Back to Journals » Infection and Drug Resistance » Volume 16
A Multicenter Epidemiological and Pathogenic Characteristics Study of Community-Acquired Bacterial Meningitis Children in China: Results from the Chinese Pediatric Bacterial Meningitis Surveillance (CPBMS) 2019–2020
Authors Wang C, Xu H, Liu G, Liu J, Yu H, Chen B, Zheng G, Shu M, Du L, Xu Z, Huang L, Li H, Shu S, Chen Y
Received 13 April 2023
Accepted for publication 25 August 2023
Published 9 October 2023 Volume 2023:16 Pages 6587—6601
DOI https://doi.org/10.2147/IDR.S413147
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Caiyun Wang,1 Hongmei Xu,2 Gang Liu,3,4 Jing Liu,5 Hui Yu,6 Biquan Chen,7 Guo Zheng,8 Min Shu,9 Lijun Du,10 Zhiwei Xu,11 Lisu Huang,1,12 Haibo Li,13 Sainan Shu,14 Yinghu Chen,1 Corporate Authors of Chinese Pediatric Bacterial Meningitis Surveillance (CPBMS) Study Group:Dong Wang, Huiling Deng, Songting Bai, Qingwen Shan, Chunhui Zhu, Jianmei Tian, Jianhua Hao, Aiwei Lin, Daojiong Lin, Jinzhun Wu, Xinhua Zhang, Qing Cao, Zhongbin Tao, Yuan Chen, Guolong Zhu, Ping Xue, Zhengzhen Tang, Xuewen Su, Zhenghai Qu, Shiyong Zhao, Lin Pang On behalf of The CPBMS Study Group
1Department of Infectious Disease, Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, National Children’s Regional Medical Center, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Infectious Disease, Children’s Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 3Department of Infectious Diseases, Key Laboratory of Major Diseases in Children, Ministry of Education, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, People’s Republic of China; 4Research Unit of Critical Infection in Children, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 5Department of Infectious Disease, Hunan Children’s Hospital, Changsha, Hunan, People’s Republic of China; 6Department of Infectious Disease, The Children’s Hospital of Fudan University, Shanghai, People’s Republic of China; 7Department of Infection, Anhui Province Children’s Hospital, Hefei, Anhui, People’s Republic of China; 8Department of Neurology, Children’s Hospital of Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 9Department of Pediatrics, West China Second University Hospital, Sichuan University/ West China Women’s and Children’s Hospital, Chengdu, Sichuang, People’s Republic of China; 10Department of Neurology, Children’s Hospital of Shanxi, Taiyuan, Shanxi, People’s Republic of China; 11Pediatric Inpatient Ward, The 2nd Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China; 12Department of Infectious Disease, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 13Outpatient Department of Pediatrics, The First Hospital of Jilin University, Changchun, Jilin, People’s Republic of China; 14Department of Pediatric Infection and Gastroenterology, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, Hubei, People’s Republic of China; The CPBMS team: the Children’s Hospital of Zhejiang University School of Medicine; National Clinical Research Center for Child Health; National Children’s Regional Medical Center; Children’s Hospital of Chongqing Medical University; Beijing Children’s Hospital; Capital Medical University; National Center for Children’s Health; Research Unit of Critical Infection in Children; Chinese Academy of Medical Sciences, 2019RU016; Hunan Children’s Hospital; the Children’s Hospital of Fudan University; Anhui Province Children’s Hospital; Children’s Hospital of Nanjing Medical University; West China Second University Hospital; Sichuan University/ West China Women’s and Children’s Hospital; Shanxi Children’s Hospital; the 2nd Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University; Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine; the First Hospital of Jilin University; the Affiliated Children’s Hospital of Xi’an Jiaotong University; Xi’an Central Hospital; the First Affiliated Hospital of Zhengzhou University; the First Affiliated Hospital of Guangxi Medical University; Jiangxi Provincial Children’s Hospital; Children’s Hospital of Soochow University; Kaifeng Children’s Hospital; Children’s Hospital Affiliated to Shandong University; Hainan Women and Children’s medical center; Women and Children’s Hospital; School of Medicine; Xiamen University; Shanghai Children’s Medical Center; National Children’s Medical Center; Shanghai Jiaotong University School of Medicine; the First Hospital of Lanzhou University; the Second Hospital of Hebei Medical University; the Women’s and Children’s Hospital of Qinghai Province; Taiyuan Maternal and Child Health Care Hospital; the First People’s Hospital of Zunyi; Inner Mongolia People’s Hospital; the Affiliated Hospital of Qingdao University; Hangzhou Children’s Hospital; Beijing Ditan Hospital; Capital Medical University; Tongji Hospital; Tongji Medical College of Huazhong University of Science and Technology
Correspondence: Yinghu Chen, Department of Infectious Disease, Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, National Children’s Regional Medical Center, 3333 Binsheng Road, Hangzhou, Zhejiang, 310052, People’s Republic of China, Tel +86 13857154891, Email [email protected]
Objective: To explore the epidemiological and pathogenic characteristics of children with community-acquired bacterial meningitis.
Methods: A multicenter, retrospective study was conducted among CABM patients under 15 years old from 33 hospitals in China from 2019 to 2020. The medical record, laboratory, and microbiological data were collected and analyzed.
Results: A total of 1610 children with CABM were identified and presented at a median onset age of 45 days of whom 955 (59.3%) were males. CABM occurred mostly in infants < 1 year of age (84.0%, 1352/1610). In etiology-confirmed cases, the pathogens were isolated from CSF culture in 515 (32.0%), 400 (24.8%) in blood culture, and 186 (11.6%) both in CSF and blood culture. In total, 126 pathogens were identified through CSF mNGS in 330 CABM cases; 21 S. pneumoniae isolates were detected in 83 CABM cases by antigen detection method. Major pathogens were E. coli (195, 24.7%), GBS (170, 21.5%), and S. pneumoniae (157, 19.9%). GBS (29.3%, 22/75) was the first pathogen of CABM in neonates aged 0– 6 days old, while E. coli (44.7%, 76/170) in 7 to 28 days of age; S. pneumoniae (96.2%, 151/157) was the most common pathogen in > 3 months old cases. About 9.7% (19/195) strains of E. coli produced ultra‑broad‑spectrum β‑lactamases. The common intracranial imaging complications were subdural effusion and (or) empyema in 349 (21.7%), hydrocephalus in 233 (14.5%), and cerebral abscess in 178 (11.1%). A total of 389 (24.2%) cases were completely cured and 1088 (67.6%) cases improved. Among 166 patients (10.3%) with adverse outcomes, 32 cases (2.0%) died, and 37 cases (2.3%) relapsed.
