Back to Journals » Cancer Management and Research » Volume 11
A Novel lncRNA MYOSLID/miR-1286/RAB13 Axis Plays a Critical Role in Osteosarcoma Progression
Received 17 September 2019
Accepted for publication 14 November 2019
Published 10 December 2019 Volume 2019:11 Pages 10345—10351
DOI https://doi.org/10.2147/CMAR.S231376
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Shouhang Yang,1 Ming Chen,1 Chuanfu Lin2
1Department of Blood Transfusion, The Third Affiliated Hospital of Wenzhou Medical University, Ruian 325200, People’s Republic of China; 2Department of Orthopedics, The Third Affiliated Hospital of Wenzhou Medical University, Ruian 325200, People’s Republic of China
Correspondence: Chuanfu Lin
Department of Orthopedics, The Third Affiliated Hospital of Wenzhou Medical University, No. 108 Wansong Road, Ruian 325200, People’s Republic of China
Email [email protected]
Background: Osteosarcoma (OS) is a quite malignant bone cancer. However, how long noncoding RNA (lncRNA) regulates OS progression remains poorly investigated. The present study aims to illustrate the potential functions of lncRNA MYOSLID in the regulation of OS progression.
Methods: The expression of YOSLID, miR-1286 and RAB13 was analyzed by qRT-PCR. Cell proliferation was determined via CCK8 and colony formation assays. Transwell assay was used to examine migration and invasion. The luciferase reporter assay, RNA pulldown and RNA immunoprecipitation (RIP) assays were utilized to detect the interactions among MYOSLID, miR-1286 and RAB13.
Results: The expression of MYOSLID was upregulated in OS tissues and cell lines. MYOSLID overexpression predicted poor prognosis in OS patients. MYOSLID knockdown suppressed proliferation, migration and invasion of OS cells. MYOSLID was the sponge for miR-1286 and inhibited its expression while miR-1286 targeted RAB13 directly. MYOSLID promoted RAB13 expression via sponging miR-1286.
Conclusion: Our work demonstrated that the MYOSLID/miR-1286/RAB13 axis is a novel regulatory signaling in promoting OS progression.
Keywords: MYOSLID, miR-1286, RAB13, osteosarcoma, progression
Introduction
Osteosarcoma (OS) is one of the most prevalent and malignant cancers among juveniles.1 OS causes a large number of deaths every year.2 The main therapeutic methods include surgery, chemotherapy and radiotherapy.3 However, metastasis and recurrence often occur among OS patients, which results in a less than 30% of the five-year survival rate.4 Thus, it is critical to investigate the pathogenesis of OS and develop novel therapeutic strategies.
Long noncoding RNAs (lncRNAs) are a novel type of noncoding RNAs and have over 200 nucleotides in length.5 LncRNAs have various biological functions, which is demonstrated by increasing references.6,7 Recent studies indicate that lncRNAs are fine competing endogenous RNAs (ceRNA) to regulate microRNA expression and participate in tumorigenesis.8 For example, lncRNA FLVCR1-AS1 sponges miR-513 to increase growth and metastasis of ovarian cancer.9 LncRNA MIAT interacts with miR-212 to enhance thyroid cancer development.10 Additionally, lncRNA GAS5 sponges miR-222 to suppress growth, migration and invasiveness in colon cancer.11 So many lncRNAs have been identified to be aberrantly expressed in tumor tissues.12 However, their functions are poorly explored. Hence, it is important to explain the correlation between lncRNA and OS progression.
LncRNA MYOSLID is firstly found to regulate smooth muscle differentiation.13 Recent findings show that MYOSLID promotes progression of head and neck squamous cell carcinoma and gastric cancer.14,15 Nevertheless, the role of MYOSLID is unclear in OS. In this study, we found that MYOSLID was upregulated in OS tissues and predicted poor prognosis. Moreover, MYOSLID knockdown suppressed proliferation, migration and invasion of OS cells. Mechanistically, MYOSLID sponges miR-1286 to promote RAB13 expression. Conclusively, our study identified a novel signaling that the MYOSLID/miR-1286/RAB13 axis regulates OS progression.
