Back to Journals » Journal of Inflammation Research » Volume 17
A Nomogram Model Based on the Inflammation-Immunity-Nutrition Score (IINS) and Classic Clinical Indicators for Predicting Prognosis in Extranodal Natural Killer/T-Cell Lymphoma
Authors He Y, Luo Z, Chen H, Ping L, Huang C, Gao Y, Huang H
Received 14 December 2023
Accepted for publication 19 March 2024
Published 4 April 2024 Volume 2024:17 Pages 2089—2102
DOI https://doi.org/10.2147/JIR.S452521
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Yanxia He,1,* Zhumei Luo,1,* Haoqing Chen,2,3,* Liqing Ping,2,3 Cheng Huang,2,3 Yan Gao,2,3 Huiqiang Huang2,3
1Department of Oncology, The Third People’s Hospital of Chengdu, Sichuan, People’s Republic of China; 2State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Sun Yat-Sen University Cancer Center, Guangzhou, People’s Republic of China; 3Department of Medical Oncology, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huiqiang Huang; Yan Gao, Department of Medical Oncology, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China, Email [email protected]; [email protected]
Background: Systemic inflammation, immunity, and nutritional status are closely related to patients’ outcomes in several kinds of cancers. This study aimed to establish a new nomogram based on inflammation-immunity-nutrition score (IINS) to predict the prognosis of extranodal natural killer/T-cell lymphoma (ENKTL) patients.
Methods: The clinical data of 435 patients with ENTKL were retrospectively reviewed and randomly assigned to training cohort (n=305) and validation cohort (n=131) at a ratio of 7:3. Cox regression analysis was employed to identify independent prognostic factors and develop a nomogram in the training cohort. Harrell’s concordance index (C-index), calibration curve, receiver operating characteristic (ROC) curve, and decision curve analysis (DCA) curve were employed to assess the performance of the nomogram and compare it with traditional prognostic systems (PINK, IPI, KPI). Internal validation was performed using 1000 bootstrap resamples in the validation cohort. Kaplan-Meier survival analyses were conducted to compare the overall survival (OS) of patients in different risk groups.
Results: In the training cohort, in addition to several classic parameters, IINS was identified as an independent prognostic factor significantly associated with the OS of patients. The nomogram established based on the independent prognostic indicators showed superior survival prediction efficacy, with C-index of 0.733 in the training cohort and 0.759 in the validation cohort compared to the PINK (0.636 and 0.737), IPI (0.81 and 0.707), and KPI (0.693 and 0.639) systems. Furthermore, compared with PINK, IPI, and IPI systems, the nomogram showed relatively superior calibration curves and more powerful prognostic discrimination ability in predicting the OS of patients. DCA curves revealed some advantages in terms of clinical applicability of the nomogram compared to the PINK, IPI, and IPI systems.
Conclusion: Compared with traditional prognostic systems, the nomogram showed promising prospects for risk stratification in ENKTL patient prognosis, providing new insights into the personalized treatment.
Keywords: inflammation-immunity-nutrition score, extranodal natural killer/T-cell lymphoma, nomogram, prognosis, overall survival
Introduction
Extranodal natural killer/T-cell lymphoma (ENKTL), characterized by malignant proliferation of natural killer cells and cytotoxic T lymphocytes, is a distinctly heterogeneous clinicopathological entity primarily occurring in Asia and South America.1,2 In recent years, with the optimization of radiotherapy and L-asparaginase-based chemotherapy regimens, the survival rate of ENKTL patients has markedly improved. However, approximately 50% of patients with advanced-stage ENKTL usually develop relapsed/refractory (r/r) disease, and the outcomes in these populations remain dismal.3,4 Several prognostic systems, such as the Prognostic Index for Natural Killer Lymphoma (PINK), International Prognostic Index (IPI), and Korean prognostic index (KPI), are widely used in ENKTL to stratify risk and predict the outcomes of patients, while these models do not produce accurate risk stratification to some extent.5–8 In addition, both the IPI and KPI prognostic systems were proposed for patients who were treated with anthracycline-containing regimens, such as CHOP and CHOP-like, and may not be fully applicable in the era of nonanthracycline chemotherapy.5,8 Therefore, it is extremely important to explore a more efficient and accurate prognosis evaluation system for ENKTL.