Conclusion: The onset age of CABM in children is usually within 1 year of age, especially < 3 months. The primary pathogens in infants less than 3 months old are E. coli and GBS, and the dominant pathogen in children older than 3 months old is S. pneumoniae. Subdural effusion and (or) empyema and hydrocephalus are common complications. CABM should not be excluded even if CSF leukocyte counts are within normal range. Due to the low detection rate of pathogens in children with CABM, standardized CSF bacteriological examination should be paid more attention to increase the pathogen detection rate. Non‑culture CSF detection methods may facilitate pathogenic diagnosis.
Keywords: meningitis, bacterial, pathogen, childhood, diagnosis, outcome
Introduction
Community-acquired bacterial meningitis (CABM) is a global serious threat to children’s health, with 54% of new cases of bacterial meningitis worldwide occurring in children younger than 5 years of age,1 especially infants and young children with a mortality rate of 5–30% and permanent neurological sequelae in 30–50% of survivors.2 Centers for Disease Control survey data from the USA also show that the incidence of ABM decreases with age: 80.69/100,000 at <2 months of age; 6.91/100,000 at 2–23 months of age; 0.56/100,000 at 2–10 years of age; and 0.43/100,000 at 11–17 years of age.3 Data from epidemiologic surveys in four provinces of China from 2006 to 2009 showed that the overall incidence rate of probable bacterial meningitis in the population was (1.84–2.93)/100,000, and the incidence rate in children under 5 years of age was (6.95–22.30)/100,000.4
The etiology of CABM is intricately related to the child’s age, immune status, and geographical region. With the introduction of vaccines, the incidence of Neisseria meningitidis and Haemophilus influenzae meningitis has witnessed a substantial decline; likewise, the prevalence of Streptococcus pneumoniae (S. pneumoniae) meningitis has also experienced a reduction.5 Currently, the positive detection rate of the pathogenic bacteria of pediatric bacterial meningitis in China is low, among cases of meningitis with identifiable pathogens in various regions, the predominant culprits include S. pneumoniae, group B Streptococci (GBS), and Escherichia coli (E. coli.)6
The adverse outcome varies with children’s age, pathogens, treatment, underlying disease, and economic status.7 The epidemiology and etiology of CABM is constantly changing due to the introduction and expansion of conjugate vaccines and changing resistance patterns of pathogenic bacteria.8,9 Therefore, a complete understanding of the epidemiological features, clinical characteristics, the composition of pathogens, common complications, and antimicrobial susceptibility of CABM in children in China, as well as early and rational pathogenic treatments are key to improving prognosis. There are already some regions in China where information on trends in the incidence of CABM in children, clinical features, epidemiological characteristics, and burden of disease is routinely collected and regularly updated, but lack of multicenter studies.10–12 From January 2019 to December 2020, Pediatric Bacterial Meningitis Surveillance (CPBMS) initiated a nationwide study in 33 tertiary hospitals in Grade A to collect clinical and laboratory data on CABM in hospitalized children to learn the profile of diagnosis and management.
Materials and Methods
Study Design and Procedures
Between 2019 and 2020, we conducted a national multicenter retrospective study of children (<15 years of age) diagnosed with etiology-confirmed and probable CABM across seven geographical divisions of China. A total of 33 tertiary Grade A hospitals in 23 provinces (27 cities) participated in this study. The location and population of participating hospitals are shown in Figure 1. All the hospitals must have laboratory bacterial culture and antimicrobial susceptibility assessment ability and volunteer to participate in the research. Participating hospitals included 13 in East China, 6 in North China, 4 each in Central and Northwest China, 3 in Southwest China, 2 in Southern China, and 1 in Northeast China. This study received approval from the Committee of Children’s Hospital of Zhejiang University School of Medicine (2019-IRB-094). The subjects included in the study were numbered in a unified manner, and all private information such as the patient’s name, ID number, address, and contact phone number was not involved. The waiver for parental consent to review medical records was granted by the hospital’s ethics committee. The handling of patient data confidentiality strictly followed the rules set by the institution and complied with the Declaration of Helsinki.
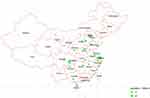 |
Figure 1 Locations of participating hospitals (dots) in this study. |
Case Definitions, Classification, and Study Populations
Suspected bacterial meningitis was defined as an illness with an acute onset of fever (usually >38.5°C rectal or 38.0°C axillary), headache, and one of the following signs: neck stiffness, altered mental status with no other alternative diagnosis, or other meningeal signs. Probable cases were defined as having no bacteria pathogen detected and CSF examination revealing at least 1 of the following (turbid appearance; CSF leukocytosis (>100 cells/μL); leukocytosis (10–100 cells/μL AND either an elevated protein (>1.0g/L) or decreased glucose (<2.2 mmol/L). If CSF protein and glucose results are not available, diagnose using the first 2 conditions (turbid appearance or leukocytosis >100 cells/μL). Etiology-confirmed cases were considered for culturing or identifying a bacterial pathogen [ie, by Gram stain or antigen detection methods or metagenomics next-generation sequencing (mNGS)] in the CSF or blood in a child with suspected or probable bacterial meningitis. Standardized case definitions for suspected, probable, and confirmed bacterial meningitis were based on World Health Organization (WHO, 2003) recommendation.13,14
Inclusion Criteria
The enrolled patients met the following three criteria.
Exclusion Criteria
The patient should be excluded if any exclusion criteria are met.
Data Collection
Medical records, laboratory, and microbiological data of all enrolled cases were systematically assessed and recorded by locally trained pediatric researchers using a standard case report form and entered into Good Clinical Data Management System. According to the International Classification of Diseases Tenth Revision diagnostic codes and Systematized Nomenclature of Medicine codes, these cases were determined by searching the electronic medical records for the discharge diagnoses “acute bacterial meningitis” or “acute purulent meningitis” or “intracranial infection” or “central nervous system infection” or “meningitis” in each collaborating hospital from January 2019 to December 2020. Only the first medical record was included for analysis if the patient was hospitalized multiple times for the same diagnosis.
