Back to Journals » Drug Design, Development and Therapy » Volume 13
A new approach of ocular nebulization with vitamin B12 versus oxytocin for the treatment of dry eye disease: an in vivo confocal microscopy study
Authors Yang J, Liu Y , Xu Y, Li X, Fu J, Jiang X, Chou Y, Ma J, Hao R, Zhang R, Qiu W, Li X
Received 30 January 2019
Accepted for publication 6 May 2019
Published 18 July 2019 Volume 2019:13 Pages 2381—2391
DOI https://doi.org/10.2147/DDDT.S203464
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Jiarui Yang,1,* Yushi Liu,1,* Yanhui Xu,1,* Xiaodan Li,1,* Jiayu Fu,1 Xiaodan Jiang,1 Yilin Chou,1 Jiahui Ma,1 Ran Hao,1 Rong Zhang,2 Weiqiang Qiu,1 Xuemin Li1
1Department of Ophthalmology, Peking University Third Hospital, Beijing, People’s Republic of China; 2Department of Neurobiology, Health Science Center, School of Basic Medical Sciences, Peking University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Purpose: To present a new ocular nebulization therapy for the treatment of dry eye disease (DED) and investigate the efficacy of vitamin B12 (VB12) and oxytocin (OXT) nebulization with clinical parameters and in vivo confocal microscopy (IVCM).
Patients and methods: Thirty-eight patients with DED were enrolled, with 19 receiving VB12 nebulization and 19 receiving OXT nebulization twice weekly for 3 months. Clinical signs and symptoms including Ocular Surface Disease Index, self-assessment of light sensitivity and dryness, tear meniscus height, tear break-up time (BUT), and corneal staining, along with IVCM data of basal epithelial cell density, sub-basal dendritic cell (DC) density, nerve density, and nerve tortuosity were acquired at baseline, 1 month, and 3 months after starting treatment.
Results: Patients treated with VB12 improved significantly in all signs and symptoms except for nerve tortuosity during the three-month treatment, while OXT demonstrated similar effects apart from BUT and nerve tortuosity. VB12 group revealed a higher BUT at 1 month and 3 months with a higher basal epithelial cell density at 3 months compared with OXT group, and a lower DC density was observed in OXT group at 1 month. Change of basal epithelial cell density was more significant at 3 months in VB12 group, with OXT group showing a significantly higher DC reduction at 1 month.
Conclusion: The nebulization therapy delivering VB12 and OXT appears to be effective in improving the symptoms and signs of dry eye, with a relatively stronger effect of BUT elevation and epithelial repair in VB12 and anti-inflammation in OXT nebulization.
Keywords: dry eye, in vivo confocal microscopy, nebulization, oxytocin, vitamin B12
Introduction
Dry eye disease (DED) is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms.1 Being the most common reason for seeking medical eye care, DED is a common public health problem, with the prevalence ranging from approximately 5–50% worldwide.2–4 Although the pathogenesis of dry eye disease is not fully understood, it is recognized that inflammation has a prominent role in the development and propagation of this debilitating condition. DED inflammation disrupts the normal homeostasis of the ocular surface, resulting in a vicious circle of ocular surface injury, and anti-inflammatory and immunosuppressive drugs are considered the pharmacologic agents of choice for controlling the inflammatory cascade of this disease.5–7 Even so, there is still a great potential for the development in anti-inflammatory and damage-repair treatments of dry eye disease.
Vitamin B12 (VB12) is a dietary essential nutrient and is important for metabolic functions of the nervous system, whose deficiency is associated with an impairment of sensory innervation, and may cause optic neuropathy, eye movement disorders, and corneal epitheliopathy with decreased vision and photophobia.8–10 Recently, several studies have reported the parenteral and topical use of VB12 for ocular pain and symptoms in dry eye treatment.11,12
Oxytocin (OXT), a nonapeptide produced in the hypothalamus, exerts a wide spectrum of central and peripheral effects. Nasal spray of OXT, which has been proved to be safe in children,13 is currently suggested as a potential treatment for various psychopathologies of social cognition and behavior in humans.14 In addition to its reproduction and psychiatry-related functions, OXT has also been proved to display a potent anti-inflammation and wound-healing effect in various inflammatory disease,15,16 as well as an analgesic and nerve healing effect in animal models.17,18 However, the anti-inflammatory and nerve healing effect of OXT has yet to be applied in ocular diseases.
