Back to Journals » Journal of Inflammation Research » Volume 16
A Novel Hematological Inflammation-Nutrition Score (HINS) and Its Related Nomogram Model to Predict Survival Outcome in Advanced Gastric Cancer Patients Receiving First-Line Palliative Chemotherapy
Authors Chen C, Wang Z , Qin Y
Received 19 April 2023
Accepted for publication 7 July 2023
Published 12 July 2023 Volume 2023:16 Pages 2929—2946
DOI https://doi.org/10.2147/JIR.S417798
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Chen Chen,* Zehua Wang,* Yanru Qin
Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yanru Qin, Department of Oncology, The First Affiliated Hospital of Zhengzhou University, No. 1 Jianshe East Road, Erqi District, Zhengzhou, Henan, 450052, People’s Republic of China, Tel +86 13676932999, Email [email protected]
Purpose: This study aims to construct a novel hematological inflammation-nutrition score (HINS) and investigate its prognostic value in patients with advanced gastric cancer (AGC). We investigated the risk stratification performance of HINS and developed a HINS-based nomogram model to predict overall survival by combining traditional predictors.
Patients and Methods: We conducted a retrospective study on 812 AGC patients who received first-line platinum- or fluoropyrimidine-containing chemotherapy at The First Affiliated Hospital of Zhengzhou University Hospital between 2014 and 2019. Patients were randomly divided into a training cohort (N=609) and a validation cohort (N=203). HINS (0– 2) was constructed based on a pre-chemotherapy systemic immune-inflammation index (SII) and albumin (ALB). Prognostic factors were screened by univariate and multivariate COX proportional regression models. Significant factors were used to construct a nomogram model. Internal validation was performed by calibration curves, time-dependent receiver operating characteristics (ROC) curves, and decision curve analysis (DCA), evaluating its prediction consistency, discrimination ability, and clinical net benefit.
Results: HINS was constructed based on SII and ALB. HINS showed a better stratification ability than JCOG prognostic index, with significant differences between groups. Multivariate analysis showed that ECOG ≥ 1 (HR: 1.379; P=0.005), Stage IV (HR: 1.581; P < 0.001), diffuse-type histology (HR: 1.586; P < 0.001), number of metastases ≥ 2 (HR: 1.274; P=0.038), without prior gastrectomy (HR: 1.830; P < 0.001), ALP ≥ULN (HR: 1.335; P=0.034), HINS (P < 0.001) were independent factors of OS. We successfully established a HINS-based nomogram model that showed a strong discriminative ability, accuracy, and clinical utility in training and validation cohorts.
Conclusion: HINS shows a superior risk stratification ability, which might be a potential prognostic biomarker for AGC patients receiving palliative first-line palliative chemotherapy. The HINS-based nomogram model is a convenient and efficient tool for managing prognosis and follow-up treatments.
Keywords: hematological inflammation-nutrition score, nomogram model, advanced gastric cancer, prognosis
Introduction
Gastric carcinoma (GC) is one of the most common malignancies and the fourth leading cause of cancer-related death worldwide.1 The global average annual incidence of GC was approximately 1806,000 cases, while Asia accounted for 689,000 cases (77.4%).1 Palliative chemotherapy remains the standard care of metastatic or recurrent GC, with the combination of fluoropyrimidine and platinum analog as the first-line treatment.2 Despite great breakthroughs in second- or late-line therapeutic options, the median survival time of advanced gastric carcinoma (AGC) patients was merely 15 months.3,4 Thus, precise prediction of individual prognosis of AGC patients is strongly warranted.
Previous studies have identified several clinicopathological indicators to predict prognosis of AGC patients. The Japan Clinical Oncology Group (JCOG) prognostic index, including Eastern Cooperative Oncology Group (ECOG) performance status (PS), primary tumor resection, number of metastases, and serum alkaline phosphatase (ALP) level, was one of the most robust prognostic scoring systems based on JCOG 9912 trial involving 760 patients.5 Moreover, the SPIRITS and G-SOX trials have externally validated the excellent stratification of JCOG prognostic index.6–8 Notably, its clinical applicability still lacks enough real-world data validation since it is originally constructed based on enrolling appropriate patients into clinical trials.9 The American Joint Committee on Cancer (AJCC) staging system is also widely applied by physicians to predict prognoses. However, patients with the same TNM stage may vary in survival. It is necessary to improve prediction accuracy by incorporating other indicators.
Systemic inflammation response plays a vital role in tumorigenesis.10 There is a complex interplay between the tumor microenvironment and cancer-related inflammation. Recently, elements of the complete blood count (CBC), either alone or in combination, have been widely investigated to mirror the inflammation status in solid tumors.11–14 CBC-derived inflammatory indices are calculated by formulas with different blood cell counts, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), neutrophil to lymphocyte and platelet ratio (NLPR), and aggregate index of systemic inflammation (AISI). Stratification models based on the abovementioned parameters have been explored in AGC and other malignancies.9,15–17 SII, a novel inflammatory index calculated by platelet (P), neutrophil (N), and lymphocyte (L) has been shown to have a strong prognostic value in multiple solid tumors, including esophageal cancer, lung cancer, colorectal cancer, and hepatocellular cancer.18–21 A recent meta-analysis confirmed that a high pretreatment SII was associated with a worse prognosis in GC.22 However, its prognostication value in GC remains controversial.23–26 Nutritional status is another critical factor affecting the response to palliative chemotherapy. Patients with malnutrition tend to show a bad tolerance to chemotherapy-related adverse events.27 Albumin (ALB), the most abundant protein in the plasma, reflects the individual’s nutritional status and is closely related to acute phase response.28 Previous studies supported that ALB was an effective prognostic biomarker in malignancies.29–31
However, there are still many unresolved research gaps. Using CBC-based inflammatory measures as GC biomarkers is becoming increasingly popular, but there is an overlap between them. A more efficient inflammatory biomarker is an urgent need. Besides, most studies only investigated one single predictor, whose clinical significance is limited. Although numerous researchers established different preoperative scoring systems to stratify prognosis after surgery, less evidence focused on their function in predicting prognosis for patients receiving first-line palliative chemotherapy. Moreover, the predictive performance of the inflammation-nutrition score is rarely validated using real-world clinical data. To our best knowledge, this is the first study to evaluate the prognostic value of HINS that combines SII and ALB in AGC. On this basis, we compare the stratification performance of HINS with JCOG prognostic index and successfully establish a HINS-based nomogram model to efficiently predict AGC patients’ overall survival (OS).
