Back to Journals » Journal of Pain Research » Volume 14
A Multidisciplinary Approach to Simultaneously Monitoring Real-Time Neuronal Activity and Pain Behaviors During Optogenetic Stimulation of Brain Neurons in Freely Moving Mice
Authors Crawford J, Liu S, Tao F
Received 14 August 2021
Accepted for publication 9 October 2021
Published 9 November 2021 Volume 2021:14 Pages 3503—3509
DOI https://doi.org/10.2147/JPR.S334256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qi Fang
Joshua Crawford, Sufang Liu, Feng Tao
Department of Biomedical Sciences, Texas A&M University College of Dentistry, Dallas, TX, 75246, USA
Correspondence: Feng Tao
Department of Biomedical Sciences, Texas A&M University College of Dentistry, 3302 Gaston Ave., Dallas, TX, 75246, USA
Tel +1-214-828-8272
Fax +1-214-874-4538
Email [email protected]
Background: Highlighted by the current opioid epidemic, identifying novel therapies to treat chronic trigeminal neuropathic pain is a critical need. To develop these treatments, it is necessary to have viable targets in the brain to act on. Historically, neural tracing studies have been extremely useful in determining connections between brain areas but do not provide information about the functionality of these connections. Combining optogenetics and behavioral observation allows researchers to determine whether a particular brain area is involved in the regulation of such behavior. The addition of multi-channel electrophysiological recording provides information on real-time neuronal activity in the specific neuronal pathway.
Methods: Male C57/BL/6J mice (8-week-old) underwent either chronic constriction injury of infraorbital nerve (CCI-ION) or a sham surgery and were injected with either channelrhodopsin (ChR2) or a control virus in the hypothalamic A11 nucleus. Two weeks after CCI-ION, they were tested in real-time place preference (RTPP), while neuronal activity in the spinal trigeminal nucleus caudalis (Sp5C) was recorded.
Results: Optogenetic excitation of the A11 neurons results in more time spent in the stimulation chamber during RTPP testing. Additionally, stimulation of the A11 results in a greater number of neuronal activity increase in the Sp5C in animals with the injection of AAV carrying ChR2 compared to animals injected with a control virus or that underwent a sham surgery.
Conclusion: In vivo multi-channel electrophysiological recording, optogenetic stimulation, and behavioral observation can be combined in a mouse model of chronic trigeminal neuropathic pain to validate brain areas involved in the modulation of such pain.
Keywords: optogenetic stimulation, multi-channel recording, real-time place preference
Introduction
Chronic trigeminal pain is a major global health problem with up to 16% of the dental visits reported being due to pain and with a large population study finding the prevalence being over 5% in adult females and over 1% in adult males.1,2 Treatment options for chronic trigeminal pain are limited. A previous study shows that only 24% of the patients feel that their treatments are successful.3 These data highlight the need for new therapies. A major barrier to developing new treatments is the need to validate brain areas directly involved in chronic trigeminal pain in order to generate prospective therapeutic targets.
Traditional methods used alone to study pathways, such as neuronal tracing, optogenetics, chemogenetics, neuronal ablation, genetically encoded viral vector injections, can infer that brain areas are linked and involved in a certain behavior but do not explicitly show that the behavior is mediated by neuronal activity of a specific pathway.4–7 Moreover, these methods provide no means to quantify the effect of modulation of neuronal activity with behavioral output.
In this study, we sought to demonstrate an efficient method to directly identify pathways involved in chronic trigeminal pain. We combine optogenetic stimulation with in vivo multi-channel electrophysiological recording and simultaneous pain behavioral measurement. This combination of multi-disciplinary techniques allows us to observe real-time effects of optogenetic stimulation on the neuronal activity in a neural circuit and reveal how those changes are related to pain behavior. Here, we chose a well-established chronic constriction injury of infraorbital nerve (CCI-ION) induced chronic trigeminal neuropathic pain mouse model,8–10 and we investigated the role of the descending dopaminergic pathway from the hypothalamic A11 nucleus to spinal trigeminal nucleus caudalis (Sp5C) in pain modulation using our combinative approaches. Our recent work10 has shown that this descending neuronal pathway contributes to the modulation of trigeminal neuropathic pain.