Nebulization, applied mainly in pneumology and rhinology diseases, is a widely used means of drug delivery to the upper and lower airways. Nebulizers are devices that convert a drug liquid in solution or suspension into small droplets.19 Its theoretic advantage over classic means of delivery is that, as a non-invasive painless treating system, it delivers drug and humidifies the target region simultaneously, enables dose modification and dose compounding, and directly reaches the target organ, which avoids systemic side-effects and enhancing local efficacy.20 To date, no applications of nebulization therapy havebeen reported in ophthalmic diseases.
In the light of the facts mentioned above, we developed a new ocular nebulization therapy delivering vitamin B12 and oxytocin separately for the treatment of DED. The main objective of this study was to estimate the efficacy of ocular nebulization in dry eye patients, as well as to compare the characteristic of VB12 and OXT aerosols using both clinical and in vivo confocal microscopy (IVCM) evaluations.
Materials and methods
Study design and participants
This prospective cohort study included 38 patients with DED recruited from the outpatient department of the Department of Ophthalmology at Peking University Third Hospital between August 2017 and June 2018. The study protocol was approved by the Human Research and Ethics Committee of Peking University Third Hospital, and the research was adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from each participant before enrollment.
Inclusion criteria included adult patients 1) with a diagnosis of DED in accordance with the criteria proposed by the Tear Film and Ocular Surface Society (TFOS) Dry Eye Workshop (DEWS);1 2) willing to cooperate during the examination and treatment procedure; and 3) with the ability to participate in follow-up examinations for at least 3 months during nebulization treatment. Exclusion criteria consisted of the following: a) patients with active allergies, infections, or inflammatory diseases of the ocular surface unrelated to dry eye within 6 months; b) patients who currently used treatments for DED (other than artificial tears); c) patients who took oral neurotrophic and anti-inflammatory drugs in the past 3 months; d) a history of ocular trauma or surgery within 1 year; e) patients with alterations in the lacrimal drainage system such as punctal occlusion; f) patients who used contact lenses within the past month; g) patients with uncontrolled systemic diseases; and h) pregnant or nursing mothers.
Clinical evaluation
Each participant had three clinic visits: before starting the nebulization therapy (baseline), 1 month after treatment, and 3 months after starting treatment. Both the patients and the observers were blinded of the nebulization group throughout the process. During each visit, all participants had a complete masked ophthalmic evaluation conducted in the following order: evaluation of symptoms using the Ocular Surface Disease Index (OSDI) questionnaire, along with a self-assessment of light sensitivity and dryness (each on a scale of 0–4, with 0 for never and 4 for severe), a complete examination of the ocular surface of both eyes, including tear meniscus height (TMH),21 tear break-up time (BUT),1 and corneal fluorescein staining (CFS) evaluated using the Van Bijsterveld scale,22 and IVCM analysis of the central cornea. Additionally, adverse events during the treatment, including pain, pressure, dizziness, blurred vision, and abdominal discomfort, were recorded during the 3-month follow-up.
TMH
The central lower TMH were measured using a slit lamp microscope (with a graticule in 0.05 mm units).21 Three consecutive readings were obtained, and the final results are presented as medians.
BUT
A total of 5 mL of 2μ sodium fluorescein was instilled onto the bulbar conjunctiva using a micropipette, without inducing reflex tearing. The patient was asked to blink naturally without squeezing three to five times and was then asked to stare straight ahead without blinking under the cobalt blue light until he or she received other instructions. A stopwatch was used to record the time between the last complete blink and the first appearance of a dry spot or disruption in the tear film.1 The procedure was repeated three times, and the final score is presented as an average value.
Corneal fluorescein staining
For the corneal staining evaluation, the cornea was divided into four sectors. Each sector was graded from 1 to 3 using the following criteria: 1 representing few separated spots; 2 representing many separated spots; and 3 representing confluent spots.22
In vivo confocal microscopy
All participants underwent laser IVCM of the central cornea in both eyes by a masked investigator (XL) using Heidelberg Retina Tomograph 3 with the Rostock Cornea Module (Heidelberg Engineering, Heidelberg, Germany). Each image represents a coronal section of 384 × 384 pixels which is equivalent to 400 × 400 μm of the cornea. Before each examination, a drop of 0.4μ oxybuprocaine chlorohydrate (Santen, Osaka, Japan) was instilled into the lower conjunctival fornix. The examination was conducted approximately at the corneal apex.