Materials and Methods
Patients
We retrospectively collected data from AGC patients who received first-line platinum- or fluoropyrimidine-containing chemotherapy at The First Affiliated Hospital of Zhengzhou University Hospital between January 2014 and December 2019.
Patients were screened based on the following inclusion criteria: 1) age ≥18 years; 2) histologically confirmed gastric adenocarcinoma or gastroesophageal junction adenocarcinoma; 3) metastatic tumor at initial diagnosis or recurrent tumor after prior surgery; 4) platinum- or fluoropyrimidine-based chemotherapy as the first-line palliative therapy; 5) ECOG performance status of 2 or less; 6) complete hospitalization records and follow-up data; 7) the expected survival at least 3 months. The exclusion criteria were as follows: 1) with other malignancies; 2) initiated on first-line chemotherapy at another hospital; 3) chemotherapy combined with immunotherapy; 4) relapsed tumor within 6 months after neoadjuvant or adjuvant therapy; 5) preexisting inflammatory conditions or autoimmune diseases; 6) incomplete medical records or lost to follow-up.
This study complies with the principle of the Helsinki Declaration. Ethic requirement was approved by the Ethics Committee of Scientific Research of the First Affiliated Hospital of Zhengzhou University (2023-KY-0369). Given the retrospective nature of this study, informed consent was waived.
Study Variables
Clinicopathological characteristics consist of age, gender, ECOG status, pathological staging at initial diagnosis, Lauren classification, number of metastases, liver metastasis, peritoneum metastasis, previous gastrectomy history. Data for peripheral blood tests prior to the initiation of palliative first-line chemotherapy were obtained. The blood samples were sent to laboratory within one hour of blood extraction. These included ALP level, ALB level, and candidate hematological inflammation indices. Inflammatory markers were calculated as follow: NLR=neutrophil/lymphocyte, PLR=platelet/lymphocyte, LMR=lymphocyte/monocyte, SII=(platelet × neutrophil)/lymphocyte, SIRI=(neutrophil × monocyte)/lymphocyte, NLPR=neutrophil/(lymphocyte × platelet), AISI=(neutrophil × platelet × monocyte)/lymphocyte.
Assessment of the Model JCOG
The JCOG prognostic index is assessed based on the following factors: ECOG PS ≥1, metastases ≥2, without gastrectomy, and elevated ALP. Each factor is marker as 1 score. GC patients with 0–1, 2–3, and 4 score were classified into good, moderate, and poor risk groups.
Study Design and Statistical Analysis
The study design is shown in Figure 1. All patients from The First Affiliated Hospital of Zhengzhou University were randomly divided into a training cohort and a validation cohort. The training cohort was used to construct HINS and a nomogram model. The validation cohort was used for internal validation of this model.
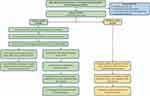 |
Figure 1 Study design and the flowchart of patient selection. |
Categorical variables were analyzed with the chi-square test or Fisher’s exact test, and continuous variables were compared by the rank sum test. To avoid multicollinearity problems, we compared similar hematological inflammatory predictors and only retained the predictor with the superior area under the curve (AUC) for subsequent evaluation. We respectively used time-dependent receiver operating characteristics (t-ROC) and X-tile software to determine the most appropriate cut-off value of inflammatory predictor. The time-dependent ROC curve analysis is an extension of the ROC curve analysis, which can be used for analyzing outcomes over time. X-tile software could assess every possible cut-off value by dividing populations into different strata. Patients were divided into groups, respectively, with high- and low-level based on the selected cut-off value.
OS was defined as the duration from diagnosis date to death from any cause or the last follow-up date. Univariate and multivariate analyses of OS were conducted with Cox proportional regression model.32 Subgroup survival analyses were displayed with Kaplan–Meier curves and compared by Log rank test.
The HINS was constructed with the cut-off value of SII and clinical threshold of hypoalbuminemia. Firstly, for comparing the risk stratification ability between model of JCOG, HINS, and a combined of them, K-M methods and time-dependent AUC were performed. Secondly, we assessed the prognostic value of HINS with Cox proportional hazards regression model.32 Variables with P <0.05 in the univariate analysis were further screened by the multivariate Cox regression analysis. And predictors with P <0.05 in multivariate analysis were selected to build a nomogram model. Internal validation of this model was performed in the training and validation cohort, respectively. The consistency between “predicted value” and “actual value” was assessed by calibration curves. The time-dependent ROC curves were used to evaluate the discriminative ability. The net benefit of this nomogram model in a clinical setting was assessed by Decision Curve Analysis (DCA). Finally, based on the risk threshold of the model, patients in two cohorts were further divided into the high- and low-risk group. Log rank test was employed to compare survival differences between two groups.
All statistical analyses were performed with SPSS 26.0 and R software (4.2.1 version). The hazard ratio (HR) and the 95% confidence interval (CI) were calculated to determine the association between predictors and survival probability. All P-values were based on a two-sided hypothesis, with P <0.05 indicating statistical significance.