We retrospectively recorded baseline information on demographics, the time of admission and discharge, clinical characteristics, craniofacial and spinal anatomical abnormalities associated with central nervous system infection, causative microorganisms, laboratory findings, cranial imaging, treatment, antibiotic susceptibility test results, and outcomes.
Laboratory Methods Analysis
All suspected bacterial meningitis patients had received LP after admission and had cerebrospinal fluid (CSF) examinations including leukocyte and red blood cell count, differential leukocyte count, CSF biochemical, CSF culture, and Gram staining), as well as blood routine test, blood cultures, and cranial imaging. CSF and blood specimens were cultured, and pathogens were identified using standard methods as previously described. Microbiological specimens were cultured by BACT/ALERT 3D 240 automatic blood culture instrument (Mérieux, France), and bacteria were identified by the automatic bacterial identification system (VITEK Compact 2, France) at each surveillance center. Drug susceptibility testing of S. pneumoniae to penicillin was supplemented with the E-test method, and the results were interpreted according to the guidelines of the American Board for Clinical Laboratory Standardization. The culture and identification procedures are carried out following the National Clinical Laboratory Procedures. Normal values of CSF parameters refer to the National Clinical Laboratory Procedures.15 Antigen detection and molecular biological detection methods in this study were performed in only some of the suspected bacterial meningitis patients who were in serious status requiring earlier identification of pathogens or patients with empirical antibiotics therapy failed or patients with negative initial CSF Gram stains whose CSF and(or) blood cultures at 72 h incubation were negative (including BinaxNOW for Streptococcus pneumoniae detection and mNGS).
Positive CSF mNGS results were interpreted according to the Expert Consensus on the Use of Cerebrospinal Fluid Macrogenomics Second-Generation Sequencing for Infectious Diseases of the Central Nervous System,16 and diagnosis of coagulase-negative staphylococcal (CoNS) CABM was based on the Centers for Disease Control and Prevention’s definition of bloodstream infection surveillance.17
Clinical Outcome
We considered the clinical situation on the discharge day as the clinical outcome. Cured was defined as relief of clinical symptoms and signs, negative CSF test results, and no cranial imaging complications during hospitalization.18 Disease improvement was defined as relief of clinical symptoms and signs, negative CSF culture, blood inflammatory indicators normal, CSF leukocyte count approximately normal, CSF protein and (or) glucose level not returned to normal, and no progress in cranial imaging complications during hospitalization.18 Unhealed was defined as no improvement in clinical symptoms, abnormal CSF test results, and neurological complications on cranial imaging. Death was defined as death that occurs during hospitalization or within 3 days of discharge from the hospital after the abandonment of treatment. Relapse was defined as CABM due to the same pathogenic bacteria after the completion of antimicrobial therapy from the initial episode.19 Adverse outcomes included unhealed, relapse, death, being discharged automatically, abandoned treatment or being transferred to other hospitals for further treatment.
Statistical Analysis
SPSS 19.0 software (IBM Corporation, Armonk, NY, USA) was used for statistical analysis. Categorical data were expressed in frequency and percentage, and the chi-square test and Fisher’s exact test were used to analyze the statistical difference. Continuous data were expressed as median (Q1, Q3) for non-normal distribution, and Kruskal–Wallis H-test was used to analyze statistical difference, with P value was set at less than 0.05 with statistical significance.
Results
Baseline Clinical Characteristics
From Jan 1, 2019, to Dec 31, 2020, 1683 children (<15 years old) with CABM were enrolled for screening. 73 cases (4.3%) were excluded due to non-bacterial, post-traumatic, hospital-acquired meningitis, and two episodes for one person. A flow diagram of the cases included is listed in Figure 2. A total of 1610 CABM patients with CABM met the inclusion criteria. A total of 955 (59.3%) of the enrolled patients were males and 655 (40.7%) were females. More than half of the patients (65.2%) were <3 months and 84.0% of patients were <1 year of age. The median age at presentation was 45 (15, 164) days. All cases were classified into five age groups: 588 (36.5%)<28 days of age (including 208 <7 days of age, 380 from 7 to ≤28 days of age), 462 (28.7%) from 28 days to <3 months of age, 302 (18.8%) from 3 months to <1 year of age, 156 (9.6%) from 1 to <5 years of age, and 102 (6.4%) from 5 to <15 years of age (Table 1). There was a sharp decline in CABM occurrence with increasing ages. Of 1610 cases, 790 were etiology-confirmed and 820 were probable cases, with a hospital stay of 24 (17, 37) d and hospital costs of 38 (22, 63) thousand yuan. The CABM patients caused by GBS had the longest hospitalization lengths (P<0.05). The total number of children diagnosed with CABM was 963 (59.8%) in 2019 and 647 (40.2%) in 2020. The proportion of CABM children in etiology-confirmed in 2020 was significantly higher than those in 2019 [53.5% (346/647) vs.46.1% (444/963), χ2=8.42, P=0.004]. The hospitalization time and cost of CABM children in the etiology-confirmed group were significantly higher than those in the probable group [26(18, 39) vs 23(16, 35) d, 41(26, 66) vs. 34(21, 59) thousand-yuan, Z=−3.58, −4.53, All P<0.001].
 |
Table 1 Age Distribution of Children with CABM, n (%) |
 |
Figure 2 Flowchart of study participants. |
CABM caused by E. coli and GBS occurred throughout the year, with S. pneumoniae meningitis concentrating in winter (November, December, and January) and 3 cases of Neisseria meningitidis meningitis occurring in July, September, and December, respectively.