In recent years, growing evidence has indicated that the inflammatory response, immunity and nutrition status of individuals have a close relationship with the occurrence, progression, and metastasis of tumours.9,10 Many studies have reported that the ratios of different peripheral blood mononuclear subtypes, such as the neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet-lymphocyte ratio (PLR), and monocyte to leukocyte ratio (MWR), play an important role in predicting the prognosis of patients with various cancers.11–14 High-sensitivity C-reactive protein (hs-CRP) is a critical inflammatory-related protein synthesized by the liver and has been shown to affect patient survival in hepatocellular carcinoma and nasopharyngeal carcinoma.15–17 The levels of serum albumin (ALB) intuitively reflect the nutritional status of individuals and have been confirmed to be an effective tool for predicting the outcomes of patients with gastric cancer and haematological malignancy.18,19 The inflammation-immunity-nutrition score (IINS), which reflects hs-CRP, lymphocytes (LYM), and ALB, is considered a superior prognostic evaluation system indicator.12,20,21 However, the relationship between IINS and the prognosis of patients with ENKTL has not been further explored.
Therefore, the aim of our study was to develop a new nomogram model based on the IINS score and traditional clinical indicators and to evaluate the performance of the evaluation system in predicting the prognosis of patients with ENKTL. Additionally, we further compared the prognosis predictive ability of the nomogram model with traditional prognostic systems, including PINK, IPI, and KPI systems.
Materials and Methods
Patients
The clinical and laboratory data of 435 consecutive patients diagnosed with ENKTL at Sun Yat-Sen University Cancer Center (SYSUCC) between January 2017 and October 2021 were retrospectively collected. The eligible patients were defined as follows: 1) histological and pathological confirmation of ENKTL; 2) newly diagnosed; and 3) complete clinicopathological and follow-up data. The main exclusion criteria were as follows: 1) complications with other malignancies; 2) definite evidence of infection; and 3) lost to follow-up. The last follow-up date was April 18, 2023, and the overall survival (OS) was calculated as the time span from the diagnosis of ENKTL to the last follow-up or patient death. The study was carried out in accordance with the 1964 Helsinki Declaration and its later amendments and approved by the institutional Ethics Committee of SYSUCC.
Study Variables and Cutoff Value Calculation
We obtained the baseline clinicopathological parameters of patients, such as age, sex, Ann Arbor stage, and haematological parameters, including hs-CRP, ALB, and LYM. According to receiver operating characteristic (ROC) curve analysis, we determined the optimal cutoff value for each indicator associated with OS and grouped each parameter as follows: hs-CRP score =0 (≤10.1 mg/L) and score=1 (>10.1 mg/L), ALB score=0 (>41.1 g/L) and score=1 (≤41.1 g/L), LYM score= 0 (>1.12×109/L), score =1 (≤1.12×109/L). The IINS scores were defined as the sum of the hs-CRP, LYM, and ALB scores.12 The patients were then allocated to three groups according to IINS scores: Low IINS (IINS=0), medium IINS (IINS=1), and high IINS (IINS=2 or 3).
Study Design and Statistical Analysis
The flowchart of this study is shown in Figure 1. All cases were randomly assigned to the training cohort (n=304) and validation cohort (n=131) at a ratio of 7:3. The Chi-square test and Fisher’s exact test were used to examine the balance of clinicopathological features and scores of several prognostic systems between the training cohort and the validation cohort. In the training cohort, a prognostic nomogram model was constructed by using univariate and multivariate Cox analyses to screen variables that independently related to OS. ROC curve analysis was utilized to evaluate the predictive efficacy of the nomogram. The performance and net clinical benefit of the nomogram were evaluated by calibration curve and decision curve analysis (DCA) and then compared with traditional prognostic models PINK, IPI, and KPI. Internal validation was adopted in the validation cohort. The R software (version 4.2.1) packages “rms”, “timeROC” and “ggDCA” were utilized to construct the nomogram, ROC curves, calibration curves and DCA curves, respectively. Kaplan–Meier analysis was conducted to compare the OS of patients between different risk groups. Statistics were performed using R software (version 4.2.1) and SPSS software (version 26.0), and a two-tailed p<0.05 was defined as statistically significant.