Results
Clinicopathological Characteristics of Patients in Two Cohorts
Among the 903 advanced gastric cancer patients who received platinum- or fluoropyrimidine-containing first-line palliative chemotherapy at the First Affiliated Hospital of Zhengzhou University between January 2014 and December 2019, 812 were eligible for inclusion. Patients were randomly divided into a training cohort (N=609) and a validation cohort (N=203) with a ratio of 3:1 (Figure 1).
The baseline characteristics of two cohorts are summarized in Table 1. The median OS of training and validation cohort were, respectively, 407 and 404 days. Of all cases, 516 (63.5%) were <65 years and 587 (72.3%) were males. A total of 506 (62.3%) and 306 (37.7%) patients had an ECOG PS of 0 and ≥1, respectively. Moreover, a total of 539 (66.4%) cases had a stage-IV tumor; 517 (63.7%) had a diffuse-type Lauren classification; 203 (25%) patients were positive for HER-2; 327 (40.3%) had ≥2 metastatic sites. A total of 304 (37.4%) patients received prior gastrectomy before chemotherapy. A total of 244 (30%) patients occurred liver metastasis; 101 (12.4%) occurred peritoneum metastasis; and 66 (8.1%) patients occurred lung metastasis. Regarding the blood detection, 154 (19%) patients had an ALP ≥upper limit of normal (ULN) before chemotherapy initiation; 53 (6.5%) patients had hypoalbuminemia. Each variable was well balanced in two cohorts with no statistical significances (P >0.05).
 |
Table 1 Baseline Clinicopathological Characteristics of Patients in Two Cohorts (N=812) |
Due to some degree of overlap among these CBC-based inflammatory indicators, time-dependent AUCs for OS were performed to identify the optimal inflammatory parameter to avoid redundancy. The results indicated that 1-Y AUC for SII (0.667) was superior to those for neutrophils (0.598), platelets (0.589), lymphocytes (0.397), NLR (0.649), PLR (0.661), LMR (0.430), SIRI (0.609), NLPR (0.580), and AISI (0.631) (Table 2). AUCs for 2- and 3-year OS also supported this finding. Moreover, albumin (ALB) is a marker implicated in nutritional status. Hypoalbuminemia (<35g/L) tend to represent malnutrition and a worse response to palliative therapy. There was no predictor similar to the ALB. Therefore, we retained SII and ALB for further analysis. The time-dependent ROC was used to determine the cut-off value of SII (540) according to the 1-year OS. X-tile software also supported this outcome (P <0.0001).
 |
Table 2 Comparisons of the Time-Dependent AUCs Among CBC-Based Inflammatory Indicators |
Survival Analysis
Table 3 presents the results of univariate and multivariate Cox regression analyses of OS using baseline characteristics. Eight significant variables related to OS were further included in the multivariate regression model. The following factors were independent factors associated with poor prognosis in the training cohort: ECOG ≥1 (HR=1.381, 95% CI: 1.101–1.734, P=0.005), stage-IV (HR=1.582, 95% CI: 1.217–2.055, P <0.001), diffuse-type Lauren classification (HR=1.587, 95% CI: 1.238–2.034, P <0.001), number of metastases ≥2 (HR=1.280, 95% CI: 1.018–1.608, P=0.035), without prior gastrectomy (HR=1.832, 95% CI: 1.462–2.354, P <0.001), ALP ≥ULN (HR=1.343, 95% CI: 1.029–1.752, P=0.030), ALB <35g/L (HR=1.707, 95% CI: 1.150–2.533, P=0.008), SII ≥540 (HR=1.568, 95% CI: 1.250–1.965, P <0.001). Consistently, as shown in Figure 2A and B, the overall prognosis of SII-H patients was statistically worse than that of SII-L patients, whereas patients in ALB-H group had longer OS compared to patients in ALB-L group.
 |
Table 3 Univariate and Multivariate Analyses of Overall Survival (OS) in the Training Cohort |
Establishment of Hematological Inflammation-Nutrition Score (HINS)
According to survival analyses above, we combined the ALB and SII level to generate four subgroups. We observed significant differences (P <0.001) among the four subgroups (Figure 2C). Besides, the OS was similar in subgroups with either SII ≥540 or ALB <35 g/L (P=0.376). Therefore, we combined those two subgroups and established the hematological inflammation-nutrient score (HINS) as follows: (1) ALB <35g/L, score 1; ALB ≥35g/L, score 0; (2) SII ≥540, score 1; SII <540, score 0 (Table 4). The HINS was defined as the sum of score of ALB and SII: HINS = score of ALB (0 or 1) + score of SII (0 or 1). Therefore, HINS was used to classify patients into good (score 0), moderate (score 1), and poor (score 2) risk groups. The predictive value of HINS was compared with other classical CBC-based indices through time-dependent ROC curves (Figure 2D–F). The 1-year, 2-year, and 3-year AUCs of HINS were 0.641, 0.632, and 0.631, respectively. Although the AUCs of HINS were not always superior to SII, the overall predictive performance of HINS was stable and favorable. Particularly, the 3-year AUC of SII was the largest among all CBC-based inflammatory indicators. Meanwhile, the HINS takes into accounts both inflammatory and nutritional states without compromising predictive ability of prognosis. Next, the distribution of HINS and its correlation with clinicopathological characteristics in the training cohort are summarized in Table 5. The HINS for most AGC patients (96.7%) were 0 or 1. The distribution of clinicopathological characteristics of patients in three groups was relatively consistent with no significant statistical difference (P ≥0.05).