Materials and Methods
Tumor Tissues
51 OS samples and their adjacent normal controls were collected from the Third Affiliated Hospital of Wenzhou Medical University. None of patients was treated with chemotherapy or radiotherapy before surgery. Samples were stored in liquid nitrogen. This study was approved by the Ethics Committee of the Third Affiliated Hospital of Wenzhou Medical University. Written informed consent was obtained from patients.
Cell Culture and Transfection
Human OS cell lines and the hFOB1.19 cells were obtained from American Type Culture Collection (ATCC) and maintained using DMEM medium containing 10% FBS at 37°C. MYOSLID siRNA (5ʹ-GACACTTAACTGATCTAAATATT-3ʹ and 5ʹ-TACATAAAGGATCTTTTCCATTG-3ʹ), miR-1286 mimics, miR-1286 inhibitors and negative controls were purchased from Gene Pharma (Shanghai, China). Plasmids were transfected into OS cells using Lipofectamine 2000 Reagent following the manual protocol.
qRT-PCR
Total RNA was extracted using Trizol reagent (Invitrogen) as previously described.16 1 μg RNA was reversely transcribed into cDNA using a Prime Script Kit (Takara Bio Inc., Otsu, Japan). qPCR was carried out using a SYBR Premix Ex Taq™ Kit (Takara Bio Inc.). U6 or GAPDH was the normalized control and relative expression was determined using the 2−ΔΔCT method. Primer sequences were as follows: MYOSLID (Forward: 5ʹ-AAGAGGGAGTGGGAGTTAGGC-3ʹ and reverse 5ʹ-CACTGTGGTGGGATCTGCAAG-3ʹ) and GAPDH (Forward: 5ʹ-TGATGACCCTTTTGGCTCCC-3ʹ Reverse: 5ʹ-GAAGCTTGTCATCAATGGAAAT-3ʹ).
Cell Proliferation
Cell proliferation was determined by Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) and colony formation assays. CCK8 assay was performed as previously reported.2 As for colony formation assay, 500 cells were seeded into the 6-well plates and cultured for 14 days. Then colonies were fixed and stained with 0.1% crystal violet. Colony numbers were finally counted.
Cell Migration and Invasion Assay
Cell migration and invasion were completed with a Transwell system (Corning, Inc., Corning, NY, USA) as reported before.2
Dual-Luciferase Reporter Assay
WT or mutant MYOSLID or RAB13 3ʹ-UTR luciferase reporter plasmid was constructed using pmirGLO vector (Promega) and transfected into OS cells along with miR-1286 mimics or negative controls. 48 h later, the relative luciferase activity was determined by the Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Renilla luciferase activity was normalized control.
Statistical Analysis
All results were calculated using SPSS 16.0 software (Chicago, IL) and expressed as the mean ± standard deviation (SD). Differences were calculated using the Student t test or and one-way ANOVA. Expression correlation was determined by Spearman correlation analysis. P < 0.05 was considered statistically significant.
Results
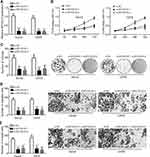
MYOSLID Was Upregulated in OS
The expression of MYOSLID was firstly analyzed. We found that MYOSLID was upregulated in OS tissues compared to normal controls (Figure 1A). Similarly, MYOSLID was also upregulated in OS cell lines compared to hFOB1.19 cells (Figure 1B). Moreover, the OS patients were divided into MYOSLID high expression and low expression groups. And MYOSLID high expression patients displayed a low survival rate compared to that of MYOSLID low expression group (Figure 1C).
