Back to Journals » Cancer Management and Research » Volume 15
A New Scoring System for Predicting Mortality in Hematological Malignancies with Sepsis: A Derivation and Validation Study
Authors Li H , Fan S, Lu D, Zhou J
Received 4 July 2023
Accepted for publication 22 September 2023
Published 29 September 2023 Volume 2023:15 Pages 1073—1083
DOI https://doi.org/10.2147/CMAR.S428930
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Haitao Li,1,* Shengjin Fan,2,* Dongxue Lu,2 Jin Zhou1
1Harbin Medical University, Harbin, 150001, People’s Republic of China; 2Department of Hematology, First Affiliated Hospital of Harbin Medical University, Harbin, 150001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jin Zhou, Harbin Medical University, No. 23 157 Health Road, Nangang District, Harbin, 150001, People’s Republic of China, Email [email protected]
Objective: This study aimed to derive and validate a prognostic scoring system to identify patients with hematological malignancies (HMs) and sepsis who have a high mortality rate.
Methods: Cohorts for derivation and validation were created from all data. Using univariate and multivariate analysis, the independent variables connected to 28-day mortality in the derivation cohort were found. A receiver operating characteristic (ROC) curve was used to compare the predictive power and determine their cutoff points. These risk variables were given a score weighted by risk prediction function, and a new scoring system was also developed. The area under the ROC curve (AUROC) and sensitivity and specificity for mortality of the risk category of the new scoring system were compared with Sequential Organ Failure Assessment (SOFA) score.
Results: 90 (45.22%) of the 199 patients passed away within 28 days. Ninety-nine patients made up the derivation cohort, with 47 (47.47%) fatalities. Ages in the non-survival group were higher (61.47 ± 14.53 vs 55.13 ± 15.66) than in the survival group. As independent predictors of death, multivariable analysis identified SOFA score (OR 1.442, 95% CI 1.035, 2.009), age (OR 1.242, 95% CI 1.026, 1.503), and prothrombin time (PT) (OR 1.213, 95% CI 1.030, 1.430). The AUROC with 95% CI of the new scoring system and its sensitivity and specificity to mortality were virtually all superior to SOFA score in both derivation and validation cohorts: AUROC (0.757 vs 0.716), Sensitivity (75 vs 67.3%), and Specificity (68.1% vs 63.8%) are the Derivation cohort; Validation cohort: Sensitivity (91.2% vs 84.2%), AUROC (0.792 vs 0.733), and Specificity (58.1% vs 58.1%). The model was correctly calibrated, according to the Hosmer–Lemeshow test.
Conclusion: The new scoring system was more accurate in predicting 28-day mortality among patients with HMs and sepsis than the SOFA score.
Keywords: sepsis, hematological malignancies, scoring system
A Letter to the Editor has been published for this article.
Introduction
Sepsis is a dysregulated immunological response to infection that can cause life-threatening organ dysfunction. In the intensive care unit (ICU), sepsis is major cause of mortality for critically ill patients. In 2020, there were 20.6% sepsis cases in ICUs in China, the mortality of sepsis was 35.5%, and the mortality of severe sepsis was over 50%.1 Early diagnosis with prompt intensive care and infection source control before organ dysfunction is the acknowledged paradigm in sepsis care.