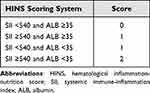 |
Table 4 Definition of HINS Scoring System |
 |
Table 5 Relationship Between the HINS and Clinicopathological Characteristics in Training Cohort (N=609) |
Comparison of Model JCOG, HINS, and a Combined of Them
Kaplan-Meier curves for OS were divided into three groups based on the JCOG prognostic index (Figure 3A). The good (N=300), moderate (N=290), and poor (N=19) risk groups exhibited a median OS of 35.53, 13.77, and 8.03 months, respectively. In comparison with the good risk group, the moderate and poor risk groups had a hazard ratio of 2.1771 (95% CI: 1.729–2.742, P <0.001) and 3.1552 (95% CI: 1.841–5.408, P <0.001) for OS. AGC patients who reached a higher JCOG score were associated with a shorter OS (P <0.001). However, no significant difference in OS was observed between moderate and poor risk groups (HR=1.462, 95% CI: 0.864–2.475, P=0.1568). Despite stratification ability validated by the real-world data, the JCOG prognostic index did not adequately classify AGC patients.
 |
Figure 3 Kaplan–Meier curves of overall survival (OS) according to the model (A) JCOG, (B) HINS, and (C) a combined one. |
Similarly, the model HINS classified patients into good (N=265), moderate (N=324), and poor (N=20) risk groups, with a median OS of 31.3, 15.9, and 6.88 months, respectively. The moderate and poor risk groups had an HR of 1.6934 (95% CI: 1.345–2.132, P <0.001) and 3.7357 (95% CI: 2.239–6.232, P <0.001) for OS relative to the good risk group. Patients with a higher HINS had worse overall prognosis with statistically significant difference (Figure 3B). The poor risk group also had significantly worse OS compared to the moderate risk group (HR=2.167, 95% CI: 1.316–3.570, P=0.0024). Therefore, HINS showed relatively better stratification ability than JCOG prognostic index, with significant differences between each risk group.
We further combined all JCOG and HINS indices to evaluate risk stratification performance. Accordingly, a total of 6 factors (ECOG ≥1, No. of metastases ≥2, no prior gastrectomy, elevated ALP, hypoalbuminemia, elevated SII) were added into the final formula, making it more suitable for patient populations in clinical practice. Thereafter, this combined index divided patients into good (score 0–2, N=369), moderate (score 3–4, N=226), and poor (score 5–6, N=14) risk groups, along with a median OS of 34.23, 10.73, and 6.78 months, respectively (Figure 3C). Evidently, this combined prognostic score had better stratification performance than either JCOG or HINS, as there was less overlapped area of 95% CI among three groups. Figure 4 displayed dynamic change for predictive abilities of JCOG, HINS, and the combined index using time-dependent AUC curves. During the observation period, the combined index was significantly superior to either JCOG or HINS for predicting OS.
 |
Figure 4 Dynamic change for predictive abilities of model JCOG, HINS, and a combined of them in training (A) and validation cohort (B). |
Screening for Independent Prognostic Factors
We performed a univariate Cox regression analysis using HINS replace ALB and SII. Thirteen indicators in relation to OS were screened in the univariate Cox regression analysis and variables with P <0.05 were further included in the multivariate Cox regression model. Results showed that seven indicators were independent prognostic factors of OS in AGC patients. They were respectively ECOG ≥1 (HR: 1.379; P=0.005), Stage IV (HR: 1.581; P <0.001), diffuse-type histology (HR: 1.586; P <0.001), No. of metastases ≥2 (HR: 1.274; P=0.038), without prior gastrectomy (HR: 1.830; P <0.001), ALP ≥ULN (HR: 1.335; P=0.034), HINS (P <0.001). We visualized the results of multivariate regression in a forest plot (Figure 5A).
Development of a Nomogram Model
Based on seven independent risk factors (P <0.05) screened by the multivariate analysis, a nomogram model was developed to predict 1-, 2-, and 3-year OS in AGC patients (Figure 5B). Each factor had a specific score using the point axis as reference, which transformed the risk of each factor into a calculable value. The total point could be calculated by adding the score of all variables. Survival probability of 1-, 2-, and 3-year OS can be estimated by locating the ‘total point axis’ down to “survival axis”. Through this HINS-based nomogram model, prognostic contribution of each predictor can be intuitively observed, as the width of the line segment represents the weight of each prognostic factor affecting the OS. Obviously, the weight of HINS was larger than other clinicopathological parameter, manifesting that HINS might play a vital in prognosis prediction of AGC patients.
Evaluation and Validation of This Nomogram Model
The predictive performance of this nomogram model was comprehensively evaluated by calibration curves and time-dependent ROC curves in both training and validation cohorts. The 1-, 2-, and 3-year calibration curves demonstrated that the “predicted survival” was highly consistent with the “actual survival” in the training cohort (Figure 6A–C) and the validation cohort (Figure 6D–F). The AUCs for predicting 1-, 2-, and 3-year OS in the training cohort were 0.744, 0.726, and 0.718, respectively (Figure 6G), and those in the validation cohort were 0.715, 0.759, and 0.789 (Figure 6H). All AUCs were over 0.7, manifesting the favorable discriminative ability of this nomogram model.
DCA was performed to further explore the superiority of HINS-based nomogram proposed in our study. Compared to the traditional AJCC staging system, it has been confirmed that clinical net benefits for HINS-based nomogram model at the time endpoint of 1-, 2-, and 3-year OS were better, with a wide range of threshold probabilities in both training (Figure 7A–C) and validation cohort (Figure 7D–F).
The Optimal Risk Thresholds of the Nomogram Model
Time-dependent ROC curve was used to determine the optimal risk threshold of 1-Y OS prediction. Based on the optimal cut-off value of risk score (1.64, AUC: 0.744), patients were divided into high- (≥1.64) and low-risk (<1.64) groups. Kaplan-Meier survival analysis (Figure 8A) showed that the OS of patients in high-risk group was significantly shorter than those in low-risk group in the training cohort (P <0.001). Consistently, this finding had been internally validated in the validation cohort (Figure 8B).
Discussion
Despite great advancements in second- and late-line therapeutic options that improved long-term prognoses,33–35 most AGC patients still fail to exhaust all treatment options.36 Precise evaluation of individual prognosis is the foundation for treatment recommendation and follow-up therapy strategy.
