Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
A New Assessment Method of Vitiligo by Combination of Dermoscopy and Reflectance Confocal Microscopy
Authors Wang HF , Wang CY, Zhou XF, Deng XF, Huang H, Wang J, Chen XQ, Zhai ZF
Received 24 July 2023
Accepted for publication 4 November 2023
Published 18 December 2023 Volume 2023:16 Pages 3615—3623
DOI https://doi.org/10.2147/CCID.S432169
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Hui-Fen Wang, Chun-You Wang, Xiao-Fang Zhou, Xiang-Fen Deng, Hui Huang, Juan Wang, Xue-Qin Chen, Zhi-Fang Zhai
Department of Dermatology, the First Affiliated Hospital of Army Medical University, Chongqing, People’s Republic of China
Correspondence: Zhi-Fang Zhai, Department of Dermatology, the First Affiliated Hospital of Army Medical University, Gaotanyan Street No. 30, Shapignba District, Chongqing, 400038, People’s Republic of China, +86 023 6876 6338, Email [email protected]
Purpose: The aim is to investigate the application value of dermoscopy combined with reflectance confocal microscopy (RCM) in assessing vitiligo disease activity and treatment response.
Patients and Methods: We enrolled 279 patients with vitiligo and evaluated the disease activity by Vitiligo Disease Activity (VIDA) score, dermoscopy, RCM and dermoscopy combined with RCM respectively. The sensitivity and specificity of different assessment techniques were compared with VIDA score by the differences and consistency. The different characteristics of dermoscopy and RCM with different treatment responses were also analyzed.
Results: The results showed that the sensitivity and specificity of dermoscopy combined RCM were higher than RCM or dermoscopy alone (P values less than 0.05). In the repigmentation process, leukotrichia, pigment network absent and perilesional hyperpigmentation under dermoscopy at the baseline suggested a poor treatment response, while the incompletely disappearing pigment rings under RCM and perifollicular hyperpigmentation under dermoscopy indicated a good treatment response. We also found the proportion of patients with telangiectasia, increased pigment at the lesions and around the hair follicles was significantly higher in the good treatment response group than that in the poor one by dermoscopy (χ 2 = 4.423, 32.471, 4.348, P = 0.035 0.000, 0.037) and by RCM the proportion of patients with both increased pigment granules and dendritic melanocytes in the good treatment response group was higher than that in the poor one (χ 2 = 38.215, 5.283, P = 0.000, 0.022, respectively).
Conclusion: With the higher sensitivity and specificity than dermoscopy or RCM alone, a combination of dermoscopy and RCM may be a new more accurate measure to assess the vitiligo disease activity and the treatment response.
Keywords: vitiligo, dermoscopy, reflectance confocal microscopy, the disease activity, the treatment response
Introduction
Vitiligo is one of the most common acquired depigmented disorders with a global incidence of 0.5–2%.1 Vitiligo is clinically characterized by chalky-white patches with clear borders, which significantly affects not only the patients’ appearance but also their psychology and daily life.2 The stability of vitiligo is pivotal in planning the treatment regimen and especially important for selecting patients eligible for surgery.3,4 Up to now, the Vitiligo Disease Activity (VIDA) score is currently the most widely used measurement instrument in clinical practice because of its convenience, but it is also limited by the strong subjectivity and poor specificity.5–7Therefore, it is essential to establish an objective and effective method to evaluate vitiligo stability and treatment response. Dermoscopy and reflectance confocal microscopy (RCM), as important auxiliary examination tools in dermatology,8,9 have been gradually recognized in evaluating vitiligo stability and treatment efficacy,10–13 though there are some limitations in the clinical application.14 The dermoscopy is easy to operate, with high acceptance of patients, but it lacks information on deep structures of lesions. RCM can generate real-time images with a resolution like histological images while it requires smooth sample preparation.15 The study was designed to assess the reliability of dermoscopy combined with RCM in assessment of vitiligo activity/stability and monitor treatment effects, of which there is little data up to now.
Materials and Methods
Patients Collection
Two hundred and seventy-nine patients clinically diagnosed as vitiligo were collected, who visited the Dermatology Department of the First Affiliated Hospital of Army Medical University from July 2021 to August 2022. All of them were reconfirmed by two or more senior dermatologists based on diagnostic criteria of consensus on the diagnosis and treatment of vitiligo (2021 version).16 Those who had received systemic or local treatment within the last 8 weeks, or who refused or were unable to cooperate with the dermoscopy and/or RCM examination were excluded. All patients (or their legal representatives) informed and consented to this study. The study was approved by the Research Ethical Committee, the First Affiliated Hospital of Army Medical University, in accordance with the Helsinki Declaration.
