Back to Journals » Drug, Healthcare and Patient Safety » Volume 13
A Narrative Review of Statin-Induced Rhabdomyolysis: Molecular Mechanism, Risk Factors, and Management
Authors Safitri N, Alaina MF, Pitaloka DAE , Abdulah R
Received 11 August 2021
Accepted for publication 1 November 2021
Published 8 November 2021 Volume 2021:13 Pages 211—219
DOI https://doi.org/10.2147/DHPS.S333738
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Siew Siang Chua
Nisa Safitri,1 Maya Fadila Alaina,1 Dian Ayu Eka Pitaloka,1,2 Rizky Abdulah1,2
1Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, 45363, Indonesia; 2Center of Excellence in Higher Education for Pharmaceutical Care Innovation, Universitas Padjadjaran, Sumedang, 45363, Indonesia
Correspondence: Dian Ayu Eka Pitaloka Tel +62 22-84288812
Email [email protected]
Abstract: Although statins are effective for treating hypercholesterolemia, they can have various side effects, including rhabdomyolysis, a potentially fatal condition. This review evaluated the incidence and underlying molecular mechanism of statin-induced rhabdomyolysis and analyzed its risk factors, prevention, and management. We focused on the clinical and randomized clinical trials of statin monotherapies and combinations with other drugs. The primary mechanism of statin therapy-induced rhabdomyolysis is believed to be a decrease in ubiquinone (coenzyme Q) produced by the HMG-CoA pathway. Additionally, different types of lipophilic and hydrophilic statins play a role in causing rhabdomyolysis. Although statin-induced rhabdomyolysis has a low incidence, there is no guarantee that patients will be free of this side effect. Rhabdomyolysis can be prevented by reducing the risk factors, such as using CYP3A4 inhibitors, using high-dose statins, and strenuous physical activities.
Keywords: statin, hypercholesterolemia, adverse effect, rhabdomyolysis, risk factor
Graphical Abstract:
Introduction
Cholesterol, a fatty substance necessary for the proper functioning of the body, is critical for the synthesis of hormones and vitamin D. Cholesterol is transported through the blood by a specific class of particles called lipoproteins. Low-density lipoproteins (LDLs) carry liver cholesterol to the cells, and high-density lipoproteins (HDLs) remove excess cholesterol from different tissues and transport it back to the liver for elimination.1 LDL, also known as bad cholesterol, is the most important factor in cardiovascular disease development. LDL can cause atherosclerosis, the formation of plaque on the artery walls that reduces the blood flow, and can even cause a heart attack.2 The maximum limit of tolerated cholesterol is 200 mg/dL for total cholesterol and 100 mg/dL for LDL cholesterol (LDL-C), and the minimum limit for HDL cholesterol (HDL-C) is 50 mg/dL. When the corresponding plasma cholesterol levels in a patient exceed these limits, the patient is defined as having a condition called hypercholesterolemia.3
Statins are well-known lipid-lowering agents and one of the world’s most prescribed drugs. Statins lower cholesterol levels through three interconnected mechanisms. The first mechanism is the selective and competitive inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase that limits the conversion speed of HMG-CoA to mevalonic acid, a precursor of sterols, including cholesterol in the mevalonate pathway, causing the substrate to bind to the active site and reducing cholesterol synthesis.4 The second indirect mechanism is to increase the receptor-mediated absorption of LDL, thereby reducing the level of plasma LDL. The third mechanism involves lowering the levels of LDL precursors, very-low-density and intermediate-density lipoproteins, to reduce the level of plasma LDL-C further.5
However, statins have been reported to exert side effects on muscles; the most common side effects are muscle pain, stiffness, myalgia, myopathy, and rhabdomyolysis.6 Rhabdomyolysis is caused by the damage to skeletal muscles that disrupts muscle integrity and releases muscle components, such as Creatine Kinase (CK), myoglobin, lactate dehydrogenase, aldolase, and electrolytes, into the bloodstream.7,8 Clinical presentations of rhabdomyolysis include muscle pain, swelling, weakness, and red urine because of increased myoglobin levels.9 Several reports have shown that the use of statins causes rhabdomyolysis.10–13 Although rhabdomyolysis rarely occurs, it remains a concern because of its severe clinical effects.