Distribution of Pathogens in Different Age Groups
To investigate the potential correlation between age and CABM pathogens, we recorded the different infectious agents found in different age groups. As shown in Table 2, the common pathogens in the <28 days old group and the 28 days to <3 months old group were E. coli and GBS. GBS (29.3%, 22/75) was the dominating pathogen, followed by E. coli (25.3%, 19/75) in 0–6 days newborns and E. Coli (44.7%, 76/170) was the leading pathogen, followed by GBS (27.1%, 46/170) in 7 to 28 days newborns. The predominant pathogens in the 3 months to <1 year of age group were S. pneumoniae, E. coli, and GBS; the first pathogen of children in the age group 1 to <5 years and 5 to <15 years was S. pneumoniae. There were 9.7% (19/195) strains of E. coli producing ultra‐broad‐spectrum β‐lactamases. Of the 26 Haemophilus influenzae strains detected, there were 80.8% (21/26) in the age group 3 months to <1 year, and 11.5% (3/26) and 7.7% (2/26) in the age group 1 to <5 years and ≥5 years, respectively.
 |
Table 2 Pathogen Composition of CABM Children in Different Age Groups |
Coagulase-negative staphylococci (CoNS) accounted for 36.4% (4/11) in each of the <28 days old group and 28 days to <3 months old group. Non-typhoid Salmonella accounted for 63.6% (7/12) of the 3 months to <1-year-old group. A total of 3 Neisseria meningitides were detected in 2019 and none in 2020.
Pathogen Testing Samples Types and Results
Of the enrolled cases, 1610 blood and 1598 (99.3%) CSF culture results were recorded, and CSF culture was not performed in 12 (0.7%) children due to certain clinical conditions (low specimen volume or coagulation). CSF and blood culture positive rates were 32.2% (515/1598) and 24.8% (400/1610), respectively, with 9 cases of positive bone marrow cultures. Both CSF and blood cultures were positive in 23.5% (186/790) of the children in the etiology-confirmed group. In 820 probable cases, a pathogen was not identified.
In this study, antigen detection and molecular biological detection methods were performed in only some of the suspected bacterial meningitis patients who were in serious status requiring earlier identification of pathogens or patients with empirical antibiotics therapy failed, or patients with negative initial CSF Gram stains whose CSF and (or) blood cultures at 72 h incubation were negative. The positivity detection rates of CSF mNGS and S. pneumoniae antigen were 38.2% (126/330) and 25.3% (21/83), respectively. Of the 126 cases with positive mNGS results, the positive rates of CSF culture and blood culture were 8.2% (27/330) and 4.2% (14/330); both CSF and blood cultures were positive in 3.6% (12/330); 22.1% (73/330) cases with negative CSF or blood culture results. Of the 21 cases with positive S. pneumoniae antigen testing results in CSF, the positive rates of CSF culture or blood culture were both 6.0% (5/83); both CSF and blood cultures were positive in 3.6% (3/83). Only 11 cases with positive Gram staining results were collected.
Laboratory Parameters
The CSF leukocyte count was recorded in 1511 (93.9%), glucose concentration in 1558 (96.8%), and protein concentration in 1548 (96.1%) in the first LP of 1610 children. We observed a median CSF leukocyte count of 440 (100, 1923) cells/μL, a median percent of neutrophils 64.0 (35.0, 77.3) %, a median CSF protein of 1.6 (0.9, 2.8) g/L, and a median CSF glucose of 1.7 (1.0, 2.2) mmol/L. The highest CSF leukocyte count (142×103/ cells /μL) was in E. coli cases in the first LP, and the lowest CSF leukocyte count (6/ cells /μL) was in L. monocytogenes cases. The median CSF leukocyte count in the first LP was 1215.0 (112.0, 6250.0) cells/μL in E. coli cases, 1000.0 (185.0, 2920.0) cells/μL in GBS cases, 780.0 (192.5, 2342.5) in the S. pneumoniae cases and 464.5 (236.8, 1066.3) cells/μL in the L. Monocytogenes group, but no significant difference was found (Z=5.720, P = 0.126).
There were 32 cases (2.1%) with normal CSF leukocyte count and 123 cases (7.9%) with normal CSF glucose concentration in the first LP in etiology-confirmed cases. Of the 32 cases with normal CSF leukocyte counts in the etiology-confirmed group, 15 cases (46.9%) were neonates; pathogens were identified: 19 (59.4%) had positive CSF cultures, 9 (28.1%) in both positive blood and CSF cultures, 4 (12.5%) in positive CSF mNGS. In 32 cases, E. coli was found in 11 cases (34.4%), Streptococcus aureus in 8 cases (25.0%), S. pneumoniae in 5 cases (15.6%), Klebsiella pneumoniae in 4 cases (12.5%), L. Monocytogenes, and non-typhoid Salmonella in every 2 cases (6.3%). Among 123 cases with CSF glucose concentration (>2.8 mmol/L) in the first LP in the etiology-confirmed group, 96 cases (78.0%) were <1 year old, including 26 cases <28 days and 31 cases 28 days to <3 months of age. Among 123 cases, pathogens were identified: only 67 cases (54.5%) by CSF culture, 37 cases (30.0%) by both the CSF and the blood cultures, 18 cases (14.6%) by CSF mNGS, and 1 case (0.8%) by CSF antigen detection.
As shown in Table 3, the proportions of CSF leukocyte count >1000 cells/μL and CSF glucose concentration (<1.1 mmol/L) in the first LP were higher in the etiology-confirmed group than in the probable group; the proportions of normal leukocyte count and glucose concentration (>2.8 mmol/L) in CSF before discharge were low.
 |
Table 3 Comparison of CSF Parameters in Children with CABM in the Etiology-Confirmed and Probable Groups |
The highest serum white blood cell (WBC) count and C-reactive protein (CRP) were documented in the S. pneumoniae group. The median WBC count and CRP were 21.54 x109/L (15.92–28.62) and 98.54g/L (42.83, 124.64) in the S. pneumoniae group.
Antimicrobial Susceptibility Analysis of Isolated Strains
There were 129 E. coli isolates recorded antimicrobial susceptibility data. E. coli were susceptible to meropenem [96.3% (104/108)], Piperacillin-tazobactam [97.6% (41/42)], and Cefoperazone-sulbactam [92.3% (24/26)]; the susceptibility rates to cefotaxime and ceftriaxone were 64.3% (36/56) and 52.4% (54/103), respectively.