Growing evidence suggests that inflammatory mediators could trigger a cascade of inflammatory responses and tissue atrophy, thereby facilitating tumor progression and metastasis.37,38 Recently, there has been a great interest in applying CBC-based measures as prognostic biomarkers,39–41 where NLR and PLR were the most well-investigated indices in GC.42–44 SII has been regarded as a more effective prognostic indicator than other inflammation indices.45 A recent meta-analysis provides strong evidence that an elevation of SII is associated with a worse prognosis in GC.22 However, there is no consensus on which hematological indicator is the best one to reflect AGC prognosis. Meanwhile, several nutritional indices, such as ALB, BMI, and PNI, have been proven to be related to GC prognosis after gastrectomy.46,47 ALB is one of the most extensively confirmed prognostic biomarkers in gastric cancer.48
Various prognostic scoring systems were established based on inflammatory or nutritional indicators,49,50 but most of them focused on a single indicator. Another unsolved question is that previous studies mainly investigated preoperative indicators rather than pre-chemotherapy indices. The limited prognosis scoring system is designed for advanced GC patients with few treatment options.36 Considering multicollinearity issues, we first evaluated which CBC-derived predictors display the greatest potential in prognosis prediction. The higher the AUC is, the higher the predictive accuracy of the prognostic indicator. This method has been widely utilized in prior studies.51,52 After comparing the AUC values of similar predictors, SII with the highest AUC value was retained for subsequent evaluation. Moreover, multivariate analysis revealed that pretreatment SII and ALB were independent predictors of OS in AGC patients, consistent with previous findings.22,53,54 Furthermore, we divided patients into four subgroups based on SII and ALB levels and observed significant differences in K–M curves among them. Therefore, we constructed a novel hematological inflammation-nutrition score, namely HINS combining SII and ALB levels, and comprehensively evaluated its prognostic significance in AGC patients. This is the first study to use the inflammation-nutrition score for prognosis prediction in metastatic or recurrent GC patients following first-line palliative chemotherapy.
On the one hand, this study validated the clinical availability of the JCOG prognostic index using real-world patient data. However, it might not adequately stratify patients between moderate and poor risk groups with a wide overlapped 95% confidence interval of two groups, which kept in line with previous findings.55 This controversial result might be attributed to involving a greater number of GC patients with diffuse-type histology. As previously reported, diffuse-type GC tends to have a worse prognosis than intestinal-type GC.56 Apart from four factors constituting the original JCOG prognostic index, a multivariate analysis of our cohort showed that ALB and SII were independent risk factors affecting OS. We herein compared the model JCOG, HINS, and a combination of them in stratification performance. HINS showed a better stratification ability than the JCOG system, with a significant difference between the moderate and poor risk groups. Unsurprisingly, the combined model demonstrated excellent risk stratification by fully considering tumor condition and inflammation-nutrition status, which showed the best time-dependent AUC compared to either JCOG or HINS alone. Of note, all three models suggested that the good risk group had a longer median OS than those included in prior studies, while the median OS of the poor risk group was less than ten months.5,8,9,55 Overall, the JCOG prognostic index combined with HINS might greatly improve stratification according to prognosis. Our study continued previous research, finding more effective indicators to optimize the original prognostic scoring system.
On the other hand, we combined HINS and traditional characteristics to establish a nomogram model. In clinical settings, a single variable only plays a limited role in estimating the risk of death, perhaps due to tumor heterogeneity.57 A visual nomogram is a good approach to improve prediction accuracy by integrating multiple clinicopathological factors.58 In our study, variables with P <0.05 in univariate analysis were further screened by multivariate analysis. We finally determined 7 variables and utilized them to construct the HINS-based nomogram model for predicting 1-, 2-, and 3-year OS among AGC patients. Calibration curves showed high consistency between the predictive and actual observed values. And the model proposed in our study displayed practical discrimination ability and satisfied clinical net benefit. To further evaluate the generalizability of the HINS-based nomogram model, we verified it in a validation cohort. Meanwhile, we divided patients into high- and low-risk death groups based on the model’s risk threshold. A considerable number of patients in the high-risk group died due to tumor or other complications, which may be attributed to the exposure to multiple risk factors. Clinicians should pay more attention to high-risk patients identified by this model. Overall, this HINS-based nomogram model could assist clinicians in making a precise, quick, comprehensive prognosis evaluation for each patient.
The biological rationale behind the prognostic value of HINS may depend on the function of platelet, neutrophil, lymphocyte, and ALB. Platelets could directly interact with tumor cells to release factors that facilitate tumor growth, invasion, and angiogenesis.59 Also, platelets could stabilize the retention of tumor cells at the endothelium and protect tumor cells from attacks by immune cells.60,61 There is mounting evidence that neutrophils not only prompt an immunosuppressive microenvironment by secreting various inflammatory mediators (VEGF, IL-8, IL-6)62–64 but also facilitate the distant metastasis of circulating tumor cells (CTC).65,66 Tumor-infiltrating lymphocytes form the cellular basis of immunosurveillance, which significantly induces cytotoxic death and suppresses tumor cell growth.67–69 It has been proven that lymphopenia is associated with poor prognosis in cancer patients.70,71 In addition, hypoalbuminemia is a medical sign which potentially affects the quality of life, chemotherapy tolerance, and performance status. Serum albumin is generated in the liver to maintain body nutrition and plasma osmotic pressure, partly reflecting the nutritional status of individuals.28 As for metastatic or recurrent GC patients, they are more likely to present hypoalbuminemia or cachexia due to side effects of chemotherapy or the malignant tumor itself. Hypoalbuminemia has been considered a predictive factor of poor prognosis in a variety of conditions.72,73
Tumor microenvironment (TME) is a sophisticated ecosystem, interacting with various immune cells and inflammatory mediators.74 Therefore, an elevation of SII and a decreased ALB imply a dominance of pro-tumor activity in the TME, manifesting a poor prognosis in patients. Compared to the costly and time-consuming gene sequencing, the peripheral blood sample was easily available.75 For those who gained a high score of HINS, clinicians should closely monitor their inflammatory status, timely adjust the application of hormones and immunosuppressants, and improve their nutritional status by adjusting diets or supplying amino acids, especially for non-normal-weight patients. Since most patients with advanced AGC present cachexia, infections should be actively prevented. Therefore, on the one hand, the HINS can help physicians to better stratify patients with AGC, which might be a transition biomarker for future molecular prognosis; on the other hand, the HINS-based prognostic model holds the potential for wide popularization and application in clinical practice, providing a more precise basis for guiding follow-up treatment.