Patients with hematologic malignancies (HMs) are more susceptible to infections that spread quickly and progress to sepsis. Immune dysfunction or neutropenia brought on by the underlying malignancies and cytotoxic chemotherapeutic medications both play a role in this.2 Compared to patients without malignancies, patients with HMs have an up to 10- to 15-fold increased risk of developing sepsis, who frequently necessitates transferring them to the ICU.3,4 In particular, sepsis in patients with neutropenia was more common (ranges from 7% to 42%)5 and was associated with a higher mortality rate (greater than 30%).4 Due to the individuals with HMs may have a multitude of comorbid ailments and underlying diseases, the signs and symptoms of sepsis may vary considerably or be less noticeable, making early diagnosis and treatment of the sepsis very challenging. However, specific clinical scoring system can predict sepsis risk before it becomes a serious condition. The SOFA score was suggested as a useful tool to evaluate organ failure and sepsis prognosis in the Third International Consensus Definitions for Sepsis-3.0 in 2016.6 The SOFA score should change by at least 2 points from baseline to indicate organ dysfunction. The SOFA score is a crucial measure for assessing the seriousness of the patient’s condition and forecasting the early death.7,8 The predictive validity of SOFA for in-hospital mortality among patients with infection in ICU was comparable with the more complex LODS and was statistically greater than systemic inflammatory response syndrome (SIRS), supporting its use in clinical sepsis criteria.9 However, the clinical use of SOFA score is still constrained and cannot be applied to HM patients without limitations: Due to thrombocytopenia caused by chemotherapy or tumor, platelet (PLT) cannot be employed; additionally, limited assessment is possible due to liver and kidney impairment brought on by chemotherapy.5 In addition, the Acute Physiology and Chronic Health Evaluation II (APACHE II) system is frequently employed to determine the severity of the patient’s condition in ICU. However, it underestimated the risk of death for septic patients.10 Thus, in order to predict mortality rate and risk stratification in patients with HMs and sepsis, we set out to develop a new scoring system.
Although coagulation and inflammation are necessary defenses against infection, sepsis-induced stimulation of these processes also results in life-threatening organ malfunction. A fatal complication of sepsis is known as sepsis-induced coagulopathy (SIC), which can range from thrombocytopenia to disseminated intravascular coagulation (DIC).11 SIC scores are based on parameters including PLT count, PT-INR and SOFA score, are easy to calculate and have a higher predictive value for 28-day mortality. However, it still is not a reliable scoring system for patients with HMs. Thrombocytopenia, as one of its main assessment indicator, can also be caused by chemotherapy and tumors, and this condition decreased its prediction accuracy. In light of this, we speculate that SOFA score in conjunction with markers of coagulation function such as PT, international normalized ratio (INR), or activated partial thromboplastin time (APTT) may be more accurate at predicting the prognosis of patients with HMs and sepsis.
Up to now, the SOFA score has been served for decades as the industry benchmark for determining the prognosis of sepsis. The SOFA score may be more helpful in the diagnosis and prognosis of sepsis patients when paired with a variety of biomarkers, such as procalcitonin (PCT),12,13 age, albumin level, and international normalized ratio,14 among others, according to studies published in recent years. Additionally, the ability of the SOFA score to predict outcomes may also be impacted by indications like coagulation indicators, blood lactate level and various underlying disorders. Those indexes and score models’ predictive significance have not, however, been systematically verified in patients with HMs and sepsis. We do not yet know whether patients with HMs and sepsis have better prognostic predictive value when these markers are combined with SOFA scores. To the best of our knowledge, there has been currently no reliable scoring system for determining when patients with HMs and sepsis should be admitted to the HICU or for early identification of severe sepsis. The aims of our study are to develop a new prognostic scoring system to identify patients with HMs and sepsis who have a high mortality rate.
Methods
Patients
A single-center retrospective study with medical chart review was performed. Data was gathered between October 2017 and May 2023. Three hundred and twelve patients diagnosed with HMs and sepsis who were admitted to the HICU, an 8-bed ICU, were identified, in the first affiliated hospital of Harbin Medical University, China. All patients were admitted directly from the hematology department ward. During the study period, only the values from their first 24-hour HICU admission were included in this analysis. All related information was gleaned from the medical records of our institution. We did not have any established criteria for admission to HICU, decisions were made in consultation between the attending HICU specialist and hematologist.
Patients from the following categories were included: adults were identified by a medical record search for all patients ≥18 years presenting with HMs and sepsis; evidence of documented infection and organ dysfunction represented by SOFA score of 2 points or more; those were admitted to the HICU and received early goal directed therapy (EGDT);15 within 28 days of receiving HICU medical care, patients who passed away or survived have been included.