For each eye, 1 image of basal epithelial layer, and 3 most representative images of the sub-epithelial layer were selected by a single masked investigator (XL). Three independent observers (JF, YL and YZ) measured each image, among which the mean value of series of the 3 sub-epithelial layer readings was recorded, and the average of the measurements by the observers was used for further analysis.
Evaluated parameters included the following:
- Basal epithelial cell density (cells/mm2): basal epithelial cells were immediately above the Bowman’s layer and identified by the lack of a visible nucleus, polygonal cell shape, hyper reflective cell borders and a relatively higher density of cells per frame.23 The counting was carried out within a region of interest of standardized size (region of interest [ROI] = 100 × 100 μm) under a manual cell counting procedure conducted using ImageJ software (http://imagej.nih.gov/ij/), in which cells that were partially within the area analyzed were counted only along the left and upper margins.24
- Dendritic cell (DC) density (cells/mm2): Dendritic cells were morphologically identified as bright individual dendritiform structures with cell bodies,25 which was manually counted in each corneal section at the level of subbasal nerves, selecting the images in which there was a greater density of DCs. The counting was carried out using the manual cell counting procedure same as basal epithelial cell density in the ROI of 400×400 μm.
- Nerve tortuosity: the grade of nerve tortuosity was classified in four grades according to the tortuosity grading scale reported previously.26
- Sub-basal nerve density (mm/mm2): defined as the total length of all nerve fibers within a frame (400×400 μm). It was traced and calculated using NeuronJ (http://www.imagescience.org/meijering/software/neuronj/), a semi-automated nerve analysis plug-in of ImageJ.27
To note, change of variables (Δ) at each follow-up was defined as the value at a certain follow-up minus that at baseline (eg, ΔOSDI at 1 month=OSDI at 1 month-OSDI at baseline).
Nebulization treatment
Recruited subjects under the age of 50 were assigned to the VB12 group due to the possible effects of OXT on reproductive age patients, and others were randomly assigned to receive 4 mg (28 mL) of VB12 or 30 International Unit (23 mL) of OXT28 (both as saline solution) nebulization twice weekly for 3months with a VGR-001 Ultrasonic Nebulizer (INSPIRED, Guangdong, China) and an eye mask (3M, St. Paul, MN, USA). This nebulization system enabled room-temperature nebulization at the rate of 1 mL/min, providing particles with a diameter of 5±1.25 μm. Nebulization was terminated when the nebulizer chamber was empty, usually within 10 minutes. A photo of the ultrasonic nebulization system used in this study is shown in Figure 1.
 |
Figure 1 Ultrasonic nebulization system developed in this study. |
For drug conservation, VB12 (Kingyork, Tianjin, China) was preserved at room temperature, and OXT (Harvest, Shanghai, China) was stored at 4°C due to its poor thermal stability. After being taken out of the refrigerator, OXT was used for nebulization within 30 minutes. Altogether, two nebulization systems were used following routine sterilization protocol and cleaning of equipment prior to each use.
Statistical analysis
For variables other than the three symptom indicators (OSDI, light sensitivity, and dryness), patient’s data for both eyes were calculated (n=76), while only one value was applied for analysis for these three factors (n=38).
Statistical analyses were conducted using SPSS software version 22.0 (SPSS, Inc., Chicago, IL, USA). The basic characteristics of the two treatment groups were described using descriptive statistics including means and standard deviations for continuous variables, and frequencies and proportions for categorical variables. An independent t-test was used to analyze the continuously numeric variables, and a chi-square test was applied in classified variables in the comparison of basic characteristics between groups. The paired t-test was applied to assess the differences of all variables at each follow-up from baseline in the two groups separately. Furthermore, an independent t-test was implemented to determine differences in all variables at each time point, as well as their changes from baseline between the two groups. Two-sided P-values <0.05 were considered statistically significant for all comparisons.