Nevertheless, there are several limitations to our study. Firstly, selection bias is inevitable due to the nature of the single-centre retrospective design. Secondly, although the sample size is sufficient to some extent, our study still needs external validation. Thirdly, even if regular blood testing is performed before each cycle of chemotherapy for GC patients, it is difficult to guarantee that blood tests are taken under a uniform standard. Hence, our study needs to be further verified in a prospective study. Moreover, blood samples were only collected before first-line chemotherapy, so the dynamic inflammatory or nutritional status changes cannot be reflected. Future studies will record blood indicators at multiple time points. Furthermore, in the future study, we will explore the impact of treatment regimens and treatment cycles on prognosis and further validate our HINS-based prognostic model based on a more extensive study population.
Conclusion
Our study is the first to establish a HINS system by combining SII and ALB levels. We demonstrated HINS’s superior risk stratification ability, which may be a potential prognostic biomarker for AGC patients. We also developed a HINS-based nomogram model combined with several clinicopathological parameters. This model is a convenient and efficient tool for pre-chemotherapy counselling and helps clinicians better to manage the prognosis and follow-up treatment of patients.
Data Sharing Statement
The dataset used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Ethic requirement was approved by the Ethics Committee of Scientific Research of the First Affiliated Hospital of Zhengzhou University (Ethics approval number: 2023-KY-0369). This study was conducted in accordance with the Declaration of Helsinki. As the First Affiliated Hospital of Zhengzhou University is a teaching hospital, all admitted patients clearly indicate that they agree to use the clinical data for relevant clinical research. Patient informed consent was waived because this study retrospectively collected patients’ information. The waiver of informed consent has been approved by the Ethics Committee of Scientific Research of the First Affiliated Hospital of Zhengzhou University. All data about the patients was anonymized or maintained with confidentiality.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82273381).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi:10.1016/S0140-6736(16)30354-3
3. Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised Phase 3 study. Lancet Oncol. 2009;10(11):1063–1069. doi:10.1016/S1470-2045(09)70259-1
4. Kang YK, Chin K, Chung HC, et al. S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin as first-line therapy in patients with advanced gastric cancer (SOLAR): a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(8):1045–1056. doi:10.1016/S1470-2045(20)30315-6
5. Takahari D, Boku N, Mizusawa J, et al. Determination of prognostic factors in Japanese patients with advanced gastric cancer using the data from a randomized controlled trial, Japan clinical oncology group 9912. Oncologist. 2014;19(4):358–366. doi:10.1634/theoncologist.2013-0306
6. Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a Phase III trial. Lancet Oncol. 2008;9(3):215–221. doi:10.1016/S1470-2045(08)70035-4
7. Koizumi W, Takiuchi H, Yamada Y, et al. Phase II study of oxaliplatin plus S-1 as first-line treatment for advanced gastric cancer (G-SOX study). Annal Oncol. 2010;21(5):1001–1005. doi:10.1093/annonc/mdp464
8. Takahari D, Mizusawa J, Koizumi W, Hyodo I, Boku N. Validation of the JCOG prognostic index in advanced gastric cancer using individual patient data from the SPIRITS and G-SOX trials. Gastric Cancer. 2017;20(5):757–763. doi:10.1007/s10120-017-0702-0
9. Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer--pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22(12):2395–2403. doi:10.1200/JCO.2004.08.154
10. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–226. doi:10.1097/MCO.0b013e32832a7902
11. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–230. doi:10.1016/j.critrevonc.2013.03.010
12. Leach M. Interpretation of the full blood count in systemic disease--A guide for the physician. J R Coll Physicians Edinb. 2014;44(1):36–41. doi:10.4997/JRCPE.2014.109
13. Velioglu Y, Yuksel A. Complete blood count parameters in peripheral arterial disease. Aging Male. 2019;22(3):187–191. doi:10.1080/13685538.2019.1588873
14. Zinellu A, Paliogiannis P, Sotgiu E, et al. Blood cell count derived inflammation indexes in patients with idiopathic pulmonary fibrosis. Lung. 2020;198(5):821–827. doi:10.1007/s00408-020-00386-7
15. D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. doi:10.1001/jama.280.11.969
16. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi:10.1182/blood-2016-08-733196
17. Zhang Y, Lu JJ, Du YP, Feng CX, Wang LQ, Chen MB. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in gastric cancer. Medicine. 2018;97(12):e0144. doi:10.1097/MD.0000000000010144
18. Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261–6272. doi:10.3748/wjg.v23.i34.6261
19. Zhang Y, Chen B, Wang L, Wang R, Yang X. Systemic immune-inflammation index is a promising noninvasive marker to predict survival of lung cancer: a meta-analysis. Medicine. 2019;98(3):e13788. doi:10.1097/MD.0000000000013788
20. Zhang Y, Xiao G, Wang R. Clinical significance of systemic immune-inflammation index (SII) and C-reactive protein-to-albumin ratio (CAR) in patients with esophageal cancer: a meta-analysis. Cancer Manag Res. 2019;11:4185–4200. doi:10.2147/CMAR.S190006
21. Wang B, Huang Y, Lin T. Prognostic impact of elevated pre-treatment systemic immune-inflammation index (SII) in hepatocellular carcinoma: a meta-analysis. Medicine. 2020;99(1):e18571. doi:10.1097/MD.0000000000018571
22. Qiu Y, Zhang Z, Chen Y. Prognostic value of pretreatment systemic immune-inflammation index in gastric cancer: a meta-analysis. Front Oncol. 2021;11:537140.