Materials and Methods
Animals
All mice used were male 8-week-old C57BL/6J mice obtained from Jackson Laboratory. Animal studies were done with approval from the Texas A&M University Institutional Animal Care and Use Committee in accordance with NIH guidelines.
Preparation of the CCI-ION-Induced Trigeminal Neuropathic Pain Model
Following anesthesia with pentobarbital (i.p., 50 mg/kg), mice were placed in the supine position and the oral cavity exposed by placing a suture through each cheek and securing to the surgical table. A 2-mm incision in the buccal mucosa starting from the first molar was made to isolate the infraorbital nerve using blunt dissection. Two 4–0 chromic gut sutures were loosely placed around the infraorbital nerve. The incision was closed by applying a small drop of tissue adhesive. The mice were allowed to recover from anesthesia in a temperature-controlled environment. For sham-operated animals, the incision was closed after nerve exposure without ligation.
Preparation of Optical Fiber and Cannula
The fiber and furcation tubing were cut, and the fiber was then threaded through the tubing. Both ends of the fiber were stripped using a micro-stripper. A 25-gauge needle was used to add epoxy (1g epoxy in 100mL epoxy hardener) into one end of the large ferrule assembly. Next, the stripped end of the fiber was inserted through the flat end of the ferrule and thread through until the unstripped part of the fiber hit the ferrule opening. The epoxy bead was heated until it turns black before scoring the round end of the ferrule with a diamond knife. Using a hemostat to hold the ferrule, the unstripped end of the fiber was placed under a piece of tape and the exposed fiber was snapped at the desired length. Finally, we tested the output of the fiber.
Virus Injection and Implantation of the Optical Fiber
Under isoflurane anesthesia, a hole was drilled on the skull at the following coordinates from bregma (AP, −2.3 mm; ML, 0.2 mm) after exposing the skull using surgical scissors and locating bregma. Next, a Hamilton syringe was lowered into the hole until it reached a depth of 3.8 mm. After 0.5 µL of AAV carrying channelrhodopsin (ChR2) (AAV5-hSyn-hChR2(H134R)-EYFP (titer: 4.6×1012 vg/mL, UNC Vector Core) or its control (AAV5-hSyn-EYFP, titer: 3.3×1012 vg/mL, UNC Vector Core) was delivered, the syringe was left in place for 5 minutes before removing. Following removal of the syringe, a pre-made optical fiber was lowered into the same hole until it reached a depth of 3.5 mm. The fiber was then secured to the skull by using dental cement. The animal was allowed to recover from anesthesia in a temperature-controlled environment.
Preparation and Implantation of Multi-Channel Electrode
Preparation of the multi-channel electrode was modified from a previous report.11 Both the top and bottom parts of the headstage were obtained by 3D print. We used a Formlabs Form 2 printer to print all pieces used in these experiments. Next, the 1.2M nut was glued on the 3D printed base piece and placed a screw through the top piece and tightened until it was tight against the bottom piece. After it was glued in place, the wire was cut into 16 pieces of approximately 15 cm in length. A single wire was placed through a single hole in the green circuit board under the microscope. Using heat and a razor blade, we removed the covering on the end of the wires to expose the bare metal. A single wire was soldered to a single leg of the Omnetics connector. These procedures were repeated for all wires and then soldered to the green board.
We implanted multi-channel electrodes 2 weeks after viral injection. Under isoflurane anesthesia, a hole was drilled on the skull at the following coordinates from bregma: AP, −8.0 mm; ML, 1.5 mm. Next, the headstage was lowered into the hole 4.5 mm and then secured to the skull with cement. The animal was allowed to recover in a temperature-controlled environment.