This review aims to study the evidence related to statin-induced rhabdomyolysis, identify the risk factors, and describe the potential prevention and management strategies. Additionally, we focus on the evidence reported in clinical trials and randomized controlled trials (RCTs) on statin monotherapies and statin-related multidrug therapies.
Methods
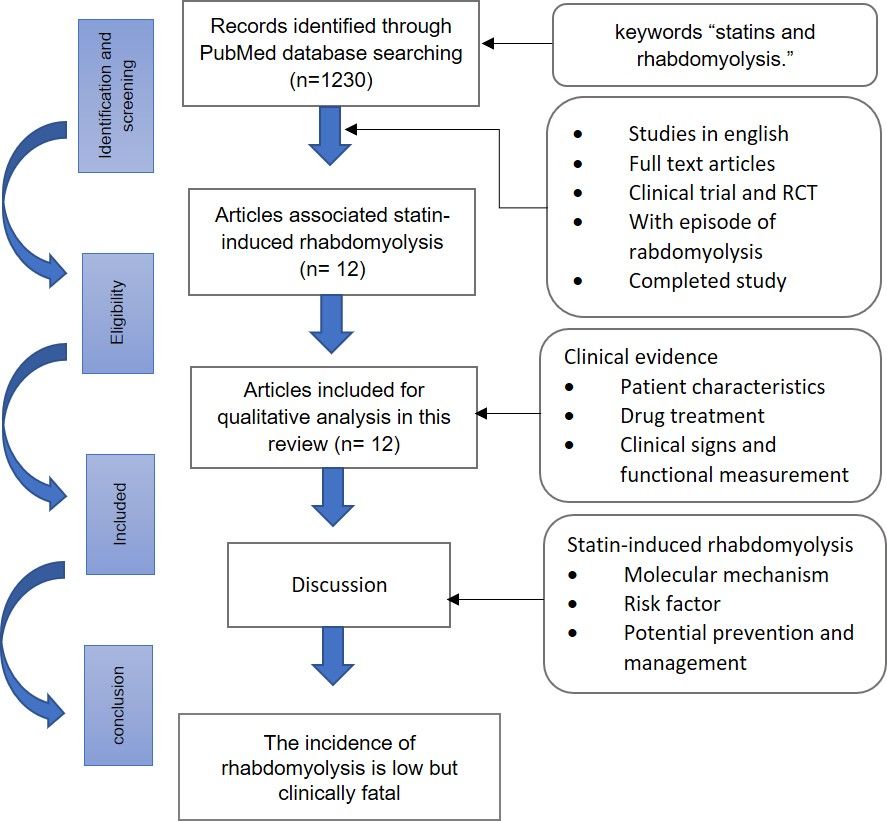
The selection for the related article published in 2001–2021 was conducted in the PubMed database using the keywords “statins and rhabdomyolysis.” The inclusion criteria were English articles on the RCTs and clinical trials on statin-associated monotherapies or multidrug therapies. A total of 1230 publications were found, of which 59 met the inclusion criteria. In addition, through full-text screening, we excluded the articles that did not describe an episode of rhabdomyolysis, study rhabdomyolysis, or have related research results. Of these 59 papers, only 12 were on the incidence of rhabdomyolysis because of statins: 7 were RCTs, 4 were clinical trials, and 1 was a Phase 1 clinical trial (Figure 1).
 |
Figure 1 Flowchart of the literature search process. |
Current Evidence of Statin-Induced Rhabdomyolysis
Patient Characteristics
The total number of patients reported in 12 studies was 1,129,477, of which 102 (0.009%) had statin-induced rhabdomyolysis (Table 1). The mean age of the patients was 63.57. Additionally, most of these patients were male (57.4%). However, other characteristics, such as the body mass index and comorbidities, were not mentioned in all 12 studies.