Results
Basic characteristics
A total of 38 DED patients were enrolled, with 19 patients in the VB12 group and 19 in the OXT group, and both eyes of these patients were evaluated. Of these subjects, 18 patients in the VB12 group and 17 patients in the OXT group finished the follow-up at 1 month after treatment, and eventually 14 patients in the VB12 group and 15 patients in the OXT group completed the 3-month follow-up. As shown in Table 1, patient characteristics were matched between VB12 and OXT at baseline, except for a higher age in the OXT group. No adverse events were observed during the 3-month therapy, either in VB12 or OXT group.
 |
Table 1 Clinical characteristics and IVCM results at baseline in VB12 and OXT groups |
Clinical and IVCM results at each follow-up
Patients receiving VB12 nebulization, as shown in Table 2, achieved significant improvements in clinical and IVCM parameters in the first month of treatment, except for DC density and nerve tortuosity. Significant differences were demonstrated from 1 to 3 months of nebulization therapy in OSDI, dryness, BUT, basal epithelial cell density and, notably, DC density. Overall, there were statistically significant improvements in all signs and symptoms of DED, except for nerve tortuosity during the 3-month treatment.
 |
Table 2 Clinical and IVCM results at each follow-up in the VB12 group |
Table 3 shows the results of subjects treated with OXT, among which all clinical and IVCM variables improved significantly within 1 month, except for nerve tortuosity. There were significant improvements in OSDI, TMH, BUT, and sub-basal nerve density at 3 months compared to that of 1 month, whereas nerve tortuosity appeared to be increased. Apart from BUT and nerve tortuosity, clinical and IVCM results demonstrated significant improvements at 3 months from baseline.
 |
Table 3 Clinical and IVCM results at each follow-up in the OXT group |
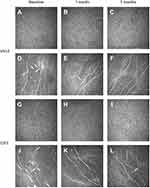
Figure 2 represents the IVCM change at each follow-up in two groups. Both VB12 and OXT nebulization induced improvement in DED conditions. Figures 2A and G represent the corneal basal epithelial layer at baseline in VB12 and OXT groups, with Figures 2B, C, H, and I showing increased basal epithelial cell density at 1 month and 3 months after treatment. Loss of nerves and multiple DCs of dry eye patients can be seen in Figures 2D and J, while Figures 2E, F, K, and L demonstrate elevated sub-basal nerve density, and decreased DC density throughout the 3-month treatment.
Differences of clinical and IVCM results between VB12 and OXT groups
The comparison of clinical and IVCM data between the VB12 and OXT groups is shown in Figure 3. Although obvious improvements were detected in OSDI, dryness, light sensitivity, TMH, CFS, and sub-basal nerve density in both groups during the ocular nebulization process, no significant difference was evident between the two treatment groups. TheVB12 group revealed a significantly higher BUT at 1 month and 3 months (P=0.041 and P=0.005, respectively), along with a higher basal epithelial cell density at 3 months (P=0.043) compared with the OXT group. Asignificant lower DC density was observed in the OXT group after 1 month of nebulization treatment (P=0.021).
Figure 4 describes the changes in clinical parameters and IVCM results of each follow-up from baseline between the two groups. Although no difference was found at 1 month (P=0.723), Δbasal epithelial cell density in the VB12 group was significantly higher at 3 months (P=0.022). Moreover, patients treated with OXT nebulization showed a significantly higher DC reduction at 1 month (P<0.001) compared to the VB12 group, with no significant difference at 3 months between groups (P=0.136). As for Δnerve tortuosity, OXT treatment induced a significantly greater reduction at 1 month (P=0.049), in contrast to a notable increase at 3 months (P=0.040), corresponding to the nerve tortuosity increase from 1 to 3 months mentioned above.
Discussion
This study proposed a new approach of ocular nebulization for the treatment of dry eye disease, which appeared to be effectively proven by clinical and in vivo confocal evaluations. Two drugs, vitamin B12 and oxytocin, were tested, and both proved to be efficient in relieving DED symptoms and signs with several differences in certain aspects.
Nebulizers have long played an important role in the treatment of diverse diseases. Based on the aqueous-deficiency and inflammation-related nature of DED, we considered nebulization on the ocular surface to be a feasible therapy and, in combination with VB12 and OXT, might achieve a remarkable relief in signs and symptoms of DED. This is the first research, to the best of our knowledge, describing a nebulization therapy in the treatment of dry eye disease, as well as the first attempt of oxytocin application on ocular surface.