Real-Time Place Preference Test, Optogenetic Stimulation, and Multi-Channel Recording Simultaneously
Before the experiment, animals were acclimated to experimental environment and equipment once a day for 3 days (10 min per day). We set up the Any-Maze software to get real-time automatic analysis of time spent in each real-time place preference (RTPP) test chamber. The RTPP apparatus was home built and its dimensions are 18” × 21” for two chambers and 10.5” × 21” for the buffer area. The length of the RTPP test was set to 15 min. To prepare the multi-channel recording equipment, we turned on the Omniplex chassis and then opened the Omniplex server software. Once there is connection between the hardware and software, “Run” on the top menu of PlexControl software was selected. Under isoflurane anesthesia, the optogenetic stimulation cable was attached to the implanted cannula. Following the animal was fully recovered from the anesthesia, it was placed in the middle chamber and we then clicked the play button on the test screen of Any-Maze to begin video tracking. The multi-channel recording was simultaneously begun by clicking “Start data acquisition” on the PlexControl software. During the experiment, we monitored the animal’s movement with Any-Maze, and when the animal entered one chamber that is assigned for stimulation, we manually triggered the laser power supply. Once the experiment was complete, the optogenetic stimulation cable was removed and the animal was returned to its cage. The experimental equipment was cleaned thoroughly before testing the next animal.
Analysis of Behavioral and Multi-Channel Recording Data
Any-Maze software was used for the analysis of behavioral data. A spreadsheet containing the data was generated by selecting “View spreadsheet” on the menu. This spreadsheet can either be copied and pasted in a data processing program or saved as a.csv file for later analysis by clicking “Save” on the menu. To get the detailed timestamps for each time an animal was in a zone, we navigated to the ‘Tests’ page and clicked on a test number and then selected Related Reports → Test Details Report from the menu. The data can be saved to a.csv file for later use in conjunction with electrophysiological recordings.
For the analysis of multi-channel recording data, Plexon Offline Sorter was used to classify the data by clicking File → Opening and selecting the intended.pl file. To begin sorting waveforms, we selected a recorded channel from the channels panel on the right-hand side of the layout. Clusters were identified by looking at the ‘2D Clusters’ panel. To specify a cluster, “Add Unit” was clicked and then a circle around a group of waveforms was drawn by using the mouse. The heterogeneity of the cluster was verified by visually inspecting the waveform recordings in the “Waveforms” panel. Individual waveforms can be added (“Add Wfs”) or deleted (“Remove Wfs”) from a cluster as necessary. These steps should be repeated for all other recorded channels. Once all channels have been sorted, the data were exported to a neuroexplorer formatted file for further analysis. We opened the exported waveform data in neuroexplorer and selected the correct file. In the main panel, we selected which units to be analyzed and then clicked the “Rate Histograms” option in the “Analyses” panel. The desired length of the recording was analyzed for generating firing rate histograms. The firing rate histograms were used in conjunction to find units that were either increased, decreased, or unchanged by optogenetic stimulation. For each animal, the time spent in stimulation chamber was overlayed onto the firing rate histogram of each unit. The statistical analysis was conducted with one-way ANOVA. The level of significance was set at p < 0.05.
Results
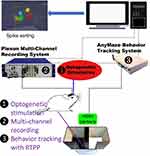
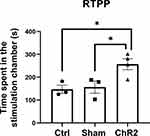
We performed real-time multi-channel electrophysiological recording and simultaneous behavioral observation during optogenetic stimulation of the A11-Sp5C pathway using our multi-disciplinary rig (Figure 1). Following CCI-ION surgery, mice exhibit pain-like behaviors for up to 28 days.8 Our RTPP test showed that the amount of time spent in stimulation chamber on day 14 post-surgery in the CCI-operated mice injected with AAV carrying ChR2 (AAV5-hSyn-hChR2(H134R)-EYFP) was significantly more than that in the CCI-operated mice injected with its control virus (AAV5-hSyn-EYFP) or the sham-operated mice injected with the AAV carrying ChR2 (Figure 2). These results indicate that optogenetic excitation of the A11 neurons can produce pain relief in mice with chronic trigeminal neuropathic pain.
Using multi-channel recording, we found that the optogenetic stimulation of A11 in the CCI-operated mice injected with AAV carrying ChR2 caused greater neuronal activity changes in the Sp5C than that in the sham-operated mice or the CCI-operated mice injected with a control virus. Of the neuron clusters showing neuronal activity changes, a significant difference was found in clusters with an increased firing rate during optogenetic stimulation between ChR2 and control or sham groups (Figure 3).