 |
Table 1 Studies on Statins and Rhabdomyolysis Included in This Review |
Drug Treatment
Although the types and doses of statins used in the trials varied, simvastatin was the most broadly used type. For example, two studies reported using simvastatin as monotherapy.14,15 Abraldes et al administered simvastatin at 20 mg/day for the first 15 days and continued with 40 mg/day as the standard therapy to increase the survival rate of patients with cirrhosis.14 Meanwhile, Wierzbicki et al compared the effects of 80 and 120 mg/day simvastatin on patients with hypercholesterolemia.15 Both studies showed simvastatin monotherapy to have a very strong effect in inducing rhabdomyolysis, as evidenced in the percentage of its occurrence (Table 1).
Meanwhile, three studies studied simvastatin in combination with other drugs. The study by HPS2-THRIVE reported a 4-week regimen of simvastatin (40 mg/day), niacin (1 g/day), and laropiprant (20 mg/day), followed by 3–6 weeks of extended-release niacin (2 g/day) and laropiprant (40 mg/day). During the study, 10 mg/day ezetimibe was given when the total cholesterol was less than 3.5 mmol/L.13 This combination therapy-induced rhabdomyolysis at the same rate as the placebo group (simvastatin 40 mg/day), at 0.109% and 0.114%, respectively. Moreover, another study compared the efficacy of simvastatin monotherapy (40 mg/day) in reducing LDL-C with that of a combination of simvastatin and ezetimibe (10 mg/day).11 Furthermore, Pose et al compared the efficacy of two different doses of simvastatin (40 and 20 mg/day) in combination with rifaximin (1200 mg/day) and demonstrated that 40 mg/day simvastatin induced rhabdomyolysis more than 20 mg/day simvastatin.16
Conversely, Pedersen et al compared the efficacy of two types of statins in two doses: 20 mg/day simvastatin and 40 mg/day high-dose atorvastatin.17 The study showed that simvastatin at a lower dose had a greater effect on rhabdomyolysis, that is, by 0.07%, than at a higher dose.17 The effectiveness of 80 mg/day atorvastatin before and 24 h after percutaneous coronary intervention followed by 40 mg/day atorvastatin for 30 days was already conducted in the RCT study. A total of three cases of rhabdomyolysis were reported in this study.10 Additionally, clinical trials were conducted to assess the safety of rosuvastatin (5–40 mg/day) in 16,874 patients18 and its side effects on skeletal muscles after fluvastatin and rosuvastatin use (dosages are unavailable). One case of rhabdomyolysis occurred in a patient who received rosuvastatin in combination with gemfibrozil.19 Furthermore, a phase 1 clinical trial study was conducted to evaluate the safety of rosuvastatin (1–8 mg/kg/day) and erlotinib (150 mg/day) in treating advanced solid malignancies; one case of rhabdomyolysis was reported.12
Lastly, other studies did not list the type or dose of statins used. Enger et al conducted an RCT study to analyze the side effects of lipid-lowering agents, such as statins and fibrates.20 Based on the results, the percentage of statin-induced rhabdomyolysis increased when a statin was combined with fibrates; additionally, there was a correlation between the use of fibrates and rhabdomyolysis. Meanwhile, another RCT study reported the incidence of rhabdomyolysis in patients taking lipid-lowering agents, including statins, but the dose of each drug was not clearly stated.21 These findings suggest that simvastatin induces rhabdomyolysis at a higher rate than other types of statins, consistent with the theory that patients taking lipophilic statins have a higher risk of side effects and that increasing statin dose causes a higher ratio of side effects.22
Clinical Signs and Functional Measurement
According to most studies, the patients with induced rhabdomyolysis experienced muscle weakness, myalgia, and fatigue. Additionally, they had CK levels above normal at more than 2000 U/L or 10 times the upper limit of normal (ULN).5,12,19 Moreover, their urine samples change to a darker color.19 However, some of the studies did not specifically list the symptoms experienced by patients and the laboratory test results.