In this study, our objective was to demonstrate the changes occurring in the ocular surface after nebulization treatment, using clinical evaluations and IVCM study. As it is increasingly used in in vivo study, IVCM has been proven to be capable of identifying different corneal structural aspects that are considered pathognomonic in DED,29 including a reduced number of corneal epithelial cells, increased number of dendritic cells, reduced sub-basal nerve density, and increased nerve tortuosity.30
The neurotrophic effect of vitamin B12 has long been known in clinical practice.11,12 Recently, Macri et al31 indicated that VB12 eye drops improved oxidative stress as well as symptoms of dry eye. Hence, in our study, it is noteworthy that DED patients responded well as the clinical signs and symptoms improved notably within 1 month after the initiation of VB12 nebulization, accompanied by a significant improvement in IVCM results (Table 2). Previous studies have shown that VB12 is able to promote corneal re-innervation and re-epithelization after mechanical injury in a rat model.32 In agreement with this, our study revealed a continuous increase in basal epithelial cell density and sub-basal nerve density at 1 month and 3 months after VB12 nebulization. This might be explained by the evidence that vitamin B12 plays an important role in the enhancement of β-III tubulin expression in neurons,33 in addition to the regulation of neurotrophic factors synthesis, such as an increased expression of TNF-α and decreased neurotrophic epidermal growth factor (NEGF),34 which support neurite outgrowth and survival and, in turn, promotes epithelial wound healing.35
Similar to VB12, our study revealed a significant effect of OXT nebulization with regard to DED symptoms and signs (Table 3). The mechanism underlying this finding is not yet fully understood, and we assumed that there could be four mechanisms of OXT contributing to the DED healing process. First, as has been well described in numerous studies, OXT treatment displays an anti-inflammatory effect by i) stimulation of nitric oxide release, leading to inhibition of the adhesion and aggregation of neutrophil leukocytes;36 ii) decreased release of IL-6 and increased release of prostacyclin, inhibiting platelet aggregation, which has been suggested to promote the inflammation process;37,38 iii) decreased M1 macrophages differentiation with no effect on the M2 population, thereby leading to an anti-inflammatory phenotype;39 and iv) increasing corticosterone levels acutely, which is capable of inhibiting neutrophil extravasation in response to different stimuli.40 This was consistent with our results, as DC density rises rapidly at 1 month after the application of OXT. The second possible role of OXT, described in a rat sciatic nerve damage model,17 is that it resulted in an accelerated nerve recovery, probably by increasing nerve growth factor (NGF) and IGF-1, which enhance nerve regeneration and promote axonal growth rate.41,42 In our study, we believed the increased sub-basal nerve density after treatment to be related to this nerve-healing effect of OXT. Another finding was the relieved dry eye symptoms including dryness and light sensitivity, which could probably be explained by a potential relationship between OXT and pain due to an activation of OXT receptors located superficially in the dorsal horn, a possible combination of OXT with opioid receptors, and a decrease of pain sensitivity by improving mood, relieving anxiety, and mitigating the stress response.18 The fourth mechanism is that OXT mediates a myoepithelial cell-driven acini contraction that contributes directly to the function of the lacrimal gland,43 corresponding to our results of TMH and BUT elevation during the OXT nebulization process.
In the comparison of VB12 and OXT nebulization, one might notice the mismatch of age at baseline, with a range of 27–81 years old in the VB12 group in contrast to 50–87 years old in the OXT group. This was due to a physiological consideration in the central and peripheral actions of oxytocin, including parturition, lactation, maternal behavior, erectile dysfunction, and ejaculation.44 Although oxytocin is a small peptide drug with only nine amino acids, there was a possibility of systemic administration through ocular route.45 It is, therefore, necessary to avoid OXT application in patients of reproductive age due to ethical considerations.
After both were demonstrated to be efficient in the treatment of DED, the characteristic of VB12 and OXT nebulization were further compared due to their different mechanism. Our study did detect their differences in certain aspects (Figures 3 and 4). In this study, individuals treated with VB12 had a better improvement in BUT and basal epithelial cell density than those who received OXT nebulization. Tear film breakup occurs mainly as a result of tear film evaporation, and BUT represents stability of tear film,46 whose homeostasis is achieved reflexly by the lacrimal functional unit (LFU). LFU consists of the ocular surface, its secretory appendages, and the connecting innervation, where the trigeminal innervation of cornea, conjunctiva, and lid margins provides the afferent limb of the feedback loop whilst the secretomotor innervation of the lacrimal gland, meibomian glands, and the conjunctival goblet cells provides the efferent limb.47 We suspect that, due to its remarkable effect on corneal re-innervation, VB12 restores corneal sensitivity and, therefore, induces secretion of the ocular appendages,47,48 thereby leading to the restoration of LFU function and tear film stability. Also, corneal nerve regeneration, as mentioned above, is a key factor in the renewal of a normal epithelium during DED treatment. At the IVCM level, our study revealed a rapid reduction of DC density at first month along with a notable increase in nerve tortuosity at 3 months among OXT patients. This is probably the result of the previously described anti-inflammatory effect of OXT that leads to a quick decrease of DC, which was proved to have a critical function in activation of the immune system in the ocular surface.49
Therefore, in addition to the promising effect of both nebulization therapies adopted in this study, drugs are recommended to be selected according to patient conditions. In this regard, we highlight the application of OXT nebulization for DED patients with inflammation-related signs and symptoms, and VB12 for those with severe epithelial damage as well as patients of reproductive age.