 |
Figure 1 Study design and flowchart of the included patients. |
Results
Clinical Characteristics and Survival
The baseline clinical characteristics of all 435 patients are summarized in Table 1. In the total population, the median age was 46 (range 7~83) years, and 67.8% were male. The majorities of the patients were in the early stage (stage I/II) (72.4%) and exhibited good status according to the Eastern Cooperative Oncology Group Performance Status (ECOG PS) assessment (93.3%). Eighty-one percent (81.4%) of individuals had nasal cavity or adjacent tissue invasion. Only a few patients presented distal lymph node involvement (12.4%), bone marrow (4.1%), or B symptoms (34.5%). Baseline characteristics were balanced between the two groups.
 |
Table 1 Clinical Characteristics of Patients |
The median follow-up times in the training cohort and validation cohort were 51.9 months (95% CI: 47.9–56.0 months) and 47.8 months (95% CI: 44.7–51.0 months), respectively. The 1-, 3-, and 5-year OS rates were 84.2%, 70.5%, and 62.6% in the training group and 81.7%, 71.5%, and 67.1% in the validation group, respectively.
Univariate and Multivariate Cox Analysis
Cox univariate and multivariate analyses were performed, and the results are presented in Table 2. Univariate analysis revealed that high IINS scores (IINS=2 or 3) had a significant influence on OS, with high hazard ratios (HR=2.093, 95% CI: 1.634–2.682, p<0.001). In addition, age (HR=1.648, 95% CI: 1.062–2.558, p=0.026), ECOG PS (HR=5.252, 95% CI: 3.105–8.885, p<0.001), B symptoms (HR=1.941, 95% CI: 1.316–2.862, p<0.001), primary sites (HR=1.952, 95% CI: 1.258–3.031, p=0.003), distal lymph nodes involvement (HR=2.654, 95% CI: 1.654–4.258, p<0.001), and Ann Arbor stage (HR=2.945, 95% CI: 1.992–4.353, p<0.001) were associated with OS of patients. Then, we included variables with p< 0.05 for Cox multivariate analysis and observed that age (HR=1.862, 95% CI: 1.170–2.965, p= 0.009), ECOG PS (HR=2.857, 95% CI: 1.537–5.313, p=0.001), Ann Arbor stage (HR=2.189, 95% CI: 1.310–3.656, p=0.003), bone marrow involvement (HR=2.587, 95% CI: 1.172–5.710, p=0.019), and IINS (HR=1.429, 95% CI: 1.051–1.943, p=0.023) were still significant independent factors of OS.
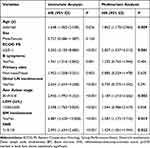 |
Table 2 Cox Univariate and Multivariable Analysis of Predictors Correlated with OS in the Training Cohort |
Construction of the Nomogram and Model Performance
In the training cohort, the prognostic nomogram was constructed according to the selected variables (Figure 2). The risk rating of each variable was assigned a corresponding point, which provides a visual representation of the scores for each risk factor. Larger points suggested a higher probability of death. By adding the scores for all risk factors, we could calculate the survival probability of patients at different time points. We observed that IINS had a greater impact on OS than ECOG PS, bone marrow involvement, and Ann Arbor stage. The constructed nomogram model based on the selected factors showed superior accuracy in predicting the OS rate of patients, with a C-index of 0.733 (95% CI: 0.684–0.781). The calibration curves revealed good performance in predicting the 1-, 3- and 5-year OS of patients in this population (Figure 3A–C). Moreover, the C-index of the nomogram for OS prediction in the validation cohort was 0.759 (95% CI: 0.680–0.838), and the calibration curves showed good agreement between 1-, 3-, and 5-year overall survival probabilities predicted by the nomogram and actual observation (Figure 3D–F).