Goss et al reported a rhabdomyolysis-related death. Of the 24 patients in the study, one experienced rhabdomyolysis after the administration of rosuvastatin and erlotinib and died.12 On day 6 (erlotinib only), this patient showed normal alanine transaminase, albumin, hepatic, renal, and muscle functions. However, on day 28 (rosuvastatin + erlotinib), the patient’s CK level increased to more than 2000 U/L. These findings demonstrate that the percentage of rhabdomyolysis due to statin therapy is relatively low. However, the authors did not mention the possibility of rhabdomyolysis in certain patients.
Molecular Mechanism of Statin-Induced Rhabdomyolysis
Although the primary mechanism of rhabdomyolysis due to statin therapy (Figure 2) remains unknown, statins are believed to induce skeletal muscle necrosis, probably because of the decrease in ubiquinone. In addition to cholesterol, the HMG-CoA pathway also produces other essential molecules, such as ubiquinone (coenzyme Q). Ubiquinone, a component of the mitochondrial respiratory chain, acts as a mitochondrial electron transport facilitator. Thus, inhibiting the HMG-CoA pathway may inhibit ubiquinone production and disrupt cellular energy production, causing muscular cell death.23
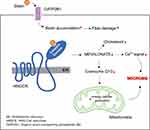 |
Figure 2 Potential mechanism underlying statin-induced rhabdomyolysis. |
The integrity of skeletal muscles; the presence of a catabolic state can lead to muscle atrophy. The pathway mediates protein turnover through several enzymes, one of which is protein ligase (atrogin-1), which increases after statin therapy and is associated with muscle loss.22
Different types of lipophilic and hydrophilic statins play a role in the occurrence of rhabdomyolysis. In vitro studies indicate that lipophilic statins have a more significant myopathic effect than hydrophilic statins. Rhabdomyolysis is a severe form of myopathy. Lipophilic statins, such as atorvastatin, simvastatin, and fluvastatin, increase cell disruption through apoptosis and proteolysis. These statins can easily pass through the lipid layer membrane by passive transport; thus, they have higher toxic effects.22 This review’s findings are in line with this theory. The incidence of rhabdomyolysis is more common in patients taking atorvastatin and simvastatin. These findings are also consistent with the research by Mendes et al,24 who examined the incidence of rhabdomyolysis due to statin therapy in the case reports published in 1990–2013 and found that simvastatin was the most widely reported statin associated with rhabdomyolysis (55 of 112 cases).
The effect of statins on myofibers has been observed in rats disease model. For example, statins can reduce intracellular adenosine triphosphate (ATP) and Ca2+ levels in the sarcoplasmic reticulum. It can also inhibit muscle contraction, causing contractile dysfunction and leading to cell damage, muscle cell death, and rhabdomyolysis.25,26
In the body, statins are also ionized into anions and get taken up by membrane transporters, such as the organic anion transporting polypeptide (OATP) transporter.27 Knauer et al reported that OATP2B1, an OATP isoform, facilitated the accumulation of statins in human myotubes and increased their myotoxicity.28 A similar result was reported by Tanaka et al who investigated the mechanism of statin myotoxicity in rats and found that OATP isoforms, OATP2b1 and OATP1a4, facilitated statin-induced myotoxicity and increased the risk of rhabdomyolysis.25
Risk Factor Associated with Statin-Induced Rhabdomyolysis
Statin-induced rhabdomyolysis may be due to statin monotherapies or combination therapies of statin and other drugs. However, certain drugs administered concurrently with statins increase the risk of rhabdomyolysis. Omar and Wilson examined the incidence of rhabdomyolysis due to statin therapy using the Adverse Event Reporting System of the Food and Drug Administration (FDA) and found that 50% of the statin-induced rhabdomyolysis cases are caused by drug–drug interactions.29
For example, combining statins with an inhibitor of the cytochrome P450 3A4 (CYP3A4) enzyme, such as verapamil, diltiazem, ritonavir, and amiodarone, potentially increases the risk of rhabdomyolysis.30–32 Since statins are oxidized by CYP3A4, which is produced by the liver, inhibiting CYP3A4 with certain drugs can reduce statin oxidation and decrease statins metabolism. Consequently, statins remain longer in the body, increasing the risk of side effects.24
Additionally, fibrates are associated with rhabdomyolysis. Fibrates can decrease LDL and increase HDL levels. However, Enger et al20 and another study have shown that fibrates, mostly fenofibrate, induce rhabdomyolysis.33 However, the mechanism underlying fibrate-induced rhabdomyolysis has not been precisely defined.34
The incidence of rhabdomyolysis may also increase with increasing statin dosage, as a higher dose elevates the plasma concentration of statin and its active metabolite.35 For example, a meta-analysis study by LaRosa36 compared the effects of administering 10 mg of atorvastatin to those of 80 mg. Subsequently, the author reported five cases of rhabdomyolysis at the higher dose and three cases at the lower dose.