Exclusion criteria for patients: patients’ medical care was discontinued or they were sent to another medical institution with an unclear prognosis; acute promyelocytic leukemia; COVID-19 and other deadly virus infections; non-septic related coagulation disorders, such as hemophilia, anticoagulant application or vitamin K deficiency in chronically undernourished patients, and so forth; non-septic related liver dysfunction was also ruled out.
Finally, 199 patients were eventually enrolled. With patient numbers of about 1:1 ratios, we randomly assigned all of the recruited patients to the “validation cohort” and the “derivation cohort”. Ninety-nine patients were included in the derivation group, and 100 patients were included in the validation group (Figure 1). The Harbin Medical University Ethics Committee gave its approval to this study, which was carried out in accordance with the Helsinki Declaration.
 |
Figure 1 Flow diagram illustrating selection of patients. |
Study Variables
For a follow-up of 28-day mortality, a retrospective study of medical records was completed. The 28-day survival rate was used to split all study participants into groups of survivors and non-survivors. The patient’s demographic information, vital status, clinical signs and parameters (peripheral blood cell count, coagulation indicators, inflammatory biomarkers, blood lactate levels, organ function characteristics, pathogens, SOFA scores and quick SOFA (qSOFA) score, etc.), as well as discharge follow-up clinical data, were extracted from our electronic medical record system within the first 24 hours of HICU admission.
Definitions and Criteria
The diagnosis of sepsis was confirmed genetically based on the Sepsis-3.0; qSOFA score is a bedside prompt that identifies patients at greater risk of death or prolonged HICU stay. Low systolic blood pressure (≤100mmHg), a rapid breathing rate (≥22 breaths per minute), and disturbed mental status were given the qSOFA score (3 points, one point each).15 Septic shock was recorded if the patient met the criteria for sepsis and had persistent hypotension after receiving enough intravenous fluids. When the absolute neutrophil count fell below 500 cells/mm,3 it was considered neutropenia.
Statistical Analysis and Calculation of the New Scoring System
IBM SPSS Statistics Version 25.0 (Chicago, IL, USA) was used to analyze the data. Give statistical advice and seek the counsel of qualified statisticians.
In the derivation study, univariate analysis was utilized to identify variables strongly associated with the 28-day mortality rate in patients with HMs and sepsis. The continuous variables with normal distribution were reported as mean ± standard deviation (SD) and compared using a t-test; the continuous variables with abnormal distribution were presented with medians and interquartile ranges and compared using a t-test; categorical variables were expressed as frequencies and compared using the chi-square test. In all statistical analyses, a p < 0.05 was considered statistically significant. Variables discovered in the univariate analysis were subjected to a logistic multivariate regression on a dichotomous dependent variable and were used to assess the odds ratio (OR) and p value for discovering independent predictors and were adjusted for confounding factors. Variables with p > 0.05 were taken into elimination criterion.
Creating an ROC curve and determining the optimum cut-off points for each independent variable. The overall discriminating accuracy for the link between each predictor and mortality was assessed using the AUROC, which is made up of the relationship between “sensitivity” and “1-specificity”. The optimum cut-off risk prediction points, sensitivity, specificity, odds ratio (OR), and specific AUROC (95% CI) values were calculated. AUROC values 0.60 are regarded as poor, 0.60–0.80 are regarded as moderate, and AUROC values >0.8 are viewed as good.16
Building a new scoring system and stratifying patients based on the optimum cut-off point. For each of the independent predictor, the optimum cut-off point was identified and the mortality OR and 95% confidence interval (CI) were calculated. Each predictor was dichotomized for the new scoring system according to the optimum cut-off point and given a score that was weighted according to risk prediction function,17 and a new scoring system was built using the sum of the component values. Then, the risk stratification was performed according to the new score and risk factor characteristics. The mortality in sepsis in the new scoring system was calculated for each patient by adding the weighted scores. Finally, we compared the performance of the new scoring system in predicting 28-day mortality with the performance of the SOFA scoring criteria.