Among 157 S. pneumoniae strains isolated, only 91, S. pneumoniae antimicrobial susceptibility data were registered (84 from CSF, 7 from blood). Seven blood-derived S. pneumoniae strains were determined for antimicrobial susceptibility according to the breakpoint of parenteral administration of meningitis strain. We found that 69 S. pneumoniae isolates that underwent penicillin susceptibility testing: 28 (40.6%) with susceptibility and 41 (59.4%) with resistance to penicillin. The susceptibility rates of S. pneumoniae to levofloxacin, moxifloxacin, rifampicin, and chloramphenicol were 81.5% (22/27), 82.4% (14/17), 96.2% (25/26), and 91.3% (21/23). The susceptibility rates of S. pneumoniae to cefotaxime, meropenem, and ceftriaxone were 56.1% (23/41), 51.1% (23/45), and 63.5 (33/52), respectively. No S. pneumoniae isolates were resistant to vancomycin, linezolid, levofloxacin, or ertapenem. S. pneumoniae were completely resistant to erythromycin [100.0% (31/31)].
81 GBS isolates recorded antimicrobial susceptibility data. No GBS was resistant to ampicillin, ceftriaxone, cefotaxime, and meropenem, and was highly susceptible to penicillin [98.6% (69/70)], vancomycin [98.5% (67/68)], and linezolid [98.4% (61/62)], which was completely resistant to erythromycin and doxycycline.
Intracranial Imaging Complications in CABM Children
Intracranial complications were detected by cranial magnetic resonance imaging (MRI) and/or cranial computed tomography (CT) during hospitalization in 760 (47.2%) children, and the common complications were subdural effusion and (or) empyema in 349 (21.7%), hydrocephalus in 233 (14.5%), brain abscess in 178 (11.1%), and other cerebrovascular diseases in 174 (10.8%) (including encephalomalacia, cerebral infarction, and encephalatrophy), which was shown in Table 4. Subdural effusion and (or) empyema and hydrocephalus occurred mainly in children <1 year old, 93.9% (325/346) and 93.0% (212/228), respectively. More than 1 intracranial imaging abnormality was present in 306 (19.0%) children. 58 cases (3.6%) were complicated by ventricular meningitis, and pathogens were identified in 36 of the children (E. coli in 16 cases).
 |
Table 4 Clinical and Laboratory Characteristics of Children with CABM in the Etiology- Confirmed and Probable Groups |
Adverse Outcome
Adverse outcomes occurred in 166 (10.3%) children, of which 137 cases (82.5%) were less than 1 year old. 32 patients (2.0%) died during hospitalization, 14 (43.8%) were under 3 months of age (12 neonates), and 24 patients (75.0%) were under 1 year of age. Deaths and intracranial imaging complications of CABM in the seven geographic divisions are shown in Table 5. In this survey, 59.4% (19/32) of CABM patients who died were positive for pathogens, and the top three pathogens causing death were S. pneumoniae, GBS, and E. coli.
 |
Table 5 Death and Intracranial Imaging Complications of CABM in Different Regions, n (%) |
Relapse occurred in 37 (2.3%) children with CABM, 25 of whom occurred within 3 weeks of antibiotic withdrawal and 12 cases occurred after 3 weeks. There were 16 relapses of CABM in the etiology-confirmed group of children, of which 9 cases were E. coli meningitis, 3 of GBS, and 5 of other rare pathogens. 35 CABM patients with improvement or recovery relapsed after antibiotic discontinuation (32 cases in improvement and 3 cases in recovery). During the investigation, 47 patients were unhealed, and 50 cases were discharged automatically, abandoned treatment, or transferred to other hospitals for further treatment. The proportion of children discharged with a diagnosis including severe sepsis and/or multiple organ dysfunction syndrome (MODS) was 34.2% (551/1610).
The incidences of subdural effusion and (or) empyema, brain abscess, and ependymitis in the etiology-confirmed group were significantly higher than those in the probable group (26.2% (207/790) vs 17.3% (142/820), 13.0% (103/790) vs 9.1% (75/820), 4.6% (36/790) vs 2.7% (22/820), χ2 =18.71, 6.19, 4.07, all P<0.05), but no significant difference in the adverse outcomes, mortality, and relapse between two groups (all P>0.05).
Treatment and Hospital Stay in CABM Children
71.7% (1155/1610) of CABM children were pretreated with antibiotics before admission. Most of the patients with infantile CABM were initially treated with a third-generation cephalosporin. 3.3% (53/1610) of CABM patients had performed LP examinations in primary hospitals. Once CSF culture results were obtained, the local clinician would choose a more appropriate antibiotic and medication time based on the pathogen, combined with the local antimicrobial susceptibility of isolated strains.
Discussion
CABM is one of the common nervous system infectious diseases with a variable clinical presentation and high disability and mortality rates in children worldwide. This research showed that the onset age of CABM in children was mostly <1 year, especially <3 months old. This finding was consistent with a multicenter retrospective study in China on pathogens of bacterial meningitis in children >28 days old and the study conducted in the United State from 1998 to 2007.3,6 The higher incidence of CABM in young infants may be due to immature humoral and cellular immunity, phagocytosis, and an incomplete blood–brain barrier.20
The pathogens of CABM are related to the age and immune status of children.21 In this study, our findings indicate that the common pathogens in neonates and young infants in the <3-months-old groups were E. coli and GBS, which were consistent with the pattern observed in Europe, the United Kingdom, Ireland, and North America.3,22–24 We likely underestimated the incidence of CABM caused by GBS due to limitations in case ascertainment and specimen collection and processing, intrapartum antibiotic prophylaxis. S. pneumoniae was the most common cause of CABM in children after the neonatal period which is consistent with current pathogenic trends.25,26 This study had shown that the composition of pediatric CABM pathogensvaried with age consistent with previous studies,6,25 and understanding of the distribution of pathogens in CABM cases of different age groups which provided the targeted selection of antimicrobial medications.
A total of 26 strains of H. influenzae were detected in this study, of which 21 strains were identified from 3 months to 1 year of age; we detected only 3 strains of Neisseria meningitidis in infants of <4 months of age, and no strain was detected in 2020. The significant decrease in the incidence of H. influenzae and Neisseria meningitidis of CABM in children may be due to the introduction of protein conjugate vaccines and measures implemented worldwide to mitigate the spread of SARS-CoV2 (such as social distancing, wearing the mask, online classes at home) after 2019.27,28 Previous literature had shown the coverage of H. influenzae and Neisseria meningitidis vaccines were 55.9% and 90% which underlined the importance of vaccination.29,30 CABM caused by E. coli and GBS occurred throughout the year, with S. pneumoniae meningitis concentrating in winter (November, December, and January) and 3 cases of Neisseria meningitidis meningitis occurring in July, September, and December, respectively.