 |
Figure 3 The calibration curves for predicting the 1-, 3-, and 5-year OS of patients with ENKTL in the training cohort (A–C) and validation cohort (D–F). |
Comparison Performances of the Nomogram
Then, we compared the predictive ability of the nomogram with the PINK, IPI, and KPI models in terms of the C-index and AUC. In the training cohort, the C-index of the nomogram was 0.733 (95% CI: 0.684–0.781), which indicated better performance than that of the PINK, IPI, and KPI systems, with values of 0.636 (95% CI: 0.584–0.688), 0.681 (95% CI: 0.630–0.732), and 0.693 (95% CI: 0.640–0.747), respectively. Similarly, the C-index of the nomogram was 0.759 (95% CI: 0.680–0.838), higher than that of the PINK (0.737, 95% CI: 0.662–0.811), IPI (0.707, 95% CI: 0.629–0.785) and KPI (0.639, 95% CI: 0.551–0.727) (Table 3). In addition, the established nomogram had the largest AUC in predicting 1-, 3- and 5-year OS compared to the PINK, IPI and KPI systems, both in the training cohort (Figure 4A–C) and the validation cohort (Figure 4D–F). The results suggested that compared with traditional prognostic systems, the nomogram model presented a trend of superior predictive capability. Moreover, DCA showed that the nomogram appeared to yield the most significant net benefit in terms of 1-, 3-, and 5-year OS compared to the PINK, IPI, and KPI prognostic systems in both the training cohort (Figure 5A–C) and validation cohort (Figure 5D–F), which indicated that the nomogram may has good clinical application value.
 |
Table 3 Comparisons of the C-Index of the Nomogram, PINK, IPI, and KPI Prognostic Systems in the Training and Validation Cohorts |
 |
Figure 5 Decision curve analysis for 1-, 3-, and 5-year OS predictions of the nomogram, PINK, IPI, and KPI prognostic systems in the training cohort (A–C) and validation cohort (D–F). |
Discrimination Ability of the Prognostic Nomogram
First, we assigned patients to three groups according to IINS scores and found that patients with high IINS scores had significantly worse 1-, 3-, and 5-year OS than those with low IINS in both the training and validation cohorts (Figure 6A and B). Then, the patients were categorized into high-risk and low-risk groups according to the median nomogram scores. The results showed that in the training cohort, the 1-, 3- and 5-year OS rates were 93%, 89%, and 85% in the low-risk group and 75%, 60%, and 54% in the high-risk group, respectively (p < 0.001). In the validation cohort, patients in the high-risk group had lower 1-, 3-, and 5-year OS rates (67%, 53%, and 49%) than those in the low-risk group (93%, 92%, and 89%) (p<0.001) (Figure 6C and D). The constructed nomogram showed significant differences in OS between the different risk groups, reflecting the good discriminative efficacy of the model in predicting patient prognosis. The results indicated that the new prognostic nomogram model might be a useful tool for predicting the survival of patients with ENKTL.
 |
Figure 6 Kaplan–Meier curves for the IINS score and nomogram model in the training cohort (A and C) and validation cohort (B and D). |
Discussion
Here, we established a new nomogram model based on IINS scores to predict the prognosis of ENKTL patients. We showed that IINS scores were a significant prognostic factor for OS and that patients with high IINS scores had significantly poorer outcomes than those with low IINS scores. In addition, for the first time, we combined IINS scores with several traditional clinical parameters that were independently associated with patient prognosis to determine the prognostic value of the new nomogram in patients with ENKTL. Our results suggested that the new nomogram appears to exhibit superior performance compared to the traditional prognostic systems PINK, IPI, and KPI, which provides important reference value for personalized treatment of ENKTL patients.