Our results contributed to open interesting prospects for applying ocular nebulization therapy in the treatment of DED. Key advantages of this new therapy are that: a) it enables continuous delivery of low doses of an aerosolized drug to its site of action for a relatively more stable effect; b) it is fully acceptable for DED patients due to its non-invasive and painless feature; c) it simultaneously achieves humidification, anti-inflammation, and neurotrophic effect in correspondence to the diverse etiology of DED, with a further potential of combining with other drugs available; d) it is a possible way of providing a home-based treatment for DED patients, although droplet sizes and the therapeutic effects of portable nebulizers are still being tested as a result of different mechanisms between commonly-used and marketed portable nebulizers.
This preliminary research has a few limitations. First, there was a mismatch of age between the two treatment groups, which had been explained earlier in the discussion. Second, this study is in the absence of a control sham group receiving, for example, nebulization with sodium chloride. Third, this was a single center research study in exploration of a new treating method of DED, and dry eye subtypes weren’t classified due to the relatively small number of enrollees. Fourth, dose and frequency of treatment were certain in each nebulization group and further dose gradient this could be estimated in the determination of an optimal therapeutic regimen. In the inspiration of the proven efficacy, future studies should focus on an effect confirmation of nebulization in the delivery of multiple kinds of drug compared with artificial tears, as well as a sub-group analysis in a diverse type of DED patients, and the key mechanisms of ocular nebulization therapy on DED requires further investigation.
Conclusion
In conclusion, this study described a new ocular nebulization therapy delivering vitamin B12 and oxytocin, which was demonstrated to be efficient for dry eye treatment. Both groups exhibited parallel improvements with respect to clinical and confocal parameters, with a relatively stronger effect of BUT elevation and epithelial repair in VB12 and DC reduction in OXT nebulization, which might mainly be explained by the neurotrophic effect of VB12 and anti-inflammatory effect of OXT.
Acknowledgments
This study was supported by the Capital’s Funds for Health Improvement and Research under grant number 2018-2-4093 (Xuemin Li) and the Peking University Medicine Seed Fund for Interdisciplinary Research under grant number BMU2018MX016 (Weiqiang Qiu).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008
2. Uchino M, Yokoi N, Uchino Y, et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: the Osaka study. Am J Ophthalmol. 2013;156(4):759–766. doi:10.1016/j.ajo.2013.05.040
3. Vehof J, Kozareva D, Hysi PG, Hammond CJ. Prevalence and risk factors of dry eye disease in a British female cohort. Br J Ophthalmol. 2014;98(12):1712–1717. doi:10.1136/bjophthalmol-2014-305201
4. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–365. doi:10.1016/j.jtos.2017.05.003
5. Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130(1):90–100. doi:10.1001/archophthalmol.2011.364
6. Rhee MK, Mah FS. Inflammation in dry eye disease: how do we break the cycle? Ophthalmology. 2017;124(11s):S14–s19. doi:10.1016/j.ophtha.2017.08.029
7. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. doi:10.1016/j.jtos.2017.05.006