Assessment of Vitiligo Disease Activity
One of the latest emerging or enlarged lesions was selected as the target lesion of every subject. Under dermoscopy, we observed the target lesions and the edges from top to bottom and from left to right in turn. As for the long operation time and slow movement of RCM, we selected multi-points for observation location under RCM, involving the center and the edge of the target skin lesions and approximate midpoint of them. The pigment network, the lesion border, leukotrichia, telangiectasia and the area of lesion were observed with a CBS-908 dermoscope (Wuhan Bose Electronic Co., Ltd, China) and the pigment rings in the basal layer, inflammatory cells and dendritic melanocytes by the RCM of VivaScope Systems (VivaScope1500, USA, Lucid Inc.).
The VIDA score was recorded as follows:16 Activity of ≤6 weeks, +4; Activity of 6 weeks–3 months, +3; Activity of 3–6 months, +2; Activity of 6–12 months, +1; Stable for ≥1 year, 0; Stable for ≥1 year with spontaneous repigmentation, −1. Score >1 suggested active stage and score ≥4 suggested rapidly active stage.
The dermoscopic score referred to “BPLeFoSK” and was calculated as the sum of the following parameters:17 the sharp border, +1; the absent/reticulate pigment network, +1; perilesional pigmentation,+1; perifollicular pigmentation, +1; satellite lesions, −1.5; micro-Koebnerization, −2. Total score ≥1.5 is considered stable.
The RCM score referred to the following criteria:18 the existence of remaining pigment in the lesion, +1 and the complete absence of pigment, −1. An indistinct border, +1 and a clear border −1. Inflammatory cell infiltration was detected at the edge of the lesion, +1 and the dendritic melanocytes appeared in the vitiligo lesion, − 1. Grading was as follows: total score <1 represented stable stage; ≥1 represented active stage; ≥2 represented rapidly active stage.
To more objectively and effectively evaluate vitiligo activity by combining dermoscopy with RCM, we analyzed the two techniques comprehensively. Based on the opposite grading pattern of dermoscopy score and RCM score, we propose “dermoscopy score minus RCM score” for the score of dermoscopy combined with RCM. Grading was as follows: total score >0.5 points suggested stable stage; ≤0.5 points suggested active stage.
Dermoscopic and RCM Imaging Assessment on Treatment Response
We defined the clinical repigmentation area ≥30% compared with the area at the baseline as effective repigmentation.19,20 The clinical, dermoscopic and RCM features of the target lesions were collected and assessed as before in the follow-up patients.
Statistical Analysis
Compared with VIDA score, the sensitivity, the specificity, the positive predict value (PPV) and the negative predict value (NPV) of dermoscopy score, RCM score, dermoscopy combined with RCM score were calculated. Paired chi-square test was performed to identify possible differences between sensitivity, specificity of dermoscopy combined with RCM score and those of dermoscopy score or RCM score. The consistency was evaluated by Kappa test. The chi-square test was performed in comparing follow-up data. Fisher exact test was used when the expected frequency is <5. Data were coded and entered using the Statistical Package for the Social Sciences (SPSS), version 26 (IBM Corp.). It was considered as statistically significant when P-value was less than 0.05.
Results
All of the 279 vitiligo patients with 160 female (57.35%) and 119 male (42.65%) were enrolled, whose ages ranged from 1 to 82 years with a mean of 29.94±15.40 years. 92 patients were followed up with different treatments. The target lesions were localized to the limbs, faces, abdomen, neck, chest, waist and back.
Dermoscopic and RCM Imaging Features of Vitiligo Patients with Different Disease Activity
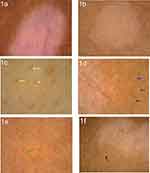
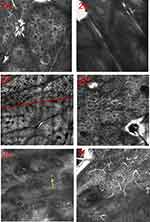
Dermoscopic features of lesions included the absent or faint pigment network within the lesions, the ill-defined or sharp border, tapioca sago, perilesional hyperpigmentation, satellite lesions, micro-Koebner phenomenon, telangiectasia, leukotrichia and perifollicular hyperpigmentation or depigmentation (Figure 1a-f). Under RCM, the absence or existence of pigment rings in the basal layer, the indistinct or clear border, inflammatory cell infiltration and dendritic melanocytes regeneration of the lesion could be observed (Figure 2a-f).