Meanwhile, Schech et al identified the risk factors for rhabdomyolysis among statin users and found that statin users aged 65 years and older had four times the risk of hospitalization for rhabdomyolysis compared with those of the younger users; the increased risk was likely correlated with a decrease in organ function.37 The authors also observed a more than twofold increase in the risk of rhabdomyolysis among females.
Meanwhile, comorbidities, such as diabetes mellitus, were not found to be related to the risk. However, the association between rhabdomyolysis and renal diseases was statistically significant. Patients with pre-existing renal impairment taking cerivastatin may be at increased risk of rhabdomyolysis since the drug is excreted through the kidneys.37 Additionally, the association between the incidence of rhabdomyolysis with other diseases was reported in 147 patients with rhabdomyolysis incidence. Two of them had a history of liver disease. Liver disease is associated with the decreased expression of SLCO1B, which encodes an anion transporter that regulates statin absorption in the liver. Thus, mutations in SLCO1B may trigger statin-induced myopathy.14
Lastly, patients who are prescribed statins but engage in strenuous exercise also have an increased risk of rhabdomyolysis. One study showed an association between statin therapy and strenuous exercise. Patients given lovastatin had 62–77% higher CK levels after a workout than patients receiving a placebo.38 Additionally, another study using rodents showed that taking cerivastatin after using a treadmill could increase muscle damage.39 However, the association between the type of exercise and the severity of the risk of statin-induced rhabdomyolysis is unclear. Nevertheless, exercise can affect the absorption, distribution, metabolism, and excretion of statins, resulting in pharmacokinetic changes that can cause specific side effects.40 Strenuous exercise also causes excessive heat production, resulting in intracellular Ca2+ increase through ATP depletion. The loss of ATP disrupts the Ca2+-ATPase and Na+/K+-ATPase pumps, increasing intracellular Ca2+, activating proteases and reactive oxygen species, and finally causing muscle damage.41 In the PubMed database, there were 17 individual cases of heatstroke-associated rhabdomyolysis. Thus, it is recommended that individuals who work in hot environments anticipate rhabdomyolysis and take reasonable precautions.42
Prevention and Management of Statin-Induced Rhabdomyolysis
The best strategy to prevent statin-induced rhabdomyolysis is to lower all risk factors. Particularly, individuals who take statins and perform strenuous exercises, such as workers of certain occupations and athletes, should take frequent breaks to relax their muscles and relieve stress to decrease the risk of rhabdomyolysis.43 Additionally, clinicians who prescribe statins can also prioritize hydrophilic-type statins such as pravastatin and rosuvastatin to reduce the potential side effects.
The FDA does not recommend prescribing simvastatin at high doses (80 mg) because of the increased risk of developing statin-induced muscle injury.44 Simvastatin at 80 mg can be prescribed if the patient exhibits no side effects after receiving the same therapy for 12 months. Additionally, the recommended dose of simvastatin is 5–40 mg once daily.39 Therefore, the administration of less than 80 mg of simvastatin or other types of statins is recommended. Although there is no guarantee that rhabdomyolysis or further muscle damage will not occur, lower dose of statin reduce the risk of muscle injury.