8. Theodoropoulos DS. Optic neuropathy in vitamin B12 deficiency. Lancet. 1998;352(9122):146–147.
9. Akdal G, Yener GG, Ada E, Halmagyi GM. Eye movement disorders in vitamin B12 deficiency: two new cases and a review of the literature. Eur J Neurol. 2007;14(10):1170–1172. doi:10.1111/j.1468-1331.2007.01824.x
10. Jurkunas UV, Jakobiec FA, Shin J, Zakka FR, Michaud N, Jethva R. Reversible corneal epitheliopathy caused by vitamin B12 and folate deficiency in a vegan with a genetic mutation: a new disease. Eye (Lond). 2011;25(11):1512–1514. doi:10.1038/eye.2011.177
11. Ozen S, Ozer MA, Akdemir MO. Vitamin B12 deficiency evaluation and treatment in severe dry eye disease with neuropathic ocular pain. Graefes Arch Clin Exp Ophthalmol. 2017;255(6):1173–1177. doi:10.1007/s00417-017-3632-y
12. Shetty R, Deshpande K, Ghosh A, Sethu S. Management of ocular neuropathic pain with vitamin B12 supplements: a case report. Cornea. 2015;34(10):1324–1325. doi:10.1097/ICO.0000000000000572
13. DeMayo MM, Song YJC, Hickie IB, Guastella AJ. A review of the safety, efficacy and mechanisms of delivery of nasal oxytocin in children: therapeutic potential for autism and prader-willi syndrome, and recommendations for future research. Paediatr Drugs. 2017;19(5):391–410. doi:10.1007/s40272-017-0248-y
14. Guastella AJ, Hickie IB, McGuinness MM, et al. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology. 2013;38(5):612–625. doi:10.1016/j.psyneuen.2012.11.019
15. Biyikli NK, Tugtepe H, Sener G, et al. Oxytocin alleviates oxidative renal injury in pyelonephritic rats via a neutrophil-dependent mechanism. Peptides. 2006;27(9):2249–2257. doi:10.1016/j.peptides.2006.03.029
16. Jankowski M, Bissonauth V, Gao L, et al. Anti-inflammatory effect of oxytocin in rat myocardial infarction. Basic Res Cardiol. 2010;105(2):205–218. doi:10.1007/s00395-009-0076-5
17. Gumus B, Kuyucu E, Erbas O, Kazimoglu C, Oltulu F, Bora OA. Effect of oxytocin administration on nerve recovery in the rat sciatic nerve damage model. J Orthop Surg Res. 2015;10:161. doi:10.1186/s13018-015-0301-x
18. Rash JA, Aguirre-Camacho A, Campbell TS. Oxytocin and pain: a systematic review and synthesis of findings. Clin J Pain. 2014;30(5):453–462. doi:10.1097/AJP.0b013e31829f57df
19. Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377(9770):1032–1045. doi:10.1016/S0140-6736(10)60926-9
20. Martin AR, Finlay WH. Nebulizers for drug delivery to the lungs. Expert Opin Drug Deliv. 2015;12(6):889–900. doi:10.1517/17425247.2015.995087
21. Pult H, Purslow C, Murphy PJ. The relationship between clinical signs and dry eye symptoms. Eye (Lond). 2011;25(4):502–510. doi:10.1038/eye.2010.228
22. van Bijsterveld OP. Diagnostic tests in the Sicca syndrome. Arch Ophthalmol. 1969;82(1):10–14.
23. Hamrah P, Qazi Y, Shahatit B, et al. Corneal Nerve and Epithelial Cell Alterations in Corneal Allodynia: An In Vivo Confocal Microscopy Case Series. Ocul Surf. 2017;15(1):139–151. doi:10.1016/j.jtos.2016.10.002
24. Villani E, Magnani F, Viola F, et al. In vivo confocal evaluation of the ocular surface morpho-functional unit in dry eye. Optom Vis Sci. 2013;90(6):576–586. doi:10.1097/OPX.0b013e318294c184
25. Kheirkhah A, Rahimi Darabad R, Cruzat A, et al. Corneal epithelial immune dendritic cell alterations in subtypes of dry eye disease: a pilot in vivo confocal microscopic study. Invest Ophthalmol Vis Sci. 2015;56(12):7179–7185. doi:10.1167/iovs.15-17433
26. Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20(4):374–384.
27. Lagali NS, Griffith M, Shinozaki N, Fagerholm P, Munger R. Innervation of tissue-engineered corneal implants in a porcine model: a 1-year in vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2007;48(8):3537–3544. doi:10.1167/iovs.06-1483
28. Okamoto Y, Ishitobi M, Wada Y, Kosaka H. The potential of nasal oxytocin administration for remediation of autism spectrum disorders. CNS Neurol Disord Drug Targets. 2016;15(5):564–577.