The Sensitivity and Specificity of Different Evaluation Techniques
Vitiligo disease activity was evaluated by four techniques respectively. There were no differences between dermoscopy combined with RCM score, dermoscopy score or RCM score and VIDA score in evaluating vitiligo stage (Kappa = 0.522, 0.503, 0.771, P = 0.200, 1.000, 0.167, respectively). The sensitivity, the specificity, PPV and NPV of dermoscopy combined with RCM, dermoscopy and RCM in assessment of vitiligo stage are described in Table 1. The sensitivity and specificity of dermoscopy scores were 93.33% and 55.56% respectively, and those of RCM scores were 90.67% and 59.26% respectively, while those of dermoscopy combined with RCM scores were 97.33% and 75.93% respectively. Dermoscopy combined with RCM presented a higher specificity than dermoscopy or RCM alone (χ2 = 4.923,13.067,5.882,5.818, P = 0.022, 0.000, 0.013, 0.012, respectively).
 |
Table 1 Comparison of Vitiligo Activity by Different Skin Imaging Techniques [Cases (%)] |
The Characteristic Changes of Dermoscopy and RCM in Vitiligo Patients with Different Treatment Responses
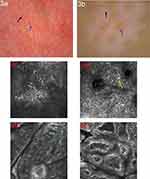
Dermoscopic and RCM assessment of target lesions was done at base-line and post-procedure and the captured images were archived. The results are shown in Table 2 and Table 3. Dermoscopic observation showed increased pigment at the lesions and around the hair follicles, changes of lesion borders, white hair restoration and telangiectasia (Figure 3a and b). The proportion of patients with telangiectasia, increased pigment at the lesions and around the hair follicles was significantly higher in the good treatment response group than that in the poor one (χ2 = 4.423, 32.471,4.348, P = 0.035, 0.000, 0.037). At the baseline, the proportion of patients with perifollicular hyperpigmentation was significantly higher in the good treatment response group than in the poor one (P = 0.018) and the proportion of patients with leukotrichia, the absent pigment network and perilesional hyperpigmentation was significantly higher in the poor response treatment group than that in the good one (P = 0.047, 0.026, 0.026, respectively). During the repigmentation process, RCM observation showed increased pigment granules in the basal layer and increased dendritic melanocytes in the epidermis (Figure 3c-f).The proportion of patients with both increased pigment granules and dendritic melanocytes in the good response treatment group was higher than in the poor one (χ2 = 38.215, 5.283, P = 0.000, 0.022, respectively). In addition, the existent pigment rings at the baseline under RCM showed better treatment response than those with absent pigment rings (χ2 = 4.089, P= 0.043).
 |
Table 2 Dermoscopic and RCM Features in Vitiligo Patients with Different Effect Response (Cases). |
 |
Table 3 Comparison of Baseline Characteristics of Dermoscopy and RCM in Vitiligo Patients with Different Treatment Effects (Cases). |
Discussion
Vitiligo is one of the most common acquired depigmented dermatoses characterized by an absence of melanocytes from the basal layer, which leads to great psychological distress for the patients. Stability assessment is the key principle in establishing a rational treatment regimen and the vitiligo disease stability is a prerequisite for successful surgery.21 There are several methods applied to evaluate vitiligo disease activity, including clinical activity, Koebner phenomenon, VIDA score and Wood’s lamp, which are thought biased or subjective in evaluating activity.6,22–24Anbar et al25 delineated and measured the distribution and surface areas of 200 vitiligo patients by the dermatologist in standardized regular light and using Wood’s lamp and concluded that Wood’s lamp can discover subclinical lesions, which is important to vitiligo patient assessment. Recently, some new clinical scoring methods (VSAS, VDAS, VDIS) have been suggested to assess vitiligo disease activity effectively while they have not been generalized in clinical practice.5–7 With the development of skin imaging technology, research shows that UV‐dermoscopy can also be used in active/progress judgment in vitiligo and the depigmented and pigmented junctional zone and perifollicular pigmentation areas could be easier and simultaneously identified via UV‐dermoscopy.11 So far, the VIDA score is the most frequently cited measurement instrument. With the application of skin imaging