23. Huang L, Liu S, Lei Y, et al. Systemic immune-inflammation index, thymidine phosphorylase and survival of localized gastric cancer patients after curative resection. Oncotarget. 2016;7(28):44185–44193. doi:10.18632/oncotarget.9923
24. Chen L, Yan Y, Zhu L, et al. Systemic immune-inflammation index as a useful prognostic indicator predicts survival in patients with advanced gastric cancer treated with neoadjuvant chemotherapy. Cancer Manag Res. 2017;9:849–867. doi:10.2147/CMAR.S151026
25. Guo J, Chen S, Chen Y, Li S, Xu D. Combination of CRP and NLR: a better predictor of postoperative survival in patients with gastric cancer. Cancer Manag Res. 2018;10:315–321. doi:10.2147/CMAR.S156071
26. Yılmaz A, Mirili C, Tekin SB, Bilici M. The ratio of hemoglobin to red cell distribution width predicts survival in patients with gastric cancer treated by neoadjuvant FLOT: a retrospective study. Ir J Med Sci. 2020;189(1):91–102. doi:10.1007/s11845-019-02153-x
27. Alwarawrah Y, Kiernan K, MacIver NJ. Changes in nutritional status impact immune cell metabolism and function. Front Immunol. 2018;9:1055. doi:10.3389/fimmu.2018.01055
28. Lu J, Xu BB, Zheng ZF, et al. CRP/prealbumin, a novel inflammatory index for predicting recurrence after radical resection in gastric cancer patients: post hoc analysis of a randomized phase III trial. Gastric Cancer. 2019;22(3):536–545. doi:10.1007/s10120-018-0892-0
29. Seebacher V, Grimm C, Reinthaller A, et al. The value of serum albumin as a novel independent marker for prognosis in patients with endometrial cancer. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):101–106. doi:10.1016/j.ejogrb.2013.07.044
30. Wei C, Yu Z, Wang G, Zhou Y, Tian L. Low pretreatment albumin-to-globulin ratio predicts poor prognosis in gastric cancer: insight from a meta-analysis. Front Oncol. 2020;10:623046. doi:10.3389/fonc.2020.623046
31. Guner A, Cho M, Kim YM, Cheong JH, Hyung WJ, Kim HI. Prognostic value of postoperative neutrophil and albumin: reassessment one month after gastric cancer surgery. Front Oncol. 2021;11:633924. doi:10.3389/fonc.2021.633924
32. Suzuki Y, Okabayashi K, Hasegawa H, et al. Comparison of preoperative inflammation-based prognostic scores in patients with colorectal cancer. Ann Surg. 2018;267(3):527–531. doi:10.1097/SLA.0000000000002115
33. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–1235. doi:10.1016/S1470-2045(14)70420-6
34. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–2471. doi:10.1016/S0140-6736(17)31827-5
35. Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437–1448. doi:10.1016/S1470-2045(18)30739-3
36. Carter GC, Kaltenboeck A, Ivanova J, et al. Real-world treatment patterns among patients with advanced gastric cancer in South Korea. Cancer Res Treat. 2017;49(3):578–587. doi:10.4143/crt.2016.001
37. Duan RD, Nilsson A. Metabolism of sphingolipids in the gut and its relation to inflammation and cancer development. Prog Lipid Res. 2009;48(1):62–72. doi:10.1016/j.plipres.2008.04.003
38. Maletzki C, Emmrich J. Inflammation and immunity in the tumor environment. Digest Dis. 2010;28(4–5):574–578. doi:10.1159/000321062
39. Lian L, Xia YY, Zhou C, et al. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer Biomark. 2015;15(6):899–907. doi:10.3233/CBM-150534
40. Hsu JT, Wang CC, Le PH, et al. Lymphocyte-to-monocyte ratios predict gastric cancer surgical outcomes. J Surg Res. 2016;202(2):284–290. doi:10.1016/j.jss.2016.01.005
41. Wang SC, Chou JF, Strong VE, Brennan MF, Capanu M, Coit DG. Pretreatment neutrophil to lymphocyte ratio independently predicts disease-specific survival in resectable gastroesophageal junction and gastric adenocarcinoma. Ann Surg. 2016;263(2):292–297. doi:10.1097/SLA.0000000000001189
42. Bilen MA, Martini DJ, Liu Y, et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer. 2019;125(1):127–134. doi:10.1002/cncr.31778
43. Zhang X, Hu D, Lin X, et al. Prognostic value of an inflammation-related index in 6865 Chinese patients with postoperative digestive tract cancers: the FIESTA study. Front Oncol. 2019;9:427. doi:10.3389/fonc.2019.00427
44. Li Z, Li S, Ying X, et al. The clinical value and usage of inflammatory and nutritional markers in survival prediction for gastric cancer patients with neoadjuvant chemotherapy and D2 lymphadenectomy. Gastric Cancer. 2020;23(3):540–549. doi:10.1007/s10120-019-01027-6
45. He K, Si L, Pan X, et al. Preoperative Systemic Immune-Inflammation Index (SII) as a superior predictor of long-term survival outcome in patients with stage I-II gastric cancer after radical surgery. Front Oncol. 2022;12:829689. doi:10.3389/fonc.2022.829689
46. Park SH, Lee S, Song JH, et al. Prognostic significance of body mass index and prognostic nutritional index in stage II/III gastric cancer. Eur J Surg Oncol. 2020;46(4 Pt A):620–625. doi:10.1016/j.ejso.2019.10.024
47. Xishan Z, Ye Z, Feiyan M, Liang X, Shikai W. The role of prognostic nutritional index for clinical outcomes of gastric cancer after total gastrectomy. Sci Rep. 2020;10(1):17373. doi:10.1038/s41598-020-74525-8