Effects of MYOSLID on Proliferation, Migration and Invasion
To investigate the function of MYOSLID, we silenced it using two independent siRNAs in Saos2 and U2OS cells (Figure 2A). CCK8 assay showed that MYOSLID knockdown suppressed the proliferation of Saos2 and U2OS cells (Figure 2B). Colony formation assay further indicated that MYOSLID knockdown impaired the potential of OS cell proliferation (Figure 2C). Additionally, we found that MYOSLID silencing decreased the number of migrated and invaded cells (Figure 2D and E), suggesting that MYOSLID regulates OS progression.
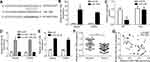
MYOSLID Sponged miR-1286
LncRNAs are classical ceRNAs in tumor.11 Thus, we analyzed the potential targets of MYOSLID via bioinformatics method. MiR-1286 ranked top among all candidates. We then constructed wild-type (WT) and mutant (Mut) MYOSLID luciferase reporters (Figure 3A). We overexpressed miR-1286 via mimic transfection (Figure 3B). MYOSLID-WT reporter activity was significantly inhibited by miR-1286 mimics (Figure 3C). In addition, RNA pulldown showed that miR-1286-WT, but not the mutant one, precipitated MYOSLID (Figure 3D), demonstrating their direct interaction. Moreover, we found that MYOSLID knockdown promoted the levels of miR-1286 in OS cells (Figure 3E). Of note, miR-1286 was downregulated in OS tissues (Figure 3F) and its expression was negatively correlated with that of MYOSLID (Figure 3G).
MiR-1286 Directly Targeted RAB13
Afterwards, we analyzed the potential targets of miR-1286. We identified RAB13. Similarly, we constructed the WT and Mut-RAB13 luciferase reporters (Figure 4A). Luciferase reporter assay demonstrated the interaction between miR-1286 and RAB13 (Figure 4B), which was further confirmed via RIP assay (Figure 4C and D). Importantly, miR-1286 mimics successfully inhibited the expression of RAB13 (Figure 4E). Moreover, MYOSLID knockdown also suppressed the expression of RAB13 (Figure 4F), which was abrogated via miR-1286 inhibitors (Figure 4F).
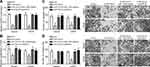
MYOSLID Promoted OS Progression via miR-1286/RAB13 Axis
To demonstrate whether MYOSLID regulates OS progression via miR-1286/RAB13, we performed rescue assays. We utilized miR-1286 inhibitors and RAB13 overexpression vectors (pcDNA3-RAB13) (Figure 5A). Through CCK8 assays, we found that miR-1286 inhibitors or oeRAB13 significantly reversed the effects of MYOSLID knockdown on proliferation (Figure 5B). Similarly, the migration and invasion were also rescued by miR-1286 inhibitors or oeRAB13 (Figure 5C and D). Therefore, MYOSLID promotes OS progression through regulating miR-1286/RAB13 axis.
Discussion
As a common cancer among children, OS results in a lot of deaths.1 However, there is an urgent requirement to improve the therapeutic strategies for OS patients. And the molecular mechanism of OS progression needs to be determined immediately. In this study, we identified a lncRNA MYOSLID that is highly expressed in OS tissues. And MYOSLID upregulation predicts poor prognosis. MYOSLID knockdown suppresses the proliferation, migration and invasion of OS cells. Thus, MYOSLID is a novel oncogene in OS.
LncRNA has been reported to regulate OS development. For example, lncRNA AFAP1-AS1 contributes to tumorigenesis of OS via p38MAPK/Twist1 axis.17 LncRNA HAND2-AS1 suppresses OS proliferation via regulating glucose metabolism.18 LncRNA BE503655 represses OS growth and metastasis through Wnt/β-catenin signaling.19 Additionally, HOXA11-AS promotes OS metastasis via miR-125a/Rab3D axis.2 MYOSLID has an oncogenic role in head and neck squamous cell carcinoma and gastric cancer.14,15 Yet, whether MYOSLID regulates OS remains unknown. In our work, we firstly found that MYOSLID was highly expressed in OS tissues. We also identified that MYOSLID may be a prognostic biomarker. Moreover, through CCK8, colony formation and Transwell assays, we demonstrated that MYOSLID is a driver for OS cell proliferation, migration and invasion. Thus, MYOSLID may be a therapeutic target for OS treatment.