technology clinically, dermoscopy and RCM have been shown to be promising tools in assessing vitiligo activity and monitoring therapeutic effects.26–28 Dermoscopy has the characteristics of strong mobility, wide observation range and good maneuverability. RCM makes use of the different refractive indexes of light in the microstructure of skin tissue, such as melanin, to present different grayscale images.29 Clinical studies have shown that the pigment network within the lesions, the lesion border, tapioca sago, perilesional hyperpigmentation, satellite and micro-Koebner phenomenon, leukotrichia, and perifollicular hyperpigmentation or depigmentation are important factors to evaluate the vitiligo activity under dermoscopy.30,31 Existence or absence of pigment rings in the basal layer of the lesion, the lesion borders, inflammatory cells and dendritic melanocytes are main focuses to evaluate the vitiligo activity under RCM.17 According to the above characteristics, the dermoscopy and RCM vitiligo activity score respectively have been developed.17,18,32 The study of Ibrahim et al32 showed the dermoscopic score was concordant with the VIDA score in 83.5% of cases and Liu et al33 found the RCM score was concordant with the VIDA score in 90% of cases. To optimally combine dermoscopy and RCM, based on the opposite grade pattern of dermoscopy score and RCM score, we proposed “dermoscopy minus RCM score” as the dermoscopy combined with RCM scoring standard, which was suggested as the comprehensive score of dermoscopy combined with RCM to be used to evaluate vitiligo activity. In our study, the sensitivity and specificity of dermoscopy combined with RCM (97.33%, 75.93%) were higher than those of dermoscopy (90.67%, 59.26%) or RCM (93.33%, 55.56%) alone in evaluating vitiligo stage. The “dermoscopy minus RCM score” presented higher accuracy than that by RCM or dermoscopy individually.
Additionally, dermoscopy and RCM have been reported to be reliable techniques to evaluate initial treatment response as they can show increases in perilesional and intralesional pigmentation that are not visible to the naked eye.34–36 In our study, the clinical, dermoscopic and RCM changes of 92 patients before and after treatment were compared and the following conclusions were found. Before the skin lesions recovered clinically, dermoscopy had observed increased pigmentation in the skin lesions. Leukotrichia, absent pigment network and perilesional hyperpigmentation under dermoscopy at the baseline suggested a poor response to treatment, while the incompletely absent pigment rings under RCM and perifollicular hyperpigmentation under dermoscopy at the baseline indicated a good treatment response. We also found several specific signs in the repigmentation process: (i) The hairs appeared “white on the upper part and black on the lower part” under dermoscopy, suggesting that the pigments at the hair follicles were in the repigmentation stage. (ii) Two types of dendritic melanocytes can be seen under RCM:dendritic-like cells and starburst-like cells. (iii) The pigment rings were bent into a “mitochondrial crista-like” structure under RCM in some patients.
There were still some limitations in our study: (i) The sample size is not large enough, especially that of patients with follow-up. (ii) Because of the inconsistency of treatment regimen for the subjects, we only compared the imaging features of skin lesions before and after treatment according to their treatment effects to the corresponding treatment. (iii) The RCM features were not confirmed by histological and cytological studies, which is necessary for us to make further studies.
Conclusion
Our study identified that both dermoscopy and RCM can be used to evaluate vitiligo disease activity and monitor treatment response, which is helpful to make optimal treatment regimens. The combination of dermoscopy and RCM can make up for the technical deficiency of each alone and present more objective evaluation than the traditional assessment method, which will be a new assessment method to evaluate vitiligo disease activity and treatment effects.
Funding
This research received the Chongqing Key Research and Develop Project, Grant/Award Number: CSTB2022TIAD-KPX0181 and Chongqing Science and Health Joint Medical Research Project, Grant/Award Number: 2021 MSXM118.
Disclosure
All the authors declare that they have no conflicts of interest in this work.