ENKTL, as a haematological malignancy originating from mature NK cells or cytotoxic T cells (CTLs), exhibits obvious heterogeneity in clinical manifestations and prognosis.1,2 Although patients with early-stage ENKTL exhibit a good prognosis after treatment with standard radiotherapy or combination chemotherapy, patients with advanced disease often present dismal outcomes, with a 5-year survival rate of approximately 70%.22,23 To identify patients’ prognostic risks more accurately, researchers have gradually explored the prognostic value of the PINK, IPI and KPI prognostic systems in ENKTL patients. However, these models failed to accurately delineate risk among different populations or to distinguish high-risk groups among early-stage patients.24,25 Previous studies have demonstrated that systemic inflammation affects the occurrence and development of tumours by inducing gene mutations, inhibiting cell apoptosis, inducing angiogenesis, and activating abnormal inflammatory signalling pathways.9,10 A growing number of studies have illustrated the diagnostic and prognostic values of inflammatory markers, such as LMR, NLR, systemic immune-inflammatory index (SII) and prognostic nutritional index (PNI), in various cancers.26–29 Recently, a study found that the Glasgow Prognostic Score (GPS), an inflammation-based prognostic evaluation system comprising measures of C-reactive protein and albumin, showed significant prognostic value in ENKTL.30 However, the prognostic system is limited to two peripheral blood indicators and may not fully reflect an individual’s inflammatory and immune status. In addition, the sample size of this study was limited. In another study. Scholars analysed the prognostic predictive value of the controlling nutritional status (CONUT) score reflecting albumin, serum total cholesterol, and absolute lymphocytes and created a workable nomogram model for personalized assessment. The results showed that the CONUT score was more efficient in predicting ENKTL prognosis than the IPI, KPI and PINK models.13 However, this study mainly focused on the impact of nutritional status on patient prognosis, while the impact of inflammation and immune status on patient prognosis was not further investigated. ENKTL is a distinct subtype of non-Hodgkin’s lymphoma (NHL) frequently characterized by prominent necrosis and inflammation.22,31 Particularly, due to its unique pathological features, ENKTL has a relatively high probability of haemophagocytic lymphohistiocytosis (HLH), a potentially fatal systemic inflammatory response syndrome caused by uncontrolled overactivation of lymphocytes and macrophages.32 Therefore, we needed to explore a more comprehensive prognostic system that could reflect the inflammatory, immune, and nutritional status of patients to predict the survival of patients more accurately.
Recently, the IINS score, which is derived from hs-CRP, ALB, and lymphocyte LYM, has been reported as a strong clinical prognostic indicator in hepatocellular carcinoma patients.20 A high IINS score represents reduced lymphocytes, hypoproteinaemia, and high hs-CRP, which often indicates high systemic inflammation and low immune function and nutritional status. At present, there have been no studies describing the prognostic effect of the IINS scoring system in ENKTL. In our study, the results revealed that patients with high IINS scores experienced a relatively shorter overall survival time than those with low IINS scores and demonstrated that IINS was a practical and effective prognostic indicator, which is consistent with those observed in some kinds of tumours12,21 Furthermore, in addition to IINS, traditional prognostic factors such as age, ECOG performance status, bone marrow involvement, and Ann Arbor stage have also been confirmed to be important factors affecting the prognosis of patients with ENKTL and have been included in several traditional prognostic models, such as the PINK, IPI, and KPI. Therefore, we constructed and validated a new nomogram model based on both IINS and independent prognostic indicators and compared the predictive effect of this model with that of the traditional prognostic systems PINK, IPI, and IPI. The results showed that compared with the PINK, IPI and KPI models, the C-index and AUC values of the IINS-based nomogram were higher, indicating that the nomogram may have more powerful predictive efficacy and better identify prognostic risks in patients with ENKTL. Our results provide insight into the relationship between inflammation, nutrition, and immunity and the survival of ENKTL patients.
However, this study has some limitations. Firstly, although the nomogram tending to have improved predictive power, it was not statistically significant enough, possibly due to the limited numbers in these cohorts. Further study of this nomogram in larger multi-institutional studies is necessary to definitively establish the effectiveness of this nomogram in comparison to other established predictive models commonly used in clinical practice. Secondly, we excluded ENKTL patients with clear infections in our study, which might be a limitation of the prognostic scoring system. However, this exclusion ensured that our observation and analysis of inflammation primarily focused on processes related to the tumor-associated inflammation, avoiding interference from infection-induced inflammation. This approach helped to reduce the impact of external factors on the study results, leading to a more accurate assessment of the characteristics and effects of tumor-related inflammation. Furthermore, different treatment regimens were included in the study, possibly impacting the survival of patients to some extent.