LncRNAs often act as ceRNA to regulate gene expression.11 Thus, we then analyzed the potential miRNA targets via bioinformatics method. We identified that miR-1286 may be targeted by MYOSLID. Through luciferase reporter assay and RNA pulldown assay, we demonstrated that MYOSLID directly interacted with miR-1286. Moreover, MYOSLID knockdown promoted the expression of miR-1286 in OS cells. And the expression of MYOSLID was negatively correlated with that of miR-1286 in OS tissues. A recent study reveals that miR-1286 promotes lung cancer development.20 Interestingly, in our study, we found that miR-1286 was downregulated in OS tissues. And miR-1286 inhibitors promoted proliferation, migration and invasion of OS cells, indicating miR-1286 is a tumor suppressor in OS.
Finally, we also utilized bioinformatics method to determine the target of miR-1286. We found that RAB13 is the most potential target miR-1286. Via luciferase reporter assay, we validated the interaction between miR-1286 and RAB13. RIP assay also showed that RAB13 and miR-1286 were in the Ago complex. RAB13 expression was suppressed by miR-1286 mimics. Notably, we found that MYOSLID knockdown inhibited the expression of RBA13, which is dependent on the existence of miR-1286. Thus, MYOSLID as a ceRNA for miR-1286 promoted RAB13 expression in OS. RAB13 is a classical oncogene in several cancers. For instance, Rab13 activation promotes metastasis of epithelial cancers.21 RAB13 enhances proliferation and chemo-resistance of gastric cancer cells.22 However, no study determines the role of RAB13 in OS. In our study, we showed that overexpression of RAB13 promoted proliferation, migration and invasion of OS cells, suggesting an oncogenic role.
In conclusion, our study identified a novel signaling pathway that MYOSLID/miR-1286/RAB13 axis promotes the progression of OS.
Disclosure
The authors have no conflicts of interest in this work.
References
1. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13.
2. Cao K, Fang Y, Wang H, Jiang Z, Guo L, Hu Y. The lncRNA HOXA11-AS regulates Rab3D expression by sponging miR-125a-5p promoting metastasis of osteosarcoma. Cancer Manag Res. 2019;11:4505–4518. doi:10.2147/CMAR.S196025
3. Meazza C, Scanagatta P. Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Rev Anticancer Ther. 2016;16(5):543–556. doi:10.1586/14737140.2016.1168697
4. Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47(1):283–292. doi:10.1016/j.ocl.2015.08.022
5. Wang J, Samuels DC, Zhao S, Xiang Y, Zhao YY, Guo Y. Current research on non-coding Ribonucleic Acid (RNA). Genes (Basel). 2017;8:12. doi:10.3390/genes8120366
6. Tian X, Gao S, Liu Y, Xuan Y, Wu R, Zhang Z. Long non-coding RNA ENST00000500843 is downregulated and promotes chemoresistance to paclitaxel in lung adenocarcinoma. Oncol Lett. 2019;18(4):3716–3722.
7. Liu B, Ye B, Yang L, et al. Long noncoding RNA lncKdm2b is required for ILC3 maintenance by initiation of Zfp292 expression. Nat Immunol. 2017;18(5):499–508.