References
1. Harris JE, Ezzedine K, Bibeau K., et al. 17755 Global survey investigating the prevalence of vitiligo and vitiligo signs among adults in Europe, Japan, and the United States. JAAD. 2020;83(6):AB198. doi:10.1016/j.jaad.2020.06.881
2. Joge RR, Kathane PU, Joshi SH. Vitiligo: a Narrative Review. Cureus. 2022;14(9):e29307. doi:10.7759/cureus.29307
3. Aboul-Fettouh N, Hinojosa J, Tovar-Garza A, et al. The majority of patients presenting with vitiligo have a clinical sign of activity. J Am Acad Dermatol. 2017;77(4):774–775. doi:10.1016/j.jaad.2017.05.027
4. Grochocka M, Wełniak A, Białczyk A, et al. Management of Stable Vitiligo-A Review of the Surgical Approach. J Clin Med. 2023;12(5):1984. doi:10.3390/jcm12051984
5. Coias J, Hynan LS, Pandya AG. Lack of correlation of the patient-derived Vitiligo Disease Activity Index with the clinician-derived Vitiligo Area Scoring Index. J Am Acad Dermatol. 2018;78(5):1015–1016. doi:10.1016/j.jaad.2017.11.034
6. van Geel N, Passeron T, Wolkerstorfer A, et al. Reliability and validity of the Vitiligo Signs of Activity Score (VSAS). Br J Dermatol. 2020;183(5):883–890. doi:10.1111/bjd.18950
7. van Geel N, Depaepe L, Vandaele V, et al. Assessing the dynamic changes in vitiligo: reliability and validity of the Vitiligo Disease Activity Score (VDAS) and Vitiligo Disease Improvement Score (VDIS). J Eur Acad Dermatol Venereol. 2022;36(8):1334–1341. doi:10.1111/jdv.18134
8. Krueger L, Saizan A, Ja S, et al. Dermoscopy of acquired pigmentary disorders: a comprehensive review. Int J Dermatol. 2022;61(1):7–19. doi:10.1111/ijd.15741
9. Levine A, Markowitz O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018;4(10):1014–1023. doi:10.1016/j.jdcr.2018.09.019
10. Kumar JA, Sonthalia S, Lallas A, et al. Dermoscopy in vitiligo: diagnosis and beyond. Int J Dermatol. 2018;57(1):50–54. doi:10.1111/ijd.13795
11. Yuan M, Xie Y, Zheng Y, et al. Novel ultraviolet-dermoscopy: early diagnosis and activity assessment of vitiligo. Skin Res Technol. 2023;29(1):e13249. doi:10.1111/srt.13249
12. AlJasser MI. dermoscopy for Facial Leukotrichia in Vitiligo: an Important Step for a Better Treatment Decision. Dermatol Pract Concept. 2022;12(3):e2022114. doi:10.5826/dpc.1203a114
13. Cortelazzi C, Pellacani G. Raposio E, et al.Vitiligo management: combination of surgical treatment and phototherapy under reflectance confocal microscopy monitoring. Eur Rev Med Pharmacol Sci. 2020;24(13):7366–7371. doi:10.26355/eurrev_202007_21904
14. Mehrabi JN, Baugh EG, Fast A, et al. A Clinical Perspective on the Automated Analysis of Reflectance Confocal Microscopy in Dermatology. Lasers Surg Med. 2021;53(8):1011–1019. doi:10.1002/lsm.23376
15. Abdi P, Anthony MR, Farkouh C, et al. Non-invasive skin measurement methods and diagnostics for vitiligo: a systematic review. Front Med. 2023;10:1200963. doi:10.3389/fmed.2023.1200963
16. van Geel N. Pigmentary Disorder Group. Consensus on the diagnosis and treatment of vitiligo (2021 version). Chine J Dermatol. 2021;54(2):105–109. doi:10.35541/cjd.20200785
17. Nirmal B, Antonisamy B, Peter CVD. Cross-sectional study of dermatoscopic findings in relation to activity in vitiligo: bPLeFoSK criteria for stability. J Cutan Aesthet Surg. 2019;12(1)::36–41. doi:10.4103/JCAS.JCAS_75_18
18. Li W, Wang S, Xu AE. Role of in vivo reflectance confocal microscopy in determining stability in vitiligo: a preliminary study. Indian J Dermatol. 2013;58(6):429–432. doi:10.4103/0019-5154.119948
19. Errichetti E, Zelin E, Pinzani C, et al. Dermoscopic and Clinical Response Predictor Factors in Nonsegmental Vitiligo Treated with Narrowband Ultraviolet B Phototherapy: a Prospective Observational Study. Dermatol Ther (Heidelb). 2020;10(5):1089–1098. doi:10.1007/s13555-020-00431-6
20. Matsuya T, Nakamura Y, Matsushita S, et al. Vitiligo expansion and extent correlate with durable response in anti-programmed death 1 antibody treatment for advanced melanoma: a multi-institutional retrospective study. J Dermatol. 2020;47(6):629–635. doi:10.1111/1346-8138.15345
21. Bae JM, Jung HM, Hong BY, et al. Phototherapy for Vitiligo: a Systematic Review and Meta-analysis. JAMA Dermatol. 2017;153(7):666–674. doi:10.1001/jamadermatol.2017.0002
22. Njoo MD, Das PK, Bos JD, et al. Association of the Kobner phenomenon with disease activity and therapeutic responsiveness in vitiligo vulgaris. Arch Dermatol. 1991;135:407–413.