Additionally, since a combination of CYP3A4 inhibitors with statins can increase the risk of rhabdomyolysis, patients with hypercholesterolemia who have certain disease complications should avoid taking CYP3A4 inhibitors in combination with statins.45 Moreover, if a patient has rhabdomyolysis due to the administration of a lipophilic statin, eg, atorvastatin and simvastatin, switching to a hydrophilic statin, eg, pravastatin and rosuvastatin, may be reasonable.
Moreover, patients suspected of having rhabdomyolysis should undergo renal examination; then, statin therapy should be discontinued. Rhabdomyolysis is typically treated with intravenous rehydration or dialysis for patients with more severe symptoms. After assessing a patient’s condition, statin can still be used at a lower dose.46 However, if the patient experiences increasing CK levels at up to five times the ULN, the statin regimen should be immediately discontinued, and the patient should be given another lipid-lowering drug.47
Lastly, Koba et al compared the efficacy of evolocumab with that of ezetimibe in patients with statin intolerance due to rhabdomyolysis. Evolocumab was more effective than ezetimibe in lowering LDL-C levels. After 12 weeks of drug administration, evolocumab decreased LDL-C levels by 59.5% of the baseline compared with ezetimibe by 20.3%.48 Thus, the use of evolocumab in a patient with statin intolerance should be recommended.
Limitation
However, our study has limitations. Although the selected articles in our study supported our study approach, we only used a single PubMed database to identify the articles that met our article selection criteria. Thus, there may be other related articles that have not been identified because they are not in PubMed. Despite the limitation, our study has described the potential risk factors for rhabdomyolysis; thus, our study can help reduce the risk of statin-induced rhabdomyolysis.
Conclusion
Although statins offer benefits in managing hypercholesterolemia, they may cause rhabdomyolysis. The findings of this review suggest that although the incidence of statin-induced rhabdomyolysis is low, its risk is still increasing because of the presence of triggering factors, such as the use of CYP3A4 inhibitors, initiation of high-dose statins, and strenuous physical activity. Therefore, the use of statins should be closely monitored, especially by measuring the CK levels that indicate rhabdomyolysis.
Acknowledgments
This study was funded by the Center of Excellence in Higher Education for Pharmaceutical Care Innovation Universitas Padjadjaran.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maxfield FR, van Meer G. Cholesterol, the central lipid of mammalian cells. Curr Opin Cell Biol. 2010;22(4):422–429. doi:10.1016/J.CEB.2010.05.004
2. Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459. doi:10.1093/EURHEARTJ/EHX144
3. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139(25):E1082–E1143. doi:10.1161/CIR.0000000000000625
4. Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. 2019;124(2):328–350. doi:10.1161/CIRCRESAHA.118.312782
5. Stancu C, Sima A. Statins: mechanism of action and effects. J Cellullar Me. 2001;5(4):378–387. doi:10.1111/j.1582-4934.2001.tb00172.x
6. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society consensus panel statement on assessment, aetiology and management. Eur Heart J. 2015;36:1012–1022. doi:10.1093/eurheartj/ehv043
7. Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40(4):188–194. doi:10.1097/JES.0b013e31826c169e
8. Torres PA, Helmstetter JA, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58–69.
9. Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013;144(3):1058–1065. doi:10.1378/chest.12-2016
10. Berwanger O, Santucci EV, de Silva PGM, et al. Effect of loading dose of atorvastatin prior to planned percutaneous coronary intervention on major adverse cardiovascular events in acute coronary syndrome: the SECURE-PCI randomized clinical trial. JAMA. 2018;319(13):1331–1340. doi:10.1001/JAMA.2018.2444
11. Giugliano RP, Wiviott SD, Blazing MA, et al. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT trial. JAMA Cardiol. 2017;2(5):547–555. doi:10.1001/JAMACARDIO.2017.0083
12. Goss GD, Jonker DJ, Laurie SA, et al. A phase I study of high-dose rosuvastatin with standard dose erlotinib in patients with advanced solid malignancies. J Transl Med. 2016;14(1):1–11. doi:10.1186/S12967-016-0836-6
13. Haynes R, Jiang L, Hopewell JC. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34(17):1279–1291. doi:10.1093/EURHEARTJ/EHT055
14. Abraldes JG, Villanueva C, Aracil C, et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology. 2016;150(5):1160–1170.e3. doi:10.1053/j.gastro.2016.01.004
15. Wierzbicki A, Lumb PJ, Chik G. Comparison of therapy with simvastatin 80 mg and 120 mg in patients with familial hypercholesterolaemia. Int J Clin Pract. 2001;55(10):673–675.