8. Xiong G, Liu C, Yang G, et al. Long noncoding RNA GSTM3TV2 upregulates LAT2 and OLR1 by competitively sponging let-7 to promote gemcitabine resistance in pancreatic cancer. J Hematol Oncol. 2019;12(1):97. doi:10.1186/s13045-019-0777-7
9. Yan H, Li H, Silva MA, et al. LncRNA FLVCR1-AS1 mediates miR-513/YAP1 signaling to promote cell progression, migration, invasion and EMT process in ovarian cancer. J Exp Clin Cancer Res. 2019;38(1):356. doi:10.1186/s13046-019-1356-z
10. Wang R, Zhao L, Ji L, Bai L, Wen Q. Myocardial infarction associated transcript (MIAT) promotes papillary thyroid cancer progression via sponging miR-212. Biomed Pharmacother. 2019;118:109298. doi:10.1016/j.biopha.2019.109298
11. Liu L, Wang HJ, Meng T, et al. lncRNA GAS5 inhibits cell migration and invasion and promotes autophagy by targeting miR-222-3p via the GAS5/PTEN-signaling pathway in CRC. Mol Ther Nucleic Acids. 2019;17:644–656. doi:10.1016/j.omtn.2019.06.009
12. Xie C, Zhang LZ, Chen ZL, et al. A novel hMTR4-PDIA3P1-miR-125/124-TRAF6 regulatory axis and its function in NF-K B signaling and chemoresistance. Hepatology. 2019. doi:10.1002/hep.30931
13. Zhao J, Zhang W, Lin M, et al. MYOSLID is a novel serum response factor-dependent long noncoding RNA that amplifies the vascular smooth muscle differentiation program. Arterioscler Thromb Vasc Biol. 2016;36(10):2088–2099. doi:10.1161/ATVBAHA.116.307879
14. Xiong HG, Li H, Xiao Y, et al. Long noncoding RNA MYOSLID promotes invasion and metastasis by modulating the partial epithelial-mesenchymal transition program in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2019;38(1):278. doi:10.1186/s13046-019-1254-4
15. Han Y, Wu N, Jiang M, et al. Long non-coding RNA MYOSLID functions as a competing endogenous RNA to regulate MCL-1 expression by sponging miR-29c-3p in gastric cancer. Cell Prolif. 2019;e12678.
16. Wang Y, Cheng Q, Liu J, Dong M. Leukemia stem cell-released microvesicles promote the survival and migration of myeloid leukemia cells and these effects can be inhibited by MicroRNA34a overexpression. Stem Cells Int. 2016;2016:9313425. doi:10.1155/2016/9313425
17. Shi D, Wu F, Mu S, et al. LncRNA AFAP1-AS1 promotes tumorigenesis and epithelial-mesenchymal transition of osteosarcoma through RhoC/ROCK1/p38MAPK/Twist1 signaling pathway. J Exp Clin Cancer Res. 2019;38(1):375. doi:10.1186/s13046-019-1363-0
18. Chen S, Xu X, Lu S, Hu B. Long non-coding RNA HAND2-AS1 targets glucose metabolism and inhibits cancer cell proliferation in osteosarcoma. Oncol Lett. 2019;18(2):1323–1329. doi:10.3892/ol.2019.10445
19. Huang Q, Shi SY, Ji HB, Xing SX. LncRNA BE503655 inhibits osteosarcoma cell proliferation, invasion/migration via Wnt/beta-catenin pathway. Biosci Rep. 2019;39:7. doi:10.1042/BSR20182200
20. Gao YW, Ma F, Xie YC, et al. Sp1-induced upregulation of the long noncoding RNA TINCR inhibits cell migration and invasion by regulating miR-107/miR-1286 in lung adenocarcinoma. Am J Transl Res. 2019;11(8):4761–4775.
21. Ioannou MS, Bell ES, Girard M, et al. DENND2B activates Rab13 at the leading edge of migrating cells and promotes metastatic behavior. J Cell Biol. 2015;208(5):629–648. doi:10.1083/jcb.201407068
22. Chen P, Chen G, Wang C, Mao C. RAB13 as a novel prognosis marker promotes proliferation and chemotherapeutic resistance in gastric cancer. Biochem Biophys Res Commun. 2019. doi:10.1016/j.bbrc.2019.08.141
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.