29. Patel DV, McGhee CN. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol. 2009;93(7):853–860. doi:10.1136/bjo.2008.150615
30. Alhatem A, Cavalcanti B, Hamrah P. In vivo confocal microscopy in dry eye disease and related conditions. Semin Ophthalmol. 2012;27(5–6):138–148. doi:10.3109/08820538.2012.711416
31. Macri A, Scanarotti C, Bassi AM, et al. Evaluation of oxidative stress levels in the conjunctival epithelium of patients with or without dry eye, and dry eye patients treated with preservative-free hyaluronic acid 0.15 % and vitamin B12 eye drops. Graefes Arch Clin Exp Ophthalmol. 2015;253(3):425–430. doi:10.1007/s00417-014-2853-6
32. Romano MR, Biagioni F, Carrizzo A, et al. Effects of vitamin B12 on the corneal nerve regeneration in rats. Exp Eye Res. 2014;120:109–117. doi:10.1016/j.exer.2014.01.017
33. Yu CZ, Liu YP, Liu S, Yan M, Hu SJ, Song XJ. Systematic administration of B vitamins attenuates neuropathic hyperalgesia and reduces spinal neuron injury following temporary spinal cord ischaemia in rats. Eur J Pain. 2014;18(1):76–85. doi:10.1002/j.1532-2149.2013.00390.x
34. Okada K, Tanaka H, Temporin K, et al. Methylcobalamin increases Erk1/2 and Akt activities through the methylation cycle and promotes nerve regeneration in a rat sciatic nerve injury model. Exp Neurol. 2010;222(2):191–203. doi:10.1016/j.expneurol.2009.12.017
35. Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–542.
36. Thibonnier M, Conarty DM, Preston JA, Plesnicher CL, Dweik RA, Erzurum SC. Human vascular endothelial cells express oxytocin receptors. Endocrinology. 1999;140(3):1301–1309. doi:10.1210/endo.140.3.6546
37. Spangelo BL, deHoll PD, Kalabay L, Bond BR, Arnaud P. Neurointermediate pituitary lobe cells synthesize and release interleukin-6 in vitro: effects of lipopolysaccharide and interleukin-1 beta. Endocrinology. 1994;135(2):556–563. doi:10.1210/endo.135.2.8033802
38. Williams KI, El Tahir KE. Effects of uterine stimulant drugs on prostacyclin production by the pregnant rat myometrium. I. Oxytocin, bradykinin and PGF2 alpha. Prostaglandins. 1980;19(1):31–38.
39. Garrido-Urbani S, Deblon N, Poher AL, et al. Inhibitory role of oxytocin on TNFalpha expression assessed in vitro and in vivo. Diabetes Metab. 2018;44(3):292–295. doi:10.1016/j.diabet.2017.10.004
40. Gibbs DM, Vale W, Rivier J, Yen SS. Oxytocin potentiates the ACTH-releasing activity of CRF(41) but not vasopressin. Life Sci. 1984;34(23):2245–2249.
41. Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27(3):277–324. doi:10.1385/MN:27:3:277
42. Johnson EO, Charchanti A, Soucacos PN. Nerve repair: experimental and clinical evaluation of neurotrophic factors in peripheral nerve regeneration. Injury. 2008;39(Suppl 3):S37–S42. doi:10.1016/j.injury.2008.06.015
43. Hawley D, Tang X, Zyrianova T, et al. Myoepithelial cell-driven acini contraction in response to oxytocin receptor stimulation is impaired in lacrimal glands of Sjogren’s syndrome animal models. Sci Rep. 2018;8(1):9919. doi:10.1038/s41598-018-28227-x
44. Arrowsmith S, Wray S. Oxytocin: its mechanism of action and receptor signalling in the myometrium. J Neuroendocrinol. 2014;26(6):356–369. doi:10.1111/jne.12154
45. Chiou GC, Shen ZF, Zheng YQ. Systemic absorption of oxytocin and vasopressin through eyes in rabbits. J Ocul Pharmacol. 1991;7(4):351–359.
46. Willcox MDP, Argueso P, Georgiev GA, et al. TFOS DEWS II tear film report. Ocul Surf. 2017;15(3):366–403. doi:10.1016/j.jtos.2017.03.006
47. Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78(3):409–416.
48. Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17(6):584–589.
49. Hamrah P, Dana MR. Corneal antigen-presenting cells. Chem Immunol Allergy. 2007;92:58–70. doi:10.1159/000099254
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.