CSF analysis was shown to remain the principal contributor to the final CABM diagnosis, and CSF culture was the gold standard for CABM diagnosis. The positive CSF culture rate of children with CABM in our study was only 32.2%, probably due to narrowing of the vertebral space in the young children which resulted in difficulty in collecting CSF, some patients were extremely uncooperative, and too small volume of CSF were collected which resulted in CSF culture failure. In addition, the antimicrobial administration before LP was also a cause of CSF culture failure.31,32 In this study, a total of 790 cases (49.1%) of pathogenic bacteria were detected by culture or molecular detection techniques, of which the positive rates of CSF and blood culture cultures were 32.2% and 25.0%, respectively, while the positive rate of both CSF and blood cultures was only 23.5%. Among CSF culture-negative cases, pathogens were detected in CSF in 73 and 8 cases by mNGS and antigen detection techniques, respectively. Molecular techniques (antigen detection, nucleic acid detection, and mNGS) should be selected as early as possible which may effectively improve the pathogens detection rate in CABM, especially when the clinical manifestations are highly suggestive of infection but the pathogens remain ambiguous.33
Gram staining is a simple, rapid, economical, and practical routine detection method in clinical laboratories. A precise description of bacterial morphology is important to determine the bacteria and their genera, therefore guiding clinical diagnosis and early therapy.34,35 One guideline recommends that all patients with suspected bacterial meningitis should have a Gram stain of cerebrospinal fluid.36 In this study, only 11 cases (0.7%) of CSF Gram staining smear results were collected, which were consistent with the results of the subsequent spinal fluid culture. Therefore, a timely smear after LP along with early positive alarm results of CSF and blood culture can be used as the basis for early treatment. Gram staining of CSF is an important supplement to CSF culture in children suspected of CABM, which can improve the detection rate of pathogens. In etiology-confirmed cases, the bacterial load is high, the infection continues to progress, the corresponding hospitalization time is longer and the hospitalization expenses are higher; the incidence of subdural effusion and/or empyema, brain abscess, and ependymitis were significantly higher than those of probable cases. These factors indicated that appropriate antibiotics should be considered as early as possible, which highlights the importance of pathogenic bacteria detection in guiding disease treatment.
In this study, 47.2% of CABM cases had intracranial imaging complications associated with CABM within hospitalization, subdural effusion and (or) empyema, hydrocephalus, and brain abscesses are common complications. Compared with older children, subdural effusion was more common in children <1 year old, which was consistent with previous studies.37,38 Subdural effusion and/or empyema occurred in 349 CABM cases, and most children are usually asymptomatic which were absorbed spontaneously during follow-up, rarely requiring medical intervention which was consistent with Snedeker’s research result.39 Systemic complications such as severe sepsis and/or multiple organ dysfunction syndrome (MODS) occurred in 34.2% of CABM cases in this study, which was higher than previous USA studies.40 CABM cases who developed systemic complications had complex clinical presentations and some patients had significantly higher rates of sequelae. Therefore, under the premise of effective anti-infection, clinicians must pay attention to monitor the changes of the condition, timely detection of complications, and active management, which is expected to reduce the disability and death rate of children’s CABM, and thus improve the prognosis of children.
Among the 790 CABM children in the etiology-confirmed group, 32 cases (4.3%) had the normal first CSF leukocyte count (including 15 neonates), which was higher than foreign results.41 Previous studies have suggested that the normal first CSF leukocyte count of CABM children was associated with a too-short time between CABM symptoms and first LP, antibiotics therapy, compromised immune function, and severity of disease.42 Once CABM is highly suspected clinically, for children with the normal first CSF leukocyte counts, considering that it takes time for the immune response to CSF leukocyte infiltration following pathogens infection, we need to consider further CSF molecular biology assays to increase the positive detection rate of pathogens, and the appropriate antimicrobial treatment in combination with the patient’s age, risk factors, the immune status, the severity of the disease, and local resistance rates of dominant pathogen as early as possible.
The issue of S. pneumoniae resistance is increasingly becoming a significant concern, particularly among pediatric patients in Asia. In the present study, the findings indicate that S. pneumoniae isolates exhibit low sensitivity to β-lactam antibiotics, with only 40.6% exhibiting sensitivity to penicillin, while 63.5%, 56.1% of isolates showed sensitivity to ceftriaxone, cefotaxime, which were all significantly lower than foreign isolates,43 slightly higher than the 2013–2017 national multicenter study.44 S. pneumoniae isolates exhibited complete resistance to erythromycin, which was following the erythromycin-resistant rate reported in China by the Asian Drug-Resistant Bacteria Detection Network.45 In this study, E. coli showed high sensitivity to Meropenem, Piperacillin-tazobactam, and Cefoperazone-sulbactam (all>90%). Approximately, 50% of E. coli isolates were sensitive to cefotaxime and ceftriaxone, which is consistent with the previous study.28,46,47 There were 9.7% (19/195) strains of E. coli producing ultra‐broad‐spectrum β‐lactamases. All GBS strains were sensitive to ampicillin, ceftriaxone, cefotaxime, and meropenem, with high susceptibility to penicillin, vancomycin, and linezolid.
One of the most significant findings of this study was that pathogens distribution differed by age, which implied diverse preferences for infection or immunity among pathogens. Further mechanistic studies should be focused on the host and pathogen interactions in individuals of different ages. Furthermore, the invasiveness of different pathogens and clinical symptoms caused by infection with different pathogens vary greatly. Monitoring the pathogen’s distribution in children with CABM is important for the development of clinical treatment. By combining epidemiological and laboratory data for bacterial diseases, we can gain insights not only into population dynamics and transmission patterns but also into how to timely adjust immunization strategies.
In total, patients suspected of central nervous system infections should receive LP for CSF microscopy, biochemistry, and culture examination when contraindications had been ruled out on clinical grounds. Non-culture tests should be considered for patients who need earlier identification of pathogens, patients who were in severe status and had previously received antibiotics, or patients who had negative initial CSF Gram stain with or without negative culture at 72 h incubation. Empiric antibiotic treatment needs to be adjusted according to the patient’s age, risk factors, and regional epidemiology.