Furthermore, we validated the results in the validation study, the new scoring system’s distinction and calibration were applied to the validation data. The Hosmer–Lemeshow X2 statistic test was used to gauge the degree of calibration. Using Log rank testing, Kaplan–Meier curves for the new scoring system were analyzed.
Results
Cohort Characteristics
During the five years of the study, there were 312 patients with HMs admitted to the HICU who also met the Sepsis-3.0 diagnostic criteria for sepsis. One hundred and thirteen patients were excluded because they failed to meet the inclusion criteria. Ultimately, this study covered 199 patients with HMs and sepsis. The 199 patients were assigned randomly to the validation cohort which included the 99 patients and the derivation cohort which included the remaining 100 patients (Figure 1). The characteristics of the patient cohort were acquired, as shown in Table 1.
 |
Table 1 Univariate Analysis of Variables in the Derivation Data Between Survivors and Nonsurvivors (N = 99) |
Derivation Study
Among the derivation study of 99 patients with HMs and sepsis, with a median age of 58 years (range 18–90 years), 47 patients died during a 28-day hospital stay (47.47%, 49/99), with a median age of 61 years (range 18–90 years), and 52 patients survived (52.52%, 52/99), with a median age of 55 years (range 18–78 years). Age differences between the non-survival and survival groups were significant (p = 0.04). The most frequent diagnosis was leukemia (43.43%, 43/99), which was followed by Lymphoma (26.26%, 26/99), MDS (19.19%, 19/99), and MM (11.11%, 11/99). Leukemia had the highest fatality rate (17/47, 36.17%). There was, however, no statistically significant difference (P = 0.306). Diabetes (p < 0.001), SOFA Scores (p = 0.009), PT (p < 0.001), and DD (p = 0.026) all showed significant differences. At the time of admission to the HICU, there were no appreciable differences in the gender distribution, pathogen, WBC, neutrophils, HGB, PLT, or APTT, FIB, DD, Cr, CRP, PCT and blood lactate levels between the two groups (All p > 0.05, Table 1).
Age (P = 0.045), SOFA score (P < 0.001), and PT (P < 0.001) were shown to be significantly different between survivors and nonsurvivors in the derivation study of 99 patients dataset by binary logistic regression analysis (Table 2). PT (OR: 1.213; 95% CI: 1.030–1.430), age (OR: 1.242; 95% CI: 1.026–1.503), and SOFA score (OR: 1.442; 95% CI: 1.035–2.009) were all identified as independent risk factors by multivariate Cox regression analysis (Table 3).
 |
Table 2 Univariate Cox Proportional Hazards Analysis for 28-Day Mortality |
 |
Table 3 Multivariate Cox Proportional Hazards Analysis for 28-Day Mortality |
The optimum cut-off points for each independent variable were established according to ROC curves. The optimum cutoff points for SOFA score, new scoring system, age and PT were 9 points, 4.5 points, 60 years and 16s, respectively. For each independent variable, the odds ratio for mortality and the 95% confidence interval (CI) were calculated (Table 4). Each independent risk factor was given a score according to risk prediction function17 (Table 5). A new scoring system was devised, with scores ranging from 0 (low risk) to 7 (very high risk); Additionally, when the PT variable assigned 0 scores, the score was 1, 2, or 3, which is classified as medium risk; when the PT variable assigned points, the score was 4, 5, or 6, which is classified as high risk. Table 6 details the New scoring system’s risk stratifying patients based on the optimum cut-off point. ROC curves were drawn for New scoring system and SOFA score (Figure 2). In comparison to SOFA score, the new scoring system showed the greater AUROC (0.757, 95% CI: 0.661–0.853 vs 0.707, 95% CI: 0.607–0.808) and greater predictive sensitivity (75.0% vs 67%) and specificity (68.1% vs 63.8%), indicating a stronger ability to predict 28-day mortality (Table 4).
