, Fiorentino M, Brandi G, Massari F. Microbiota and prostate cancer. Semin Cancer Biol. 2022;86(Pt 3):1058–1065. doi:10.1016/j.semcancer.2021.09.007
11. Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50(6):1306–1314. doi:10.1016/j.eururo.2006.09.019
12. Magistro G, Keller P, Westhofen T, et al. The significance of a high preoperative PSA level for the detection of incidental prostate cancer in LUTS patients with large prostates. World J Urol. 2021;39(5):1481–1487. doi:10.1007/s00345-020-03321-w
13. Zhang T, Wu H, Liu S, et al. Clinical evaluation of tamsulosin in the relief of lower urinary tract symptoms in advanced prostate cancer patients. Int Urol Nephrol. 2017;49(7):1111–1117. doi:10.1007/s11255-017-1591-1
14. Panaiyadiyan S, Kumar R. Prostate cancer nomograms and their application in Asian men: a review. Prostate Int. 2023;2287–8882. doi:10.1016/j.prnil.2023.07.004
15. Camp R, Dolled-Filhart M, Rimm D. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi:10.1158/1078-0432.CCR-04-0713
16. Ma CG, Ye D-W, Li C-L, et al. [Epidemiology of prostate cancer from three centers and analysis of the first-line hormonal therapy for the advanced disease]. Zhonghua Wai Ke Za Zhi. 2008;46(12):921–925. Chinese.
17. Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized Phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36. 1080.
18. Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, Phase 3 trial. Lancet Oncol. 2019;20(5):686–700. doi:10.1016/S1470-2045(19)30082-8
19. Andersson SO, Rashidkhani B, Karlberg L, Wolk A, Johansson JE. Prevalence of lower urinary tract symptoms in men aged 45–79 years: a population-based study of 40,000 Swedish men. BJU Int. 2004;94(3):327–331. doi:10.1111/j.1464-410X.2004.04930.x
20. Xia BW, Zhao SC, Chen ZP, et al. Relationship between serum total testosterone and prostate volume in aging men. Sci Rep. 2021;11(1):14122. doi:10.1038/s41598-021-93728-1
21. Duarsa GW, Sari YA, Oka AA, et al. Serum testosterone and prostate-specific antigen levels are major risk factors for prostatic volume increase among benign prostatic hyperplasia patients. Asian J Urol. 2021;8(3):289–297. doi:10.1016/j.ajur.2020.06.001
22. Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol. 2015;193(2):403–413. doi:10.1016/j.juro.2014.07.123
23. Miura N, Mori K, Mostafaei H, et al. Prognostic value of testosterone for the castration-resistant prostate cancer patients: a systematic review and meta-analysis. Int J Clin Oncol. 2020;25(11):1881–1891. doi:10.1007/s10147-020-01747-1
24. Xu SS, Li S, Xu HX, et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol. 2020;26(8):828–838. doi:10.3748/wjg.v26.i8.828
25. Shen XB, Zhang YX, Wang W, Pan YY. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score in patients with small cell lung cancer before first-line treatment with etoposide and progression-free survival. Med Sci Monit. 2019;25:5630–5639. doi:10.12659/MSM.917968
26. Kaya C, Caliskan S, Sungur M, Aydın C. HALP score and albumin levels in men with prostate cancer and benign prostate hyperplasia. Int J Clin Pract. 2021;75(3):e13766. doi:10.1111/ijcp.13766
27. Guo Y, Shi D, Zhang J, et al. The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) score is a novel significant prognostic factor for patients with metastatic prostate cancer undergoing cytoreductive radical prostatectomy. J Cancer. 2019;10(1):81–91. doi:10.7150/jca.27210
28. Heetman JG, Soeterik TFW, Wever L, et al. A side-specific nomogram for extraprostatic extension may reduce the positive surgical margin rate in radical prostatectomy. World J Urol. 2022;40(12):2919–2924. doi:10.1007/s00345-022-04191-0
29. Lin Z, Li Y, Wu J, et al. Nomogram for prediction of prolonged postoperative ileus after colorectal resection. BMC Cancer. 2022;22(1):1273. doi:10.1186/s12885-022-10377-x
30. Nguyen HT, Xue H, Firlej V, et al. Reference-free transcriptome signatures for prostate cancer prognosis. BMC Cancer. 2021;21(1):394. doi:10.1186/s12885-021-08021-1
31. Zhang S, Xu Y, Hui X, et al. Improvement in prediction of prostate cancer prognosis with somatic mutational signatures. J Cancer. 2017;8(16):3261–3267. doi:10.7150/jca.21261
32. Zhou E, Zhang B, Zhu K, Schaafsma E, Kumar RD, Cheng C. A TMPRSS2-ERG gene signature predicts prognosis of patients with prostate adenocarcinoma. Clin Transl Med. 2020;10(8):e216. doi:10.1002/ctm2.216
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