This was a retrospective multicenter study that did not involve the onset, disease progression, and antibiotic application of CABM for various reasons. Certain children had been hospitalized in other hospitals before this admission, so the clinical and laboratory data (including antimicrobial susceptibility) in some cases are incomplete; lack of follow-up data. Further large-scale prospective research should be carried out in the future.
Conclusion
The onset age of CABM in children is usually within 1 year of age, especially <3 months. The primary pathogens in infants less than 3 months old are E. coli and Group B Streptococcus, and the dominant pathogen in children older than 3 months old is S. pneumoniae. Subdural effusion and (or) empyema and hydrocephalus are common complications. CABM should not be excluded even if CSF leukocyte counts are within the normal range. Due to the low detection rate of pathogens in children with CABM, standardized CSF bacteriological examination should be paid more attention to increase the pathogen detection rate. Non‑culture CSF detection methods may facilitate pathogenic diagnosis.
Abbreviations
CABM, Community-acquired bacterial meningitis; CSF, cerebrospinal fluid; mNGS, Metagenomic Next-Generation Sequencing; LA, Latex agglutination; E. coli, Escherichia coli; S. pneumoniae, Streptococcus pneumoniae; GBS, Group B Streptococcus; LP, lumbar puncture.
Ethics Approval and Consent to Participate
All patients’ identifiers were removed from the data before extraction from the database. No personal identifiers were used during the analysis and presentation. This study received approval from the Committee of Children’s Hospital of Zhejiang University School of Medicine (2019-IRB-094).
Acknowledgments
We thank all the clinical and research staff for their participation in the CPBMS Work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by a grant from the National Science Foundation of China (82071812 and 82371829) and a Special fund of the Central guiding local Scientific and Technological Development [The Independent Design Project of National Clinical Research Center for Child Health] (S20A0003).
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. Feigin VL, Nichols E, Alam T. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):459–480. doi:10.1016/S1474-4422(18)30499-X
2. Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(5):317–328. doi:10.1016/S1473-3099(10)70048-7
3. Thigpen MC, Whitney CG, Messonnier NE, et al. Bacterial meningitis in the United States, 1998–2007. N Engl J Med. 2011;364(21):2016–2025. doi:10.1056/NEJMoa1005384
4. Li Y, Yin Z, Shao Z, et al. Population-based surveillance for bacterial meningitis in China, September 2006-December 2009. Emerg Infect Dis. 2014;20(1):61–69. doi:10.3201/eid2001.120375
5. Lundbo LF, Benfield T. Risk factors for community-acquired bacterial meningitis. Infect Dis. 2017;49(6):433–444. doi:10.1080/23744235.2017.1285046
6. Li C, Feng WY, Lin AW, et al. Clinical characteristics and etiology of bacterial meningitis in Chinese children >28 days of age, January 2014-December 2016: a multicenter retrospective study. Int J Infect Dis. 2018;74:47–53. doi:10.1016/j.ijid.2018.06.023
7. Kim KS. Acute bacterial meningitis in infants and children. Lancet Infect Dis. 2010;10(1):32–42. doi:10.1016/S1473-3099(09)70306-8
8. Rodgers E, Bentley SD, Borrow R, et al. The global meningitis genome partnership. J Infect. 2020;81(4):510–520. doi:10.1016/j.jinf.2020.06.064
9. Wall EC, Chan JM, Gil E, Heyderman RS. Acute bacterial meningitis. Curr Opin Neurol. 2021;34(3):386–395. doi:10.1097/WCO.0000000000000934
10. Dong BQ, Yang JY, Lin M, et al. 广西急性脑炎脑膜炎症候群的监测与研究 [Surveillance and research on acute meningitis, encephalitis syndrome in Guangxi, China]. Zhonghua Yu Fang Yi Xue Za Zhi. 2011;45(6):527–530. Chinese
11. Yang Y, Leng Z, Shen X, et al. Acute bacterial meningitis in children in Hefei, China 1990–1992. Chin Med J. 1996;109(5):385–388.
12. Mao FF, Wang J, Li JP, Yu XF. Aetiological spectrum and antibiotic susceptibility pattern of bacterial meningitis in infants and children in Hangzhou, China. Acta Paediatr. 2005;94(8):1162–1163. doi:10.1111/j.1651-2227.2005.tb02065.x
13. World Health Organization. WHO-recommended standards for surveillance of selected vaccine preventable diseases; 2003. Available from: http://www.measlesrubellainitiative.org/wp-content/uploads/.
14. Nakamura T, Cohen AL, Schwartz S, et al. The global landscape of pediatric bacterial meningitis data reported to the world health organization-coordinated invasive bacterial vaccine-preventable disease surveillance network, 2014–2019. J Infect Dis. 2021;224(3):S161–s173. doi:10.1093/infdis/jiab217
15. Hong S, Yusan W, Ziyu S. National Guide to Clinical Laboratory Procedures.
16. Cytology CSo IDaCF. Expert consensus on clinical application of metagenomic next‑generation sequencing of cerebrospinal fluid in the diagnosis of infectious diseases of the central nervous system. Chin J Neurol. 2021;54(12):1234–1240.
