Back to Journals » Clinical Interventions in Aging » Volume 16
A Nomogram for Identifying Subclinical Atherosclerosis in Chronic Kidney Disease
Authors Xiong J, Yu Z, Zhang D, Huang Y, Yang K, Zhao J
Received 23 March 2021
Accepted for publication 20 June 2021
Published 8 July 2021 Volume 2021:16 Pages 1303—1313
DOI https://doi.org/10.2147/CIA.S312129
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Jiachuan Xiong,* Zhikai Yu,* Daohai Zhang, Yinghui Huang, Ke Yang, Jinghong Zhao
Department of Nephrology, The Key Laboratory for the Prevention and Treatment of Chronic Kidney Disease of Chongqing, Chongqing Clinical Research Center of Kidney and Urology Diseases, Xinqiao Hospital, Army Medical University (Third Military Medical University), Chongqing, 400037, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinghong Zhao
Department of Nephrology, The Key Laboratory for the Prevention and Treatment of Chronic Kidney Disease of Chongqing, Chongqing Clinical Research Center of Kidney and Urology Diseases, Xinqiao Hospital, Army Medical University (Third Military Medical University), Chongqing, 400037, People’s Republic of China
Tel/Fax +86 023 68774321
Email [email protected]
Purpose: Atherosclerosis contributes substantially to cardiovascular mortality in patients with chronic kidney disease (CKD). But precise risk model for subclinical atherosclerosis in the CKD population is still lacking. The study aimed to develop and validate a nomogram for screening subclinical atherosclerosis among CKD patients without dialysis.
Patients and Methods: A total of 1452 CKD stage 1‒5 has been recruited in this cross-sectional study. Subclinical atherosclerosis was diagnosed with carotid ultrasonography. Patients were divided into the training set and validation set. The risk factors of atherosclerosis were identified by the training set and confirmed by the validation set. The receiver operating characteristic (ROC) curves and decision curve analyses (DCA) were executed to evaluate the accuracy of fitted logistic models in training and validation sets. Finally, a nomogram based on constructed logistic regression model in all participants was plotted.
Results: A total of 669 (46.1%) patients were diagnosed with subclinical carotid atherosclerosis. Binary logistic regression analysis showed that males, age, hypertension, diabetes, CKD stages, calcium, platelet, and albumin were risk factors for atherosclerosis. The accuracy of fitted logistic models was evaluated by the area under the ROC curve (AUC), which showed good predictive accuracy in the training set (AUC=0.764 (95% Confidence interval (CI): 0.733– 0.794) and validation set (AUC=0.808 (95% CI: 0.765– 0.852). A high net benefit was also proven by the DCA. Finally, these predictors were all included to generate the nomogram.
Conclusion: This proposed nomogram shows excellent predictive ability and might have a significant clinical implication for detecting subclinical atherosclerosis in patients with CKD.
Keywords: atherosclerosis, age, sex, risk factors
Introduction
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality in patients with chronic kidney disease (CKD) with or without dialysis,1 even in patients who received kidney transplantation.2 Previous studies showed that the mortality risk of CVD in CKD patients aged 25–34 years is 100–150 times higher than that in other age groups,3,4 while in the end-stage kidney disease stage, CVD deaths account for more than 50% of the total mortality of CKD patients.5 Additionally, CKD has been proven to be one of the main risk factors for CVD, independently related to its occurrence and development. Recent studies have suggested that abnormal mineral bone metabolism, protein energy-wasting, and FGF23/Klotho axis are emerging risk factors for adverse renal outcomes. The incidence of atherosclerosis, a common and frequently-occurring CVD,6 is observed in younger patients recently, especially in the CKD population.7 An epidemiologic study indicated that atherosclerosis is common in patients with advanced CKD.8 However, in recent years, it has been suggested that the atherosclerosis risk is increased even in early CKD stages.9 Evidence indicates that the occurrence of atherosclerosis is accelerated in the CKD population due to factors involving systemic calcium/phosphate imbalance, vascular endothelial injury, inflammation, and uremic toxicity.10 An increasing number of studies show that atherosclerosis is regarded as the initial cardiovascular abnormality in the CKD setting.11 Therefore, identifying the risk factors and interventions for the pathogenesis of atherosclerosis is crucial.