Discussion
In the present study, we demonstrate a robust multidisciplinary approach to simultaneously monitor real-time neuronal activity and pain behaviors during optogenetic stimulation of brain neurons in freely moving mice. We used CCI-ION induced trigeminal neuropathic pain mouse model. When performing the CCI surgery, it is crucial that the researcher does not transect the nerve when attempting to suture and that the sutures are tight enough to cause nerve injury. After implanting the optical fiber and the multi-channel electrode, it is critical that they are well secured with cement and that mice are singly housed to prevent the mice from removing or damaging the devices.
In addition to the above-mentioned precautions, we also modified the electrode headstage such that the top and bottom pieces are glued together to increase stability. One part of the protocol that may require troubleshooting is the RTPP behavior test. After implanting the devices, some animals may not initially explore the apparatus sufficiently to generate meaningful data. We trained the animals for 3 days prior to testing to help alleviate this but that number may need to be increased if after 3 days the animals are still not wanting to move in the apparatus.
The main limitation of this method is that the multi-channel recordings are not readily able to identify specific types of neurons. There are multiple strategies that can be used to overcome this limitation. One such way is to deliver drugs to the recording sites that inhibit or deplete a class of neurons. Another way would be to take advantage of genetic approaches available in mice to deplete a specific subset of neurons in the recording area. It is hopeful in the future that as we learn more about the electrophysiological properties of classes of neurons, the recordings alone will be able to identify the specific type of neurons. Additionally, while we demonstrate this method on a pain model, it could easily be used on any animal models where modulation of a brain neural circuit is thought to affect behaviors by simply substituting the RTPP test with a different test (eg rotarod for motor function).
Alternative methods for multi-channel recording would be fiber photometry12 or miniatured microscope imaging.13 These methods rely on injecting a virus encoding either a fluorescent calcium indicator protein or voltage indicator protein into the brain region of interest. After expression, the protein is targeted to the membrane where it inserts. The protein’s confirmation will change depending on the calcium influx or voltage change, which causes a change in the fluorescence. The changes in fluorescence allow for the real-time visualization of brain activity. A benefit to using a virus-based system is that it can be combined with other genetic tools available for rodents and specific types of neurons can be studied. For in vivo recordings, currently calcium indicators are more widely used than voltage indicators.14 Voltage indicators do hold several advantages over calcium indicators though, including faster kinetics as well as allowing the detection of hyperpolarization and subthreshold depolarization.15 As new voltage indicators are engineered that can readily be targeted to the cell membrane, it will be advantageous to explore their use over calcium indicators. However, these methods do come with drawbacks, and a major limitation is that the quality of the data is dependent on the virus expression. Differences in expression between animals could introduce a source of bias and the virus expression does not last forever therefore the amount of time a specific animal could be studied is limited.16 Additionally, the calcium indicators used for these studies are not as accurate as electrophysiological recording in measuring neural activity.17
Despite the limitations of the method, we believe that it will provide valuable insights into the validation of novel analgesic targets. This method allows quantification of how much activity level change is required to cause a change in behavior. This could help better determine effective drug dosages. One potential use of this combined method is to identify neural activity in which part of brain the drug can specifically regulate to produce analgesic effects. With this knowledge, it is possible that we are able to combine with other new technologies (such as nanoparticles) to inject the drug to a specific area, so that potentially eliminating off-target effects. Additionally, this method can also be used to identify new targets for neuromodulation. One of the current challenges limiting clinical use of deep brain stimulation is lack of validated specific targets.18 Using this combined method, we can identify specific nuclei directly and further define how much stimulation is necessary to mediate analgesia. This information could be used to guide deep brain stimulation for chronic pain treatment. Besides different brain areas, this multidisciplinary approach could also be used to validate novel analgesic targets in other areas of both central and peripheral nervous systems.
Conclusion
Here, we show a multidisciplinary method to study real-time neural activity and pain behaviors in freely moving mice. By combining multi-channel recording, optogenetic stimulation and behavioral observation, real-time electrical activity in a targeted brain area and relevant pain behaviors can be monitored simultaneously, while optogenetic manipulation of a specific neuronal pathway is carried out.