17. O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51(Rr–10):1–29.
18. Department CPsLAGL. Clinical Disease Diagnostic Basis Criteria for Cure and Improvement.
19. Expert consensus on diagnosis and treatment of community acquired bacterial meningitis in children. Zhonghua Er Ke Za Zhi. 2019;57(8):584–591. doi:10.3760/cma.j.issn.0578-1310.2019.08.003
20. Philbin VJ, Levy O. Developmental biology of the innate immune response: implications for neonatal and infant vaccine development. Pediatr Res. 2009;65(5 Pt 2):98r–105r. doi:10.1203/PDR.0b013e31819f195d
21. van de Beek D, Brouwer MC, Koedel U, Wall EC. Community-acquired bacterial meningitis. Lancet. 2021;398(10306):1171–1183. doi:10.1016/S0140-6736(21)00883-7
22. Holt DE, Halket S, de Louvois J, Harvey D. Neonatal meningitis in England and Wales: 10 years on. Arch Dis Child Fetal Neonatal Ed. 2001;84(2):F85–89. doi:10.1136/fn.84.2.F85
23. Okike IO, Johnson AP, Henderson KL, et al. Incidence, etiology, and outcome of bacterial meningitis in infants aged <90 days in the United Kingdom and Republic of Ireland: prospective, enhanced, national population-based surveillance. Clin Infect Dis. 2014;59(10):e150–157. doi:10.1093/cid/ciu514
24. Snoek L, Gonçalves BP, Horváth-Puhó E, et al. Short-term and long-term risk of mortality and neurodevelopmental impairments after bacterial meningitis during infancy in children in Denmark and the Netherlands: a nationwide matched cohort study. Lancet Child Adolesc Health. 2022;6(9):633–642. doi:10.1016/S2352-4642(22)00155-9
25. Agrawal S, Nadel S. Acute bacterial meningitis in infants and children: epidemiology and management. Paediatr Drugs. 2011;13(6):385–400. doi:10.2165/11593340-000000000-00000
26. Ouchenir L, Renaud C, Khan S, et al. The epidemiology, management, and outcomes of bacterial meningitis in infants. Pediatrics. 2017;140(1). doi:10.1542/peds.2017-0476
27. Pinto TCA, Costa NS, Oliveira LMA. World meningitis day and the world health organization’s roadmap to defeat bacterial meningitis in the COVID-19 pandemic era. Int J Infect Dis. 2021;107:219–220. doi:10.1016/j.ijid.2021.04.070
28. Fu P, Xu H, Jing C, et al. Bacterial epidemiology and antimicrobial resistance profiles in children reported by the ISPED program in China, 2016 to 2020. Microbiol Spectr. 2021;9(3):e0028321. doi:10.1128/Spectrum.00283-21
29. Lei C, Huaqing W, Jingshan Z, et al. National immunization coverage survey in China after integrated more vaccines into EPI since 2008. Chin J Vaccines Immun. 2012;18(5):419–424.
30. Ning G, Yin Z, Li Y, Wang H, Yang W. Cost-effectiveness of the Haemophilus influenzae type b vaccine for infants in mainland China. Hum Vaccin Immunother. 2018;14(1):36–44. doi:10.1080/21645515.2017.1385687
31. Prasad K, Sahu JK. Cerebrospinal fluid lactate: is it a reliable and valid marker to distinguish between acute bacterial meningitis and aseptic meningitis? Crit Care. 2011;15(1):104. doi:10.1186/cc9396
32. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23(3):467–492. doi:10.1128/CMR.00070-09
33. Wilson MR, Sample HA, Zorn KC, et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med. 2019;380(24):2327–2340. doi:10.1056/NEJMoa1803396
34. Nesher L, Hadi CM, Salazar L, et al. Epidemiology of meningitis with a negative CSF Gram stain: under-utilization of available diagnostic tests. Epidemiol Infect. 2016;144(1):189–197. doi:10.1017/S0950268815000850
35. Polage CR, Cohen SH. State-of-the-art microbiologic testing for community-acquired meningitis and encephalitis. J Clin Microbiol. 2016;54(5):1197–1202. doi:10.1128/JCM.00289-16
36. Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39(9):1267–1284. doi:10.1086/425368
37. Namani SA, Koci BM, Milenković Z, et al. Early neurologic complications and long-term sequelae of childhood bacterial meningitis in a limited-resource country (Kosovo). Childs Nerv Syst. 2013;29(2):275–280. doi:10.1007/s00381-012-1917-3
38. Zainel A, Mitchell H, Sadarangani M. Bacterial meningitis in children: neurological complications, associated risk factors, and prevention. Microorganisms. 2021;9(3):535. doi:10.3390/microorganisms9030535
39. Snedeker JD, Kaplan SL, Dodge PR, Holmes SJ, Feigin RD. Subdural effusion and its relationship with neurologic sequelae of bacterial meningitis in infancy: a prospective study. Pediatrics. 1990;86(2):163–170. doi:10.1542/peds.86.2.163
40. Adil SM, Hodges SE, Charalambous LT, et al. Paediatric bacterial meningitis in the USA: outcomes and healthcare resource utilization of nosocomial versus community-acquired infection. J Med Microbiol. 2021;70(1). doi:10.1099/jmm.0.001276
41. van Soest TM, Chekrouni N, van Sorge NM, Brouwer MC, van de Beek D. Bacterial meningitis presenting with a normal cerebrospinal fluid leukocyte count. J Infect. 2022;84:615–620. doi:10.1016/j.jinf.2022.02.029
42. Brouwer MC, Thwaites GE, Tunkel AR, van de Beek D. Dilemmas in the diagnosis of acute community-acquired bacterial meningitis. Lancet. 2012;380(9854):1684–1692. doi:10.1016/S0140-6736(12)61185-4
43. Awulachew E, Diriba K, Awoke N. Bacterial isolates from CSF samples and their antimicrobial resistance patterns among children under five suspected to have meningitis in dilla university referral hospital. Infect Drug Resist. 2020;13:4193–4202. doi:10.2147/IDR.S264692
44. Wang CY, Xu HM, Deng JK, et al. A multicentric clinical study on clinical characteristics and drug sensitivity of children with pneumococcal meningitis in China. Zhonghua Er Ke Za Zhi. 2019;57(5):355–362. doi:10.3760/cma.j.issn.0578-1310.2019.05.008
45. Kim SH, Song J-H, Chung DR, et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012;56(3):1418–1426. doi:10.1128/AAC.05658-11
46. Wang CQ, Wang AM, Yu H, et al. 2016年儿童细菌耐药监测 [Report of antimicrobial resistance surveillance program in Chinese children in 2016]. Zhonghua Er Ke Za Zhi. 2018;56(1):29–33. Chinese. doi:10.3760/cma.j.issn.0578-1310.2018.01.008
47. Valian SK, Mahmoudi S, Pourakbari B, Banar M, Ashtiani MTH, Mamishi S. The causative organisms of bacterial meningitis and their antimicrobial resistance profiles in Iranian children in 2011–2016. Infect Disord Drug Targets. 2020;20(2):229–236. doi:10.2174/1871526519666181123130101
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