 |
Table 4 Receiver Operating Characteristic Curve for the Different Indicators in Predicting the 28d-Mortality Rates in Septic Patients |
 |
Table 5 Determine Points Associated with Each of the Categories of the Risk Factors |
 |
Table 6 Risk Stratification Using a New Scoring System |
Validation Study
Another 100 patients made up the validation study, with a median age of 56 years (range 19–80 years), a male gender ratio of 59% (59/100), and a mortality rate of 43% (43/100). The 28-day mortality rates between the derivation and validation groups did not change significantly (P = 0.528). The illness source distribution resembles the derivation data in terms of distribution (Figure 3). Similar mortality rate and predictive power were found in the derivation and validation study based on the risk stratification of the New scoring system (Table 6).
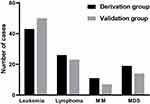 |
Figure 3 The distribution of the source of sepsis in the derivation and validation samples. Abbreviations: MDS, myelodysplastic syndrome; MM, multiple myeloma. |
There was no difference between the expected and observed results, according to the Hosmer–Lemeshow X2 value of 4.73 (P = 0.45), which indicated that the model fit the data well. We evaluated the capacity of the new scoring system in predicting mortality in comparison to the SOFA score. The results are shown in Table 4: the ROC of the new scoring system had a higher AUROC than that of the SOFA score (0.792, 95% CI 0.70–0.88 vs 0.733, 95% CI 0.63–0.84). The new scoring system has a higher sensitivity (0.91 vs 0.842) than that of SOFA score. However, the specificity is no higher than SOFA (0.58 vs 0.58). Additionally, Kaplan–Meier survival analysis revealed that survival fell as the risk level of the new scoring system rose (p < 0.001) (Figure 4). The findings validated the proposed new scoring system as a useful tool for identifying severe sepsis among critically ill patients with MHs.
 |
Figure 4 Kaplan–Meier survival curves of patients with HMs and sepsis with different levels of risk in the new scoring system. |
Discussion
In this study, we have derived and validated a new scoring system using three independent variables to identify septic patients with HMs who are at a risk of early death. Our analysis has identified age 60 years or older, PT 27s or more and SOFA Score of 4.5 points or more were the three independent predictors. We found that the new scoring system performed better than SOFA.
Sepsis is a life-threatening syndrome, the total mortality rate is above 22.5%.18 Patients with HMs and sepsis tend to a comparatively higher mortality rate, and this is frequently attributed to immune dysfunction caused by the malignancies and cytotoxic chemotherapy treatments. The SOFA score was recommended to identify sepsis and evaluate organ function damage in HMs, a higher SOFA score is typically associated with increased mortality.19 According to Cornet et al retrospective study of the ICU stays of 58 patients with HMs and sepsis, nonsurvivors on the first four days of ICU admission had significantly higher SOFA scores than survivors.20 Furthermore, Price et al found in a study of acute leukemia patients requiring invasive ventilation that a 1 point increase in SOFA score at the time of intubation was associated with a 17% increase in mortality.21 However, there are shortcomings in the clinical application of SOFA scores in HMs. The reasons are as follows: chemotherapy-related or tumor-related thrombocytopenia, PLT count cannot be used; Chemotherapy-related elevations in bilirubin and creatinine may have an impact on how the SOFA score is calculated; Assessment is sometimes also hampered by the fact that mental status can alter independently of sepsis.5 The aforementioned reasons may cause the SOFA score to be overestimated in patients with HMs and sepsis. In addition, APACHE II, the most widely used scoring system for the prognosis of severe patients, can accurately assess the prognosis of sepsis.22 However, its intricate evaluation metrics and calculation do not support the quick implementation of emergency bedside. To the best of our knowledge, there is the first scoring system that specifically predicts mortality and risk stratification for patients with HMs and sepsis. Our research highlights the importance of establishing a new scoring system that minimizes the shortcomings of SOFA scores in HM application.