In summary, we first established and validated a new nomogram based on the IINS score and traditional prognostic indicators and evaluated the prognostic value of the nomogram in ENKTL patients. Our findings suggest that IINS may act as a strong prognostic predictor in patients with ENKTL. Specifically, IINS combined with traditional prognostic factors presents better prognostic performance than PINK, IPI, and KPI, possibly providing an easy way to identify patients with a poor prognosis and an opportunity to guide treatment and follow-up strategies to improve the outcomes of ENKTL patients.
Data Sharing Statement
The data sets used and/or analyzed in this study are accessible and can be obtained from the corresponding authors if reasonably requested.
Ethics Approval
This study was supported by the Ethics Committee of Sun Yat-sen University Cancer Center (Ethics approval number: B202353601) and complied with the Declaration of Helsinki. All patients agreed to informed consent involved in the study.
Acknowledgment
We thank all patients and clinical staff who participated in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the funding of National Natural Science Foundation of China (No. 81970176); National Natural Science Foundation of China (No.82170188).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tse E, Kwong YL. The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol. 2017;10(1):85. doi:10.1186/s13045-017-0452-9
2. Tse E, Kwong YL. How I treat NK/T-cell lymphomas. Blood. 2013;121(25):4997–5005. doi:10.1182/blood-2013-01-453233
3. Bu S, Yuan F, Wei X, et al. L-asparaginase-based regimen as a first-line treatment for newly diagnosed nasal type extranodal natural killer cell/T-cell lymphoma. Exp Ther Med. 2016;11(6):2437–2445. doi:10.3892/etm.2016.3249
4. Vittayawacharin P, Khuhapinant A. A five-year retrospective study of treatment outcomes using the L-asparaginase-based regimen as a first-line chemotherapy protocol for patients diagnosed with extranodal NK/T-cell lymphoma, nasal-type, in Thailand. Hematology. 2021;26(1):75–82. doi:10.1080/16078454.2020.1867783
5. Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016;17(3):389–400. doi:10.1016/s1470-2045(15)00533-1
6. Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123(6):837–842. doi:10.1182/blood-2013-09-524108
7. Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24(4):612–618. doi:10.1200/JCO.2005.04.1384
8. Sun J, Ke X, Zhang M, et al. New prognostic models for extranodal natural killer T-cell lymphoma, nasal-type using Cox regression and machine learning. Transl Cancer Res. 2021;10(2):613–626. doi:10.21037/tcr-20-3017
9. Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi:10.1038/nrc3611
10. Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
11. Li YJ, Li ZM, Xia Y, et al. Serum C-reactive protein (CRP) as a simple and independent prognostic factor in extranodal natural killer/T-cell lymphoma, nasal type. PLoS One. 2013;8(5):e64158. doi:10.1371/journal.pone.0064158
12. Zhang Z, Liang Y, Zhong D, et al. Prognostic value of inflammation-immunity-nutrition score in patients with hepatocellular carcinoma treated with anti-PD-1 therapy. J Clin Lab Anal. 2022;36(5). doi:10.1002/jcla.24336
13. Zhang S, Sun C, Chen X, et al. The prognostic value of controlling nutritional status (CONUT) score–based nomogram on extranodal natural killer/T cell lymphoma patients. Ann Hematol. 2023;102(6):1433–1442. doi:10.1007/s00277-023-05232-3
14. Wang L, Si H, Wang J, et al. Blood cell parameters as prognostic predictors of disease development for patients with advanced non-small cell lung cancer. Oncol Lett. 2020;20(2):1101–1110. doi:10.3892/ol.2020.11655