Abbreviations
AAV, adeno-associated virus; ANOVA, analysis of variance; CCI-ION, chronic constriction injury of infraorbital nerve; ChR2, channelrhodopsin; RTPP, real-time place preference; Sp5C, spinal trigeminal nucleus caudalis.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
Acknowledgments
We thank Dr Brad Pfeiffer (UT Southwestern) for helpful comments regarding multi-channel recordings. We also thank John Cowan (3Dallas Printing) for printing of the headstage pieces.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed on the journal to which the article will be submitted; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Institutes of Health Grants K02 DE023551 (F.T.) and F31 DE029686 (J.C.).
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Häggman-Henrikson B, Liv P, Ilgunas A, et al. Increasing gender differences in the prevalence and chronification of orofacial pain in the population. Pain. 2020;161(8):1768–1775.
2. Horst OV, Cunha-Cruz J, Zhou L, Manning W, Mancl L, DeRouen TA. Prevalence of pain in the orofacial regions in patients visiting general dentists in the Northwest Practice-based REsearch Collaborative in Evidence-based DENTistry research network. J Am Dent Assoc. 2015;146(10):721–728 e3.
3. Beecroft EV, Durham J, Thomson P. Retrospective examination of the healthcare ‘journey’ of chronic orofacial pain patients referred to oral and maxillofacial surgery. Br Dent J. 2013;214(5):E12.
4. Iyer SM, Vesuna S, Ramakrishnan C, et al. Optogenetic and chemogenetic strategies for sustained inhibition of pain. Sci Rep. 2016;6:30570.
5. Kobbert C, Apps R, Bechmann I, Lanciego JL, Mey J, Thanos S. Current concepts in neuroanatomical tracing. Prog Neurobiol. 2000;62(4):327–351.
6. Peirs C, Seal RP. Neural circuits for pain: recent advances and current views. Science. 2016;354(6312):578–584.
7. Stirling LC, Forlani G, Baker MD, et al. Nociceptor-specific gene deletion using heterozygous NaV1.8-Cre recombinase mice. Pain. 2005;113(1–2):27–36.
8. Kim YS, Chu Y, Han L, et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron. 2014;81(4):873–887.
9. Liu S, Shu H, Crawford J, Ma Y, Li C, Tao F. Optogenetic activation of dopamine receptor D1 and D2 neurons in anterior cingulate cortex differentially modulates trigeminal neuropathic pain. Mol Neurobiol. 2020;57(10):4060–4068.
10. Liu S, Tang Y, Shu H, et al. Dopamine receptor D2, but not D1, mediates descending dopaminergic pathway–produced analgesic effect in a trigeminal neuropathic pain mouse model. Pain. 2019;160(2):334–344. doi:10.1097/j.pain.0000000000001414
11. Mukherjee N, Wachutka J, Katz DB. Python meets systems neuroscience: affordable, scalable and open-source electrophysiology in awake, behaving rodents.
12. Li L, Tang YJ, Sun LQ, et al. In vivo fiber photometry of neural activity in response to optogenetically manipulated inputs in freely moving mice. J Innov Opt Health Sci. 2017;10(5):1743001. doi:10.1142/S1793545817430015
13. Aharoni D, Hoogland TM. Circuit investigations with open-source miniaturized microscopes: past, present and future. Front Cell Neurosci. 2019;13:141. doi:10.3389/fncel.2019.00141
14. Baker BJ, Lee H, Pieribone VA, et al. Three fluorescent protein voltage sensors exhibit low plasma membrane expression in mammalian cells. J Neurosci Methods. 2007;161(1):32–38.
15. Kannan M, Vasan G, Pieribone VA. Optimizing strategies for developing genetically encoded voltage indicators. Front Cell Neurosci. 2019;13:53.
16. Penrod RD, Wells AM, Carlezon WA, Cowan CW. Use of adeno-associated and herpes simplex viral vectors for in vivo neuronal expression in mice. Curr Protoc Neurosci. 2015;73:4–37.
17. Hartung JE, Gold MS. GCaMP as an indirect measure of electrical activity in rat trigeminal ganglion neurons. Cell Calcium. 2020;89:102225.
18. Lozano AM, Lipsman N, Bergman H, et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019;15(3):148–160.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.