In this retrospective study of 199 consecutive patients admitted to the HICU, we identified SOFA score, age and PT as independent predictors for 28-day mortality in patients with HMs and sepsis. Numerous studies have revealed that age factor, in particular, age greater than 65, is significant independent variable.23,24 However, in this study, we defined age greater than 60 to be the critical cut-off value. The coagulation system and the inflammatory response are both activated by infection and engage in dynamic interaction. It has been shown that the activation of the coagulation system and inflammation aid the host in eliminating pathogens, but if the infection is not promptly controlled, the sepsis-induced coagulopathy (SIC) and SIRS will eventually become out of control, posing a life-threatening threat to multiple organ function. Recent advances in sepsis offered light on the role of SIC in the progression of sepsis, and SIC is closely associated with multiple organ failure and sepsis mortality rate.11 Studies have shown that the SOFA score and coagulation indicator PT are excellent indicators of sepsis prognosis.25 There are also research reports, high mortality in sepsis, when INR >1.5.26,27 Our finding of an PT ≥16s associated with high mortality in patients with HMs and sepsis has not been reported previously. The current scoring systems for coagulopathy provide advantages for its specific applications. Using the diagnostic criteria for SIC that Iba proposed in 2017. Scores on INR, PLT, and SOFA are included in this criterion. The SIC criteria are suitable for detecting sepsis cases with a greater mortality risk.28 However, it is not feasible to apply it in patients with HMs and sepsis. The Japanese Ministry of Health and Welfare’s (JMHW) DIC diagnostic scoring system included PLT, PT, fibrinogen degradation products (FDP) and FIB. The criteria say that secondary DIC in hematological disorders can be detected with ≥4 points and that PLT are not scored in the diagnosis of hematological diseases. However, its sensitivity for the diagnosis of SIC is poor.1 The International Society of Thrombosis and Haemostasis (ISTH) and Japanese Association for Acute Medicine (JAAM) criteria for SIC demonstrate a relatively high diagnostic efficacy. PLT count, PT, FDP and FIB were used in both scoring systems which can be used to judge the prognosis of patients with trauma or sepsis. However, the sensitivity of both scoring systems is low, making it difficult to diagnose DIC in the early stage, although the effect is significant for the diagnosis of typical DIC.29
In our study, we demonstrated that the proposed simple new scoring system performed better in identifying critically ill septic patients with HMs than SOFA. An earlier diagnosis of critically ill patients allows for the faster start of rational and effective care. Compared with the current researches, we did not identify PLT, FDP and FIB as independent predictors of the mortality. Yet, we demonstrated that PT was trending towards being a significant independent predictor of mortality. In addition, our study revealed that when the SOFA score paired with PT and age, they complement each other, and the new scoring system of sensitivity improves to over 75%, and the discrimination of new scoring system was much better than the SOFA score. However, the specificity of the scoring system was not materially improved by the limited predictors, so it should be used to identify critically ill patients with sepsis and HMs in the general hematology ward and receive timely intensive care. In the new scoring system, a score of 0 indicates a mortality rate that is comparatively low, whereas a score greater than 0 indicates a larger mortality rate. The mortality rate increases to over 50% when the new scoring system receives a score of 4 or more and prompt admission to intensive care may be considered. Our study contributes to the existing data by providing the new scoring system for predicting mortality among patients with HMs and sepsis. This new scoring system may serve as a useful guide for selecting variables for the creation of sepsis definition specifically for HMs in the future.
However, it may not be a perfect scoring system of mortality on its own. First of all, the current study’s single-centered retrospective study design was biased; Secondly, the new scoring system’s ability to detect severe sepsis has been successfully validated using an independent validation cohort; however, internal validation strategies were unable to assure the generalizability of the results to other patient populations whose fundamental characteristics differed from the current cohorts; Thirdly, all the patient population were critically ill patients in HICU. These findings may not apply to patients who are not in life-threatening condition in the general ward; finally, it is necessary to conduct further researches to determine whether changes in prognostic scores in the days after HICU admission are significant in determining the prognosis rather than at admission to HICU alone. Due to the limited sample size of this study, future multicentered researches of scoring system validation also need in broader and more diverse patient populations.