It is urgent to build a prediction model based on these risk factors as a precise risk model for subclinical atherosclerosis in the CKD population remains lacking. In this study, we aimed to develop and validate a nomogram for screening subclinical atherosclerosis among CKD patients without dialysis.
Patients and Methods
Participant Selection
The study participants were patients with non-dialysis dependent CKD stages 1–5 from the research in evaluation and treatment aimed renal disease (RETARD) cohort. The RETARD study is a multi-center cohort study in southwest China, which aimed to evaluate the risk factors for the progression of CKD and the effect of treatment on prognosis. The baseline characteristics of the RETARD study were used for analysis in the current study. The estimated glomerular filtration rate (eGFR) was calculated according to the CKD-EPI formula.12 CKD staging was performed using the grading criteria from the KDIGO guideline.13 Inclusion criteria are the following: (1) non-dialysis patients with stages 1–5 CKD; (2) adults (age≥18 years); (3) complete medical records and follow-up data; and (4) signed informed consent. Exclusion criteria are: (1) acute renal failure and (2) previous or current diagnosis of a malignant tumor.
Clinical Data Collection and Laboratory Analysis
Demographic information and laboratory data of patients meeting the enrollment requirements were extracted from the Electronic Medical Record System of Xinqiao Hospital. Blood samples for laboratory data measurement were collected between 7 am and 9 am, after the patients fasted for at least 8 hours. The blood sample was centrifuged at a speed of 3000 r/min for 10 min and stored at −80°C until analysis. The laboratory data included serum hemoglobin, platelets, albumin, creatinine, uric acid, calcium, phosphorus, and intact parathyroid hormone (iPTH), which were measured using the Beckman AU5800 automatic biochemical analyzer (Beckman Coulter, Inc., USA) in the Department of Clinical Laboratory.
Carotid Ultrasonography
All participants underwent a carotid ultrasonography examination between January 2015 and August 2019. Bilateral carotid arteries were analyzed by Color Doppler ultrasound at a frequency of 10 MHz on linear array transducer (Model Vinno 70; VINNO Technology (Suzhou) Co., Ltd., China) by an experienced sonographer.14 Smooth of the carotid artery was defined as normal. Additionally, an atherosclerotic plaque was defined as a focal structure encroaching into the arterial lumen by at least 0.5 mm or 50% of the surrounding intima-media thickness (IMT) value, or with a thickness >1.5 mm as measured from the media-adventitia interface to the intima-lumen interface.15 Both atherosclerotic plaque and significant IMT were regarded as carotid atherosclerosis.
Statistical Methods
Means and standard deviations were used for quantitative variables with normal distribution, while medians (25th, 75th percentiles) were determined for quantitative variables with non-normal distribution. Frequencies (%) were indicated in parentheses for qualitative variables. The independent samples t-test and Wilcoxon rank-sum test were used to compare quantitative variables between atherosclerosis and no- atherosclerosis; the chi-square test was used for qualitative variables. Then, logistic regression was used to screen risk factors, whereas receiver operating characteristic (ROC) curves analysis and decision curve analysis (DCA) were conducted to evaluate the performance of the model. Finally, the nomogram based on the constructed logistic regression model was plotted to display the risk of atherosclerosis. Statistical significance level was set at 0.05, and all analyses were performed with SPSS statistics (version 25.0, IBM Corporation).
Results
Basic Information of the Included Participants
A total of 1452 CKD participants with stages 1–5 who received carotid ultrasonography were involved in this study, of which 669 (46.1%) patients were diagnosed with subclinical carotid atherosclerosis. The mean age of patients was 54.4 (standard deviation: 14.4) years, and the majority (60.2%) were males. As the frequently present comorbidity in patients with CKD, the prevalence of CVD was 34.4%, second only to hypertension (75.9%) and followed by diabetes mellitus (26.2%). Due to the limited patients in CKD stage 1 (4% of the total CKD patients), we combined the CKD stage 1 and stage 2 into CKD stage 1–2 for analysis. Interestingly, the prevalence of subclinical carotid atherosclerosis in CKD stages 1–2 was 34.5%, which markedly increased in CKD stage 3 (50.2%), and slightly decreased in stages 4 and 5; the prevalence was 46.9% and 47% for stages 4 and 5, respectively (Figure 1). To prevent the model from over-fitting in the analysis of influencing factors, participants were divided into the training and validation sets according to the ratio of 7:3. The basic information of the two sets is detailed in Table 1. No differences in all characteristics were found between the two sets, indicating that the division of the dataset was reasonable and comparable. Participants were then divided into two groups according to whether they experienced atherosclerosis or not. Compared with patients without atherosclerosis, patients with atherosclerosis were older, predominantly male, and more likely to be known smokers and alcohol drinkers, and are thus more inclined to develop CVD, diabetes mellitus, hypertension, and worse CKD staging. Notably, there was no difference in serum lipid levels, including total cholesterol, triglyceride, high-density lipoprotein, and low-density lipoprotein levels, between the two groups (Table 2).