15. Murata M. Inflammation and cancer. Environ Health Preventative Med. 2018;23(1). doi:10.1186/s12199-018-0740-1.
16. Liao M, Chen P, Liao Y, et al. Preoperative high-sensitivity C-reactive protein to lymphocyte ratio index plays a vital role in the prognosis of hepatocellular carcinoma after surgical resection. Onco Targets Ther. 2018;11:5591–5600. doi:10.2147/ott.S167857
17. Li J, Chen S, Peng S, et al. Prognostic nomogram for patients with Nasopharyngeal Carcinoma incorporating hematological biomarkers and clinical characteristics. Int J Bio Sci. 2018;14(5):549–556. doi:10.7150/ijbs.24374
18. Zhang Y, Chen Q, Lu C, et al. Prognostic role of controlling nutritional status score in hematological malignancies. Hematology. 2022;27(1):653–658. doi:10.1080/16078454.2022.2078040
19. Lu J, Xu BB, Zheng ZF, et al. CRP/prealbumin, a novel inflammatory index for predicting recurrence after radical resection in gastric cancer patients: post hoc analysis of a randomized Phase III trial. Gastric Cancer. 2018;22(3):536–545. doi:10.1007/s10120-018-0892-0
20. Liang Y, Zhang Z, Zhong D, et al. The prognostic significance of inflammation-immunity-nutrition score on postoperative survival and recurrence in hepatocellular carcinoma patients. Front Oncol. 2022;12:913731. doi:10.3389/fonc.2022.913731
21. Jiang P, Wang J, Gong C, et al. A nomogram model for predicting recurrence of stage I–III endometrial cancer based on Inflammation-Immunity-Nutrition Score (IINS) and traditional classical predictors. J Inflamm Res. 2022;15:3021–3037. doi:10.2147/JIR.S362166
22. He X, Gao Y, Li Z, et al. Review on natural killer/T-cell lymphoma. Hematol Oncol. 2021;41(2):221–229. doi:10.1002/hon.2944
23. Kim SJ, Yoon SE, Kim WS. Treatment of localized extranodal NK/T cell lymphoma, nasal type: a systematic review. J Hematol Oncol. 2018;11(1):1–8.
24. Qi S-N, Xu LM, Yuan ZY, et al. Effect of primary tumor invasion on treatment and survival in extranodal nasal-type NK/T-cell lymphoma in the modern chemotherapy era: a multicenter study from the China Lymphoma Collaborative Group (CLCG). Leukemia Lymphoma. 2019;60(11):2669–2678. doi:10.1080/10428194.2019.1602265
25. Yamaguchi M, Suzuki R, Oguchi M, et al. Treatments and outcomes of patients with extranodal natural killer/T-cell lymphoma diagnosed between 2000 and 2013: a cooperative study in Japan. J Clin Oncol. 2017;35(1):32–39. doi:10.1200/jco.2016.68.1619
26. Wan L, Wu C, Luo S, et al. Prognostic value of Lymphocyte-to-Monocyte Ratio (LMR) in cancer patients undergoing immune checkpoint inhibitors. Dis Markers. 2022;2022:3610038. doi:10.1155/2022/3610038
27. Gawinski C, Holdakowska A, Wyrwicz L. Correlation between Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Extramural Vascular Invasion (EMVI) in locally advanced rectal cancer. Curr Oncol. 2022;30(1):545–558. doi:10.3390/curroncol30010043
28. Ding P, Guo H, Sun C, et al. Combined systemic immune-inflammatory index (SII) and prognostic nutritional index (PNI) predicts chemotherapy response and prognosis in locally advanced gastric cancer patients receiving neoadjuvant chemotherapy with PD-1 antibody sintilimab and XELOX: a prospective study. BMC Gastroenterol. 2022;22(1):121. doi:10.1186/s12876-022-02199-9
29. Wang HK, Wei Q, Yang YL, et al. Clinical usefulness of the lymphocyte-to-monocyte ratio and aggregate index of systemic inflammation in patients with esophageal cancer: a retrospective cohort study. Cancer Cell Int. 2023;23(1):13. doi:10.1186/s12935-023-02856-3
30. Li Y-J, Jiang WQ, Huang JJ, et al. The Glasgow Prognostic Score (GPS) as a novel and significant predictor of extranodal natural killer/T-cell lymphoma, nasal type. Am J Hematol. 2013;88(5):394–399. doi:10.1002/ajh.23422
31. Tse E, Fox CP, Glover A, et al. Extranodal natural killer/T-cell lymphoma: an overview on pathology and clinical management. Semin Hematol. 2022;59(4):198–209. doi:10.1053/j.seminhematol.2022.10.002
32. He Y, Gao Y, Ping L, et al. The emerging role of anti-PD-1 antibody-based regimens in the treatment of extranodal NK/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis. J Cancer Res Clin Oncol. 2022. doi:10.1007/s00432-022-04147-2
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