Conclusion
Our study provided a new scoring system that is more accurate in predicting mortality among patients with HMs and sepsis than the SOFA score. By combining the SOFA score with age and PT, two independent predictors, the new scoring system may be able to overcome its limitations in the application of HMs.
Abbreviations
AUROC, area under the receiver operating characteristic curve; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; APTT, activated partial thromboplastin time; BP, blood pressure; BSIs, bloodstream infections; CRP, C-reactive protein; DIC, disseminated intravascular coagulation; FIB, fibrinogen; HGB, haemoglobin; HSCT, hematopoietic stem cell transplantation; MM, multiple myeloma; MDS, myelodysplastic syndrome; PT, prothrombin time; PCT, procalcitonin; PLT, platelet; TBIL, total bilirubin; TT, thrombin time; WBC, white blood cell count.
Compliance with Ethical Standards
Ethics approval was obtained from the ethics committee of the participant hospital, and the study was conducted in adherence to the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Acknowledgments
We value the integrity, precision, and hard effort put in by Dandan Li, Qian Zhang, Jinyue Fu and Dongyang Zhang (Department of Hemato-Oncological ICU, First Affiliated Hospital, Harbin Medical University) in gathering the data; We appreciate the advice given by teacher Zhang Yingmei regarding statistics and article writing.
Funding
No funding received.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Asakura H, Takahashi H, Uchiyama T, et al. Proposal for new diagnostic criteria for DIC from the Japanese society on thrombosis and hemostasis. Thromb J. 2016;14(1):42. doi:10.1186/s12959-016-0117-x
2. Huoi C, Vanhems P, Nicolle MC, Michallet M, Bénet T. Incidence of hospital-acquired pneumonia, bacteraemia and urinary tract infections in patients with haematological malignancies, 2004–2010: a surveillance-based study. PLoS One. 2013;8(3):e58121. doi:10.1371/journal.pone.0058121
3. Suárez I, Böll B, Shimabukuro-Vornhagen A, Michels G, von Bergwelt-Baildon M, Kochanek M. Letalität hämatoonkologischer patienten in neutropenie auf der intensivstation [Mortality of hematology-oncology patients with neutropenia in intensivecare]. Med Klin Intensivmed Notfmed. 2016;111(2):84–91. German. doi:10.1007/s00063-015-0039-6
4. Malik IA, Cardenas-Turanzas M, Gaeta S, et al. Sepsis and acute myeloid leukemia: a population-level study of comparative outcomes of patients discharged from texas hospitals. Clin Lymphoma Myeloma Leuk. 2017;17(12):e27–e32. doi:10.1016/j.clml.2017.07.009
5. Kochanek M, Schalk E, von Bergwelt-Baildon M, et al. Management of sepsis in neutropenic cancer patients: 2018 guidelines from the Infectious Diseases Working Party (AGIHO) and Intensive Care Working Party (iCHOP) of the German Society of Hematology and Medical Oncology (DGHO). Ann Hematol. 2019;98(5):1051–1069. doi:10.1007/s00277-019-03622-0
6. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
7. Li Y, Yan C, Gan Z, et al. Prognostic values of SOFA score, qSOFA score, and LODS score for patients with sepsis. Ann Palliat Med. 2020;9(3):1037–1044. doi:10.21037/apm-20-984
8. Karakike E, Kyriazopoulou E, Tsangaris I, Routsi C, Vincent JL, Giamarellos-Bourboulis EJ. The early change of SOFA score as a prognostic marker of 28-day sepsis mortality: analysis through a derivation and a validation cohort. Crit Care. 2019;23(1):387. doi:10.1186/s13054-019-2665-5
9. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):762–774. doi:10.1001/jama.2016.0288