23. Parsad D, Sahni K. Stability in vitiligo: is there a perfect way to predict it. J Cutan Aesthet Surgery. 2013;6(2):75–82. doi:10.4103/0974-2077.112667
24. Wang YJ, Chang CC, Cheng KL. Wood’s lamp for vitiligo disease stability and early recognition of initiative pigmentation after epidermal grafting. Int Wound J. 2017;14(6):1391–1394. doi:10.1111/iwj.12800
25. Tag A, Mona A, Rasha AA et al. Subjective versus objective recognition of facial vitiligo lesions: detection of subclinical lesions by Wood’s light. J Egyptian Women’s Dermatologic Soc. 2022;19(1):7–13. doi:10.4103/jewd.jewd_42_21
26. Gupta P, Vinay K, Bishnoi A, et al. A prospective observational study to sequentially determine the dermoscopic features of vitiligo and its association with disease activity in patients on medical treatment: dermoscopy and disease activity in vitiligo. Pigment Cell Melanoma Res. 2023;36(1):33–41. doi:10.1111/pcmr.13069
27. Errichetti E. Dermoscopy in Monitoring and Predicting Therapeutic Response in General Dermatology (Non-Tumoral Dermatoses): an Up-To-Date Overview. Dermatol Ther (Heidelb). 2020;10(6):1199–1214. doi:10.1007/s13555-020-00455-y
28. Abdallah HY, Abdelhamid NR, Mohammed EA, et al. Investigating melanogenesis-related microRNAs as disease biomarkers in vitiligo. Sci Rep. 2022;12(1):13526. doi:10.1038/s41598-022-17770-3
29. Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84(1):1–14. doi:10.1016/j.jaad.2020.05.153
30. Jha AK, Sonthalia S, Lallas A. Dermoscopy as an evolving tool to assess vitiligo activity. J Am Acad Dermatol. 2018;78(5):1017–1019. doi:10.1016/j.jaad.2017.12.009
31. Vinay K, Ankad BS. Dermatoscopic Features of Pigmentary Diseases in Ethnic Skin. Indian Dermatol Online J. 2021;12(1):24–33. doi:10.4103/idoj.IDOJ_561_20
32. Ibrahim S, Hegazy RA, Gawdat HI, et al. Differentiating active from stable vitiligo: the role of dermoscopic findings and their relation to CXCL10. J Cosmet Dermatol. 2022;21(10):4651–4658. doi:10.1111/jocd.14922
33. Liu T, Xu AE. Vitiligo staging based on clinical and skin computed tomography features.Chin. J Dermatol. 2015;48(6):404–407.
34. Dev A, Vinay K, Bishnoi A, et al. Dermatoscopic assessment of treatment response in patients undergoing autologous non-cultured epidermal cell suspension for the treatment of stable vitiligo: a prospective study. Dermatol Ther. 2021;34(5):e15099. doi:10.1111/dth.15099
35. Zhang D, Wei X, Hong W, et al. A retrospective study of long term follow-up of 2283 vitiligo patients treated by autologous, non-cultured melanocyte-keratinocyte transplantation. Aging. 2021;13(4):5415–5425. doi:10.18632/aging.202472
36. Serban ED, Farnetani F, Pellacani G, et al. Role of In Vivo Reflectance Confocal Microscopy in the Analysis of Melanocytic Lesions. Acta Dermatovenerol Croat. 2018;26(1):64–67.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Syringocystadenoma Papilliferum and Basal Cell Carcinoma Arising in Nevus Sebaceous
Jiang J, Chen Y, He Q, Yang J, Zhang Z, Yang H, Zhang H, Yang C
Clinical, Cosmetic and Investigational Dermatology 2022, 15:2021-2026
Published Date: 23 September 2022
A Prospective Study of Dermoscopic and Ultrastructural Features of Vitiligo-Associated Leukotrichia
Li M, Wang F, Li X, Ding X, Du J
Clinical, Cosmetic and Investigational Dermatology 2023, 16:3673-3680
Published Date: 21 December 2023