48. Nozoe T, Iguchi T, Egashira A, Adachi E, Matsukuma A, Ezaki T. Significance of modified Glasgow prognostic score as a useful indicator for prognosis of patients with gastric carcinoma. Am J Surg. 2011;201(2):186–191. doi:10.1016/j.amjsurg.2010.01.030
49. Banna GL, Friedlaender A, Tagliamento M, et al. Biological rationale for peripheral blood cell-derived inflammatory indices and related prognostic scores in patients with advanced non-small-cell lung cancer. Curr Oncol Rep. 2022;24(12):1851–1862. doi:10.1007/s11912-022-01335-8
50. Hacker UT, Hasenclever D, Baber R, et al. Modified Glasgow prognostic score (mGPS) is correlated with sarcopenia and dominates the prognostic role of baseline body composition parameters in advanced gastric and esophagogastric junction cancer patients undergoing first-line treatment from the phase III EXPAND trial. Annal Oncol. 2022;33(7):685–692. doi:10.1016/j.annonc.2022.03.274
51. Kinoshita A, Onoda H, Imai N, et al. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer. 2012;107(6):988–993. doi:10.1038/bjc.2012.354
52. Kuroda D, Sawayama H, Kurashige J, et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21(2):204–212. doi:10.1007/s10120-017-0744-3
53. Liu BZ, Tao L, Chen YZ, et al. Preoperative body mass index, blood albumin and triglycerides predict survival for patients with gastric cancer. PLoS One. 2016;11(6):e0157401. doi:10.1371/journal.pone.0157401
54. Yin X, Fang T, Wang Y, et al. Prognostic significance of serum inflammation indexes in different Lauren classification of gastric cancer. Cancer Med. 2021;10(3):1103–1119. doi:10.1002/cam4.3706
55. Shimozaki K, Nakayama I, Takahari D, et al. A novel clinical prognostic index for patients with advanced gastric cancer: possible contribution to the continuum of care. ESMO open. 2021;6(5):100234. doi:10.1016/j.esmoop.2021.100234
56. Chon HJ, Hyung WJ, Kim C, et al. Differential prognostic implications of gastric signet ring cell carcinoma: stage adjusted analysis from a single high-volume center in Asia. Ann Surg. 2017;265(5):946–953. doi:10.1097/SLA.0000000000001793
57. Yan HHN, Siu HC, Law S, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018;23(6):882–897.e811. doi:10.1016/j.stem.2018.09.016
58. Graesslin O, Abdulkarim BS, Coutant C, et al. Nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J Clin Oncol. 2010;28(12):2032–2037. doi:10.1200/JCO.2009.24.6314
59. Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33(1):231–269. doi:10.1007/s10555-014-9498-0
60. Goubran HA, Burnouf T, Radosevic M, El-Ekiaby M. The platelet-cancer loop. Eur J Intern Med. 2013;24(5):393–400. doi:10.1016/j.ejim.2013.01.017
61. Gao QF, Qiu JC, Huang XH, et al. The predictive and prognostic role of a novel ADS score in esophageal squamous cell carcinoma patients undergoing esophagectomy. Cancer Cell Int. 2018;18:153. doi:10.1186/s12935-018-0648-2
62. Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol. 1985;134(1):230–234. doi:10.4049/jimmunol.134.1.230
63. Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci. 2007;98(11):1652–1658. doi:10.1111/j.1349-7006.2007.00606.x
64. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431–446. doi:10.1038/nrc.2016.52
65. Cools-Lartigue J, Spicer J, McDonald B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123(8):3446–3458. doi:10.1172/JCI67484
66. Najmeh S, Cools-Lartigue J, Rayes RF, et al. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Inter J Cancer. 2017;140(10):2321–2330. doi:10.1002/ijc.30635
67. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi:10.1016/j.immuni.2004.07.017
68. Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30(21):2678–2683. doi:10.1200/JCO.2011.37.8539
69. Santoiemma PP, Powell DJ Jr. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. 2015;16(6):807–820. doi:10.1080/15384047.2015.1040960
70. Fogar P, Sperti C, Basso D, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32(1):22–28. doi:10.1097/01.mpa.0000188305.90290.50
71. Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69(13):5383–5391. doi:10.1158/0008-5472.CAN-08-3845
72. Carvalho JR, Verdelho Machado M. New insights about albumin and liver disease. Ann Hepatol. 2018;17(4):547–560. doi:10.5604/01.3001.0012.0916
73. Gounden V, Vashisht R, Jialal I. Hypoalbuminemia. In: Disclosure: Rishik Vashisht Declares No Relevant Financial Relationships with Ineligible Companies. Disclosure: Ishwarlal Jialal Declares No Relevant Financial Relationships with Ineligible Companies. Treasure Island (FL) Ineligible Companies: StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC; 2023.
74. Hirahara N, Tajima Y, Fujii Y, et al. Prognostic nutritional index as a predictor of survival in resectable gastric cancer patients with normal preoperative serum carcinoembryonic antigen levels: a propensity score matching analysis. BMC Cancer. 2018;18(1):285. doi:10.1186/s12885-018-4201-4
75. Jiang P, Jia M, Hu J, Huang Z, Deng Y, Hu Z. A nomogram model involving immunohistochemical markers for predicting the recurrence of stage I-II endometrial cancer. Front Oncol. 2020;10:586081. doi:10.3389/fonc.2020.586081
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