 |
Table 1 Basic and Clinical Features of the Training and Validation Sets in CKD Patients |
 |
Table 2 Comparisons of Basic and Clinical Features of Training and Validation Set According to Atherosclerosis |
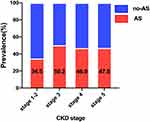 |
Figure 1 Prevalence of atherosclerosis by CKD stages. |
Risk Factors Associated with the Prevalence of Atherosclerosis
To investigate the risk factors associated with atherosclerosis in CKD patients, two models (model 1: basic characteristics; model 2: model 1 plus clinical features) were constructed by binary logistic regression. This showed that the male sex, presence of hypertension and diabetes mellitus, advanced age, CKD stage, higher calcium levels, lower platelet counts, and albumin levels were more inclined to lead to a higher prevalence of atherosclerosis (Table 3). The ROC curves were plotted to evaluate the accuracy of fitted logistic models in the training and validation sets, whose areas under the curves were 0.7636 and 0.8083, respectively (Figure 2), indicating good predictive accuracy. Moreover, the DCA for two models in two sets were also performed, revealing good net benefits (Figure 3). Finally, a nomogram based on a constructed logistic regression model in all participants was plotted to display the risk of atherosclerosis. The graph showed that males, known hypertensives and diabetics, elderly, and patients with high calcium, low platelet counts, or low albumin corresponded to higher points, which implies a higher risk of atherosclerosis (Figure 4).
 |
Table 3 Risk Factors Associated with the Prevalence of Atherosclerosis in the Training Set |
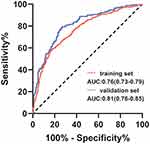 |
Figure 2 The ROC curve of the two constructed models by logistic regression. |
 |
Figure 3 The DCA for 2 models to predict the occurrence of atherosclerosis in CKD patients. Abbreviation: DCA, decision curve analysis. |
 |
Figure 4 The nomogram for prediction of the occurrence of atherosclerosis in terms of constructed model. |
Subgroup Analysis for CKD, Age, and Sex Stratification
Stratification based on sex and age was performed due to the possible effect of these factors on the occurrence of atherosclerosis. Results showed that subclinical carotid atherosclerosis risk increases in patients over 65 years of age with CKD stages 4 and 5, while male patients had a higher percentage of atherosclerosis in all CKD stages (Figure 5A and B). CKD plays a critical role in the occurrence of atherosclerosis, and we stratified the patients with atherosclerosis into the early CKD stage group (stages 1–3) and advanced CKD stage group (stages 4 and 5) (Supplementary Table 1), and compared the baseline characteristics of the CKD patients with atherosclerosis between the early stage and advanced stage of CKD. Furthermore, subgroup analysis based on sex and age was performed, and the results are shown in Supplementary Table 2. The adjusted odds ratio (OR) was shown in Figure 6, indicating that in each CKD stage, male patients had a higher OR than female patients, and patients aged over 65 years had a higher OR than those aged under 65 years.
Discussion
Our study enrolled more than 1000 CKD patients to analyze the risk factors for subclinical atherosclerosis and use them to build a nomogram model. To our knowledge, it is the first precise risk-model nomogram for identifying subclinical atherosclerosis in CKD patients that was rigorously assessed and internally validated.
Experimental study and radiological analyses have demonstrated that CKD dramatically accelerates the growth of atherosclerotic lesions and amplifies atherosclerosis,16,17 which involve the renin-angiotensin system (RAS),18 augmentations of inflammation, perturbation of lipid metabolism, endothelial impairment, and other mechanisms.19 Nonetheless, strategies to prevent activation of the pro-atherosclerotic signal pathway can reduce the incidence of atherosclerosis and CVD events in CKD populations.