10. Huang CT, Ruan SY, Tsai YJ, Ku SC, Yu CJ. Clinical trajectories and causes of death in septic patients with a low APACHE II score. J Clin Med. 2019;8(7):1064. doi:10.3390/jcm8071064
11. Iba T, Umemura Y, Wada H, Levy JH. Roles of coagulation abnormalities and microthrombosis in sepsis: pathophysiology, diagnosis, and treatment. Arch Med Res. 2021;52(8):788–797. doi:10.1016/j.arcmed.2021.07.003
12. Tsui TL, Huang YT, Kan WC, et al. A novel procalcitonin-based score for detecting sepsis among critically ill patients. PLoS One. 2021;16(1):e0245748. doi:10.1371/journal.pone.0245748
13. Liu Z, Meng Z, Li Y, et al. Prognostic accuracy of the serum lactate level, the SOFA score and the qSOFA score for mortality among adults with sepsis. Scand J Trauma Resusc Emerg Med. 2019;27(1):51. doi:10.1186/s13049-019-0609-3
14. Sivayoham N, Rhodes A, Cecconi M. The MISSED score, a new scoring system to predict mortality in severe sepsis in the emergency department: a derivation and validation study. Eur J Emerg Med. 2014;21(1):30–36. doi:10.1097/MEJ.0b013e328364a8d4
15. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247. doi:10.1007/s00134-021-06506-y
16. Koç M, Yoldaş O, Kiliç YA, et al. Comparison and validation of scoring systems in a cohort of patients treated for perforated peptic ulcer. Langenbecks Arch Surg. 2007;392(5):581–585. doi:10.1007/s00423-007-0156-7
17. Sullivan LM, Massaro JM, D’Agostino RB. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23(10):1631–1660. doi:10.1002/sim.1742
18. Liu D, Huang SY, Sun JH, et al. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med Res. 2022;9(1):56. doi:10.1186/s40779-022-00422-y
19. Liu C, Suo S, Luo L, Chen X, Ling C, Cao S. SOFA score in relation to sepsis: clinical implications in diagnosis, treatment, and prognostic assessment. Comput Math Methods Med. 2022;2022:7870434. doi:10.1155/2022/7870434
20. Cornet AD, Issa AI, van de Loosdrecht AA, Ossenkoppele GJ, Strack van Schijndel RJ, Groeneveld AB. Sequential organ failure predicts mortality of patients with a haematological malignancy needing intensive care. Eur J Haematol. 2005;74(6):511–516. doi:10.1111/j.1600-0609.2005.00418.x
21. Price KJ, Cardenas-Turanzas M, Lin H, Roden L, Nigam R, Nates JL. Prognostic indicators of mortality of mechanically ventilated patients with acute leukemia in a comprehensive cancer center. Minerva Anestesiol. 2013;79(2):147–155.
22. Sadaka F, EthmaneAbouElMaali C, Cytron MA, et al. Predicting mortality of patients with sepsis: a comparison of APACHE II and APACHE III scoring systems. J Clin Med Res. 2017;9(11):907–910. doi:10.14740/jocmr3083w
23. Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21. doi:10.1097/01.ccm.0000194535.82812.ba
24. VLim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi:10.1136/thorax.58.5.377
25. Zhang Y, Khalid S, Jiang L. Diagnostic and predictive performance of biomarkers in patients with sepsis in an intensive care unit. J Int Med Res. 2019;47(1):44–58. doi:10.1177/0300060518793791
26. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi:10.1007/s00134-012-2769-8
27. Fischer CM, Yano K, Aird WC, Shapiro NI. Abnormal coagulation tests obtained in the emergency department are associated with mortality in patients with suspected infection. J Emerg Med. 2012;42(2):127–132. doi:10.1016/j.jemermed.2010.05.00
28. Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7(9):e017046. doi:10.1136/bmjopen-2017-017046
29. Iba T, Umemura Y, Watanabe E, et al. Diagnosis of sepsis-induced disseminated intravascular coagulation and coagulopathy. Acute Med Surg. 2019;6(3):223–232. doi:10.1002/ams2.411
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