16. Pose E, Napoleone L, Amin A, et al. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5(1):31–41. doi:10.1016/S2468-1253(19)30320-6
17. Pedersen TR, Faergeman O, Kastelein JJP, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294(19):2437–2445. doi:10.1001/JAMA.294.19.2437
18. Shepherd J, Vidt DG, Miller E, Harris S, Blasetto J. Safety of rosuvastatin: update on 16,876 rosuvastatin-treated patients in a multinational clinical trial program. Cardiology. 2007;107(4):433–443. doi:10.1159/000100908
19. Drobný M, Pullmann R, Odalos I, Skerenova M, Sániova B. Incidence of skeletal muscle disorders after statins’ treatment: consequences in clinical and EMG picture. Neuroendocrinol Lett. 2014;35(2):123–128.
20. Enger C, Gately R, Ming EE, Niemcryk SJ, Williams L, McAfee AT. Pharmacoepidemiology safety study of fibrate and statin concomitant therapy. Am J Cardiol. 2010;106(11):1594–1601. doi:10.1016/j.amjcard.2010.07.041
21. Cziraky MJ, Willey VJ, McKenney JM, et al. Risk of hospitalized rhabdomyolysis associated with lipid-lowering drugs in a real-world clinical setting. J Clin Lipidol. 2013;7(2):102–108. doi:10.1016/j.jacl.2012.06.006
22. Di Stasi SL, MacLeod TD, Winters JD, Binder-Macleod SA. Effects of statins on skeletal muscle: a perspective for physical therapists. Phys Ther. 2010;90(10):1530–1542. doi:10.2522/PTJ.20090251
23. Westwood FR, Bigley A, Randall K, Marsden AM, Scott RC. Statin-induced muscle necrosis in the rat: distribution, development, and fibre selectivity. Toxicol Pathol. 2016;33(2):246–257. doi:10.1080/01926230590908213
24. Mendes P, Robles PG, Mathur S. Statin-induced rhabdomyolysis: a comprehensive review of case reports. Physiother Can. 2014;66(2):124–132. doi:10.3138/ptc.2012-65
25. Tanaka S, Sakamoto K, Yamamoto M, et al. Mechanism of statin-induced contractile dysfunction in rat cultured skeletal myofibers. J Pharmacol Sci. 2010;114(4):454–463. doi:10.1254/JPHS.10229FP
26. Sakamoto K, Honda T, Yokoya S, Waguri S, Kimura J. Rab-small GTPases are involved in fluvastatin and pravastatin-induced vacuolation in rat skeletal myofibers. FASEB J. 2007;21(14):4087–4094. doi:10.1096/FJ.07-8713COM
27. Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug–drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther. 2006;112(1):71–105. doi:10.1016/J.PHARMTHERA.2006.03.003
28. Knauer MJ, Urquhart BL, Schwabedissen HEM, et al. Human skeletal muscle drug transporters determine local exposure and toxicity of statins. Circ Res. 2010;106(2):297–306. doi:10.1161/CIRCRESAHA.109.203596
29. Omar MA, Wilson JP. FDA adverse event reports on statin-associated rhabdomyolysis. Ann Pharmacother. 2002;36(2):288–295. doi:10.1345/aph.1A289
30. Ezad S, Cheema H, Collins N. Statin-induced rhabdomyolysis: a complication of a commonly overlooked drug interaction. Oxf Med Case Rep. 2018;2018(3):86–88. doi:10.1093/OMCR/OMX104
31. Rowan CG, Brunelli SM, Munson J, et al. Clinical importance of the drug interaction between statins and CYP3A4 inhibitors: a retrospective cohort study in The Health Improvement Network. Pharmacoepidemiol Drug Saf. 2012;21(5):494–506. doi:10.1002/pds.3199