Currently, population studies show a high prevalence of atherosclerosis in CKD patients. An observational study showed that about 25% of the individuals who had eGFR <60 mL/min/1.73 m2 had femoral artery plaque.20 Furthermore, more than half of CKD patients without diabetes and 69% of those with diabetes have atherosclerosis.21 Imaging analysis also confirmed a high prevalence in non-diabetic CKD patients.22 However, the incidence may be varied based on the parts of the artery, different confirmatory methods, and CKD staging.23–25 In our study, we showed that the prevalence of atherosclerosis is about 46%, and that in CKD stages 1–2, nearly 34.5% of the patients have subclinical atherosclerosis. It is highly suggested that screening and early detection for subclinical atherosclerosis are essential for its management.
Concurrently, cross-sectional studies from general populations have shown an inverse association between carotid IMT (c-IMT) and renal function.26 Recent studies mainly focused on the prevalence of atherosclerosis in the general population, while evidence in the CKD population remains limited. We summarized the related studies which investigated atherosclerosis in the general and CKD populations (Supplementary Table 3). In adults with no known kidney disease, the c-IMT was significantly correlated with eGFR and serum creatinine.27 However, the association between atherosclerosis and eGFR was slightly different for sex. A study showed that c-IMT showed a significant negative correlation with eGFR in both male (r= -0.346, p<0 0.001) and female (r =-0.253, p < 0.001) subjects.28 In turn, subclinical carotid atherosclerosis can increase the risk of CKD. In the Kyushu and Okinawa Population Study with a five-year follow-up, 224 of the 1824 (12.9%) participants who developed CKD had higher carotid IMT.29 This indicates that CKD and atherosclerosis may interact with, and reinforce, each other, although specific mechanisms need to be further explored.
Atherosclerosis has been proven to be associated with myocardial infarction, stroke, and peripheral artery disease.30 In the CKD population, carotid plaque, even subclinical, was closely associated with a poor prognosis in non-diabetic CKD patients.31 The risk factors for atherosclerosis and its thrombotic complications include high blood pressure, smoking, diabetes, increased age, and increased high sensitivity C-reactive protein level. A significant positive correlation was found between CRP and c-IMT values (r=0.327; p<0.001).32 The National Observatory of Atherosclerosis in Nephrology (NEFRONA) study indicated that age, diabetes, smoking history, and phosphate levels are significantly associated with atherosclerosis in non-dialysis dependent CKD patients, wherein they noted sex to be another influencing factor.33 The multivariable-adjusted maximum c-IMT was significantly greater in CKD patients than in non-CKD patients, although only in patients with hypertension,34 thereby implying that hypertension is a strong indicator for atherosclerosis. Subgroup analysis further showed that the atherosclerosis risk increased with eGFR decline in male patients, which was not observed in female patients, suggesting marked differences in the pathophysiological process of atherosclerosis between sexes. A previous animal study showed that estrogens are a major determinant of atherosclerosis progression, complications, and plaque vulnerability in the reproductive stage.35 Several epidemiological studies documented an inverse relationship between plasma estrogen levels and the incidence of cardiovascular disease, with higher plasma estrogen levels contributing to the inhibition of atherosclerosis.36 This was confirmed in the results of the study wherein patients aged over 65 years, above postmenopausal age, were shown to have increased atherosclerosis risk. The burden of subclinical atherosclerosis is the strongest predictor of future CVD events in diabetic CKD patients.37 In our study, we further confirmed these risk factors and built a risk-model nomogram to achieve an accurate early diagnosis and facilitate target intervention.
Previous rodent animal studies indicated that lipid levels and lipid oxidation products were associated with atherosclerosis.38 Linear regression analysis showed that low-density lipoprotein cholesterol (LDL-C) and total cholesterol were risk factors for early-stage atherosclerosis.39 However, in our study, a significant relationship between blood lipid and subclinical atherosclerosis was not observed. Nevertheless, we found that among all the CKD patients with subclinical atherosclerosis, a significant difference in blood lipids levels was observed between early-stage CKD and advance-stage CKD, age over 65 and under 65, and male and female sexes. This indicates that CKD staging, age, and sex have a strong interaction with atherosclerosis. We speculated that dyslipidemia might contribute to the occurrence and acceleration of atherosclerosis development in CKD conditions, although the exact mechanism needs to be verified.