32. Yang BR, Seong JM, Choi NK, et al. Co-medication of statins with contraindicated drugs. PLoS One. 2015;10(5):1–11. doi:10.1371/journal.pone.0125180
33. Danis R, Akbulut S, Ozmen S, Arikan S. Rhabdomyolysis-induced acute renal failure following fenofibrate therapy: a case report and literature review. Case Rep Med. 2010;2010:2–5. doi:10.1155/2010/537818
34. Wang D, Wang Y. Fenofibrate monotherapy-induced rhabdomyolysis in a patient with hypothyroidism. Med US. 2018;97(14):1–3. doi:10.1097/MD.0000000000010318
35. Newman CB, Preiss D, Tobert JA, et al. Statin safety and associated adverse events a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39(2):E38–E81. doi:10.1161/ATV.0000000000000073
36. LaRosa JC, Grundy SM, Waters DD, et al. intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425–1435. doi:10.1056/NEJMoa050461
37. Schech S, Graham D, Staffa J, et al. Risk factors for statin-associated rhabdomyolysis. Pharmacoepidemiol Drug Saf. 2007;16(3):352–358. doi:10.1002/pds.1287
38. Unnikrishnan D, Satish B. Exertion-induced rhabdomyolysis in a patient on statin therapy. Nephrol Dial Transplant. 2005;20(1):244–247. doi:10.1093/ndt/gfh578
39. Seachrist JL, Loi CM, Evans MG, Criswell KA, Rothwell CE. Roles of exercise and pharmacokinetics in cerivastatin-induced skeletal muscle toxicity. Toxicol Sci. 2005;88(2):551–561. doi:10.1093/toxsci/kfi305
40. Lenz TL, Lenz NJ, Faulkner MA. Potential interactions between exercise and drug therapy. Sports Med. 2004;34(5):293–306. doi:10.2165/00007256-200434050-00002
41. Tietze DC, Borchers J. exertional rhabdomyolysis in the athlete: a clinical review. Sports Health. 2014;6(4):336–339. doi:10.1177/1941738114523544
42. Yoshizawa T, Omori K, Takeuchi I, et al. Heat stroke with bimodal rhabdomyolysis: a case report and review of the literature. J Intensive Care. 2016;4(1):1–5. doi:10.1186/s40560-016-0193-9
43. NIOSH. Prevention: rhabdomyolysis | NIOSH | CDC; 2019. Available from: https://www.cdc.gov/niosh/topics/rhabdo/prevention.html.
44. Food and Drug Administration. FDA drug safety communication: new restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury | FDA; 2021. Available fromhttps://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-new-restrictions-contraindications-and-dose-limitations-zocor.
45. Khalilieh S, Yee KL, Sanchez RI, et al. Results of a doravirine-atorvastatin drug-drug interaction study. Antimicrob Agents Chemother. 2017;61(2):e01364–16. doi:10.1128/AAC.01364-16
46. Barry AR, Beach JE, Pearson GJ. Prevention and management of statin adverse effects: a practical approach for pharmacists. Can Pharm J. 2018;151(3):179–188. doi:10.1177/1715163518768534
47. Ballantyne CM, Corsini A, Davidson MH, et al. Risk for myopathy with statin therapy in high-risk patients. Arch Intern Med. 2003;163(5):553–564. doi:10.1001/ARCHINTE.163.5.553
48. Koba S, Inoue I, Cyrille M, et al. Evolocumab vs. ezetimibe in statin-intolerant hyperlipidemic Japanese patients: phase 3 GAUSS-4 trial. J Atheroscler Thromb. 2020;27(5):471–484. doi:10.5551/JAT.50963
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