Limitation
There were a few limitations that should be addressed. Firstly, we only performed duplex ultrasonography of carotid vessels for detecting subclinical atherosclerosis, with no other test for confirmation. Secondly, we performed a cross-sectional study with a limited number of patients to build the risk model. Some factors that may influence atherosclerosis were not identified in the risk model, such as drug use, race, and geographic differences. Thirdly, the time variable was not accounted for in this cross-sectional study; thus, we could not determine the cause and effect of the disease as well as the interaction between CKD and atherosclerosis. Therefore, a prospective cohort study is warranted. Lastly, the precise nomogram risk model was only validated by internal data, while verification was not performed with external data. Further multi-center studies with larger study populations are necessary for its verification.
Clinical Trial Registration
Current Trials number: ChiCTR2000034569.
Conclusion
The current study constructed a nomogram for identifying subclinical atherosclerosis in the CKD population with good performance, which could be used for early screening and facilitating the target intervention for subclinical atherosclerosis in CKD patients.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author [email protected]) upon request.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards, and was approved of the ethics committee of Xinqiao Hospital (IRB approval number 2018-006-01) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study. Written informed consent was obtained for the publication of patient data.
Acknowledgments
We would like to thank all the members of the RETARD cohort study group for their assistance in recruiting participants and data collection. We are grateful to all the participants for their cooperation.
Author Contributions
X.J.C. prepared and wrote the paper; Y.Z.K. analyzed the data; Z.D.H., H.Y.H., Y.K., collected the data; Z.J.H. conceived the idea of the study; All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by research grants from the National Key R&D Program of China (2018YFC1312700), the Natural Science Foundation of China (No. 81700379), Personal Training Program for Clinical Medicine Research of Army Medical University (2018XLC1007).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kahn MR, Robbins MJ, Kim MC, et al. Management of cardiovascular disease in patients with kidney disease. Nat Rev Cardiol. 2013;10:261–273.
2. Gajjala PR, Fliser D, Speer T, et al. Emerging role of post-translational modifications in chronic kidney disease and cardiovascular disease. Nephrol Dial Transplant. 2015;30:1814–1824.
3. Ene-Iordache B, Perico N, Bikbov B, et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health. 2016;4(5):e307–19.
4. Rhee CM, Kovesdy CP. Epidemiology: spotlight on CKD deaths—increasing mortality worldwide. Nat Rev Nephrol. 2015;11:199–200.
5. Tonelli M, Karumanchi SA, Thadhani R. Epidemiology and mechanisms of uremia-related cardiovascular disease. Circulation. 2016;133:518–536.
6. Neeland IJ, Ross R, Després JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–725.
7. Shoji T, Abe T, Matsuo H, et al. Chronic kidney disease, dyslipidemia, and atherosclerosis. J Atheroscler Thromb. 2012;19(4):299–315.
8. Valdivielso JM, Rodríguez-Puyol D, Pascual J, et al. Atherosclerosis in chronic kidney disease: more, less, or just different? Arterioscler Thromb Vasc Biol. 2019;39(10):1938–1966.
9. Zalba G, Fortuño A, Díez J. Oxidative stress and atherosclerosis in early chronic kidney disease. Nephrol Dial Transplant. 2006;21(10):2686–2690.
10. Swaminathan S, Shah SV. Novel inflammatory mechanisms of accelerated atherosclerosis in kidney disease. Kidney Int. 2011;80(5):453–463.
11. Drüeke TB, Massy ZA. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol. 2010;6:723–735.
12. Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79(5):555–562.
13. Stevens PE, Levin A. Kidney disease: improving global outcomes chronic kidney disease guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830.
14. Zaid M, Fujiyoshi A, Kadota A, et al. Coronary artery calcium and carotid artery intima media thickness and plaque: clinical use in need of clarification. J Atheroscler Thromb. 2017;24:227–239.
15. Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21(2):93–111.
16. Bro S, Bentzon JF, Falk E, et al. Chronic renal failure accelerates atherogenesis in apolipoprotein E-deficient mice. J Am Soc Nephrol. 2003;14:2466–2474.
17. Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–1605.
18. Yamamoto S, Zhong J, Yancey PG, et al. Atherosclerosis following renal injury is ameliorated by pioglitazone and losartan via macrophage phenotype. Atherosclerosis. 2015;242(1):56–64.
19. Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 2017;13:368–380.
20. Hsu S, Rifkin DE, Criqui MH, et al. Relationship of femoral artery ultrasound measures of atherosclerosis with chronic kidney disease. J Vasc Surg. 2018;67(6):1855–1863.e1.
21. Palanca A, Castelblanco E, Perpiñán H, et al. Prevalence and progression of subclinical atherosclerosis in patients with chronic kidney disease and diabetes. Atherosclerosis. 2018;276:50–57.
22. Choi IJ, Lim S, Choo EH, et al. Differential impact of chronic kidney disease on coronary calcification and atherosclerosis in asymptomatic individuals with or without diabetes: analysis from a coronary computed tomographic angiography registry. Cardiorenal Med. 2018;8(3):228–236.
23. Arroyo D, Betriu A, Martinez-Alonso M, et al. Observational multicenter study to evaluate the prevalence and prognosis of subclinical atheromatosis in a Spanish chronic kidney disease cohort: baseline data from the NEFRONA study. BMC Nephrol. 2014;18(15):168.
24. Pitha J, Teplan V, Kalousova M, et al. Gender-related determinants of advanced subclinical atherosclerosis in patients undergoing kidney transplantation. Kidney Blood Press Res. 2010;33(3):227–234.
25. Gracia M, À B, Martínez-Alonso M, et al. Predictors of subclinical atheromatosis progression over 2 years in patients with different stages of CKD. Clin J Am Soc Nephrol. 2016;11(2):287–296.
26. Kokubo Y. Carotid atherosclerosis in kidney disease. Contrib Nephrol. 2013;179:35–41.
27. Buscemi S, Geraci G, Massenti FM, et al. Renal function and carotid atherosclerosis in adults with no known kidney disease. Nutr Metab Cardiovasc Dis. 2017;27(3):267–273.
28. Wu Y, Hou J, Li J, et al. Correlation between carotid intima-media thickness and early-stage chronic kidney disease: results from asymptomatic polyvascular abnormalities in community study. J Stroke Cerebrovasc Dis. 2016;25(2):259–265.
29. Shimizu M, Furusyo N, Mitsumoto F. T et al. Subclinical carotid atherosclerosis and triglycerides predict the incidence of chronic kidney disease in the Japanese general population: results from the Kyushu and Okinawa Population Study (KOPS). Atherosclerosis. 2015;238(2):207–212.
30. Muscogiuri G, Annweiler C, Duval G, et al. Vitamin D and cardiovascular disease: from atherosclerosis to myocardial infarction and stroke. Int J Cardiol. 2017;230:577–584.
31. Kim JK, Song YR, Kim MG, et al. Clinical significance of subclinical carotid atherosclerosis and its relationship with echocardiographic parameters in non-diabetic chronic kidney disease patients. BMC Cardiovasc Disord. 2013;13:96.
32. Patel ML, Sachan R, Singh GP, et al. Assessment of subclinical atherosclerosis and endothelial dysfunction in chronic kidney disease by measurement of carotid intima media thickness and flow-mediated vasodilatation in North Indian population. J Family Med Prim Care. 2019;8:1447–1452.
33. Martín M, Valls J, Betriu A, et al. Association of serum phosphorus with subclinical atherosclerosis in chronic kidney disease. Sex makes a difference. Atherosclerosis. 2015;241(1):264–270.
34. Ohara T, Kokubo Y, Toyoda K, et al. Impact of chronic kidney disease on carotid atherosclerosis according to blood pressure category: the Suita study. Stroke. 2013;44(12):3537–3539.
35. Clarkson TB. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause. 2018;25(11):1262–1274.
36. Nofer JR. Estrogens and atherosclerosis: insights from animal models and cell systems. J Mol Endocrinol. 2012;48(2):R13–29.
37. Palanca A, Castelblanco E, À B, et al. Subclinical atherosclerosis burden predicts cardiovascular events in individuals with diabetes and chronic kidney disease. Cardiovasc Diabetol. 2019;18(1):93.
38. Ahotupa M. Oxidized lipoprotein lipids and atherosclerosis. Free Radic Res. 2017;51(4):439–447.
39. Pan J, Liu J, Wang H, et al. Association of carotid atherosclerosis with Lipid components in asymptomatic low-income Chinese: a population-based cross-sectional study. Front Neurol. 2020;11:276.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


