Back to Journals » Cancer Management and Research » Volume 15
A Metastatic Pulmonary Sarcomatoid Carcinoma Patient Harboring KIF5B-RET Fusion Responds to First-Line Pralsetinib Treatment: A Case Report
Authors Qin H, Wan Y, Dong Y, Sun Q
Received 30 March 2023
Accepted for publication 10 July 2023
Published 26 July 2023 Volume 2023:15 Pages 765—769
DOI https://doi.org/10.2147/CMAR.S414077
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Hao Qin,1,* Yuxiang Wan,2,* Yuchao Dong,1 Qinying Sun1
1Department of Respiratory and Critical Care Medicine, Shanghai Changhai Hospital, The First Affiliated Hospital of Second Military Medical University, Shanghai, People’s Republic of China; 2Department of Laboratory Diagnosis, Shanghai Changhai Hospital, The First Affiliated Hospital of Second Military Medical University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuchao Dong; Qinying Sun, Department of Respiratory and Critical Care Medicine, Shanghai Changhai Hospital, The First Affiliated Hospital of Second Military Medical University, Shanghai, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Pulmonary sarcomatoid carcinoma (PSC) is a rare subtype of non-small cell lung cancer (NSCLC), accounting for about 1% of cases. These tumors are characterized by their high malignancy and frequent resistance to chemotherapy, resulting in a worse prognosis compared to other NSCLC subtypes. Currently, there is no established therapeutic strategy for PSC. Recent advancements in targeted therapies have led to the development of ret proto-oncogene (RET) inhibitors, such as selpercatinib and pralsetinib, which have been approved for the treatment of RET fusion-positive NSCLC patients. Despite their effectiveness in RET fusion-positive NSCLC is observed, the efficacy of these inhibitors in PSC remains unclear. In this context, we present a case of metastatic PSC harboring de novo KIF5B-RET fusion. The patient responded to first-line trametinib treatment. These findings suggest that RET inhibitors could be a potential treatment option for metastatic PSC patients with RET fusion-positive tumors.
Keywords: pulmonary sarcomatoid carcinoma, RET, pralsetinib, efficacy
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a rare form of non-small cell lung cancer (NSCLC) with high aggressiveness and poor differentiation, accounting for approximately 1% of NSCLC cases.1 Radical surgery is the standard treatment for early-stage PSC, while patients often experience rapid recurrence after surgical resection. Additionally, traditional palliative chemotherapy has shown limited efficacy in advanced or metastatic PSC.2 Studies have indicated that PSC patients have a poor prognosis, with a median overall survival (OS) of 7–12 months and 5-year OS rates of 19.5–25.1%.1,3
Recent research by Schrock et al has shed light on potential treatment options for PSC. These findings suggest that PSC frequently exhibits either targetable alterations or a high tumor mutational burden, opening up possibilities for targeted therapy or immunotherapy.4,5 Alterations in Ret proto-oncogene (RET), such as RET amplification and fusion, are relatively uncommon in PSC.4,5 RET amplification is observed in only 0.8% of cases,4 while RET fusion occurs in 0–6.3% of PSC patients.4–6
Currently, two RET inhibitors selpercatinib and pralsetinib have been approved for the treatment of metastatic RET fusion-positive NSCLC.7,8 However, their efficacy in RET fusion-positive PSC patients has not been extensively studied. In this report, we present the case of a stage IV PSC patient with the canonical KIF5B-RET (K15:R12) rearrangement, who exhibited tumor response to first-line treatment with pralsetinib.
Case Presentation
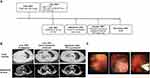
A 67-year-old never-smoker woman with Parkinson’s disease lasting for over 10 years presented with chest pain and cough for more than half a month in June 2021. The patient’s treatment history is summarized in Figure 1A. She had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 3. Chest computed tomography (CT) scans revealed the presence of a mass in the right upper lobe, nodular thickening of the right pleura, and massive accumulation of pleural fluid on the right side (Figure 1B). The tumor marker carcinoembryonic antigen (CEA, normal range: 0–5 ng/mL) was measured at 18.85 ng/mL. The biopsy sample taken from the right supraclavicular lymph node indicated the presence of an N3 tumor. Subsequently, a pleuroscopy was performed, which revealed multiple nodules measuring 1.0–1.2 cm in diameter on the right parietal pleura, visceral pleura, and phrenic pleura (Figure 1C). Further examination of the biopsy sample taken from the right parietal pleura using hematoxylin-eosin (HE) examination and immunohistochemistry (IHC) staining with vimentin (+), Ki-67 (+, 90%), CAM5.2 (small part+), CK7 (small part+), and TTF1 (+), confirmed the presence of PSC (Figure 2). IHC staining on the tumor tissue sample also revealed a high expression of programmed cell death ligand-1 (tumor proportion score: 80%, clone number for PD-L1 antibody: 22C3). To further analyze the sample, next-generation sequencing (NGS) was conducted using the Nextseq500 platform (Illumina, Inc., San Diego, CA, USA) with paired-end reads and an average sequencing depth of 2165×. The NGS analysis employed a panel consisting of 68 cancer related-genes (LungCoreTM, Burning Rock Biotech, Guangzhou, China, Table S1). The results of NGS revealed a canonical RET rearrangement known as KIF5B-RET (K15:R12) (Figure 3), with an allele frequency of 23.9%. Based on these findings, the patient was diagnosed with stage IV (T4N3M1a) PSC that was positive for KIF5B-RET fusion in July 2021 (Figure 1B). The patient was subsequently prescribed first-line treatment with pralsetinib at a daily dose of 400 mg. After undergoing treatment for 2 months, a reduction in the size of the tumor in the right lung was observed in chest CT scans (Figure 1B). According to the RECIST criteria v1.1, the treatment response was evaluated as a partial response (Figure 1B). In October 2021, the dosage of pralsetinib was reduced to 200 mg per day due to the occurrence of treatment-related adverse events, including grade I diarrhea and grade II low albumin (less than 30g/L, normal range: 40–50g/L). The reduction in the oral pralsetinib dosage effectively relieved the low albumin level, which were classified as grade 1. Comparing the chest CT scans from September 2021, the patient’s tumor remained stable in October 2021. However, in November 2021, during a follow-up call with the patient, their family informed us that the patient had passed away at another hospital in the presence of heart failure.
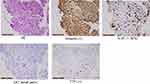 |
Figure 2 Hematoxylin-eosin (HE) and immunohistochemistry staining on the biopsy sample of the right parietal pleura. The rulers in Figure 2 indicate 50 μm. |
 |
Figure 3 The integrative genomics viewer (IGV) screenshots revealed the harboring of KIF5B-RET fusion. |
Discussion
RET encodes for a transmembrane receptor tyrosine kinase (RTK) that plays a crucial role in cell signaling. In normal conditions, RET is activated by binding to specific ligands, leading to the activation of downstream signaling pathways involved in cell growth, differentiation, and survival. However, in certain cases, the RET gene can undergo rearrangement, resulting in abnormal activation of the receptor. These rearrangements typically involve the fusion of the 3’ fragment of the RET, which encodes the intracellular tyrosine kinase domain, with the 5’ fragment of other genes. This can occur through chromosomal inversion or translocation events.9,10 Aberrant activation of RET due to these rearrangements has been implicated in the development and progression of various solid tumors. The most extensively studied tumors associated with RET fusion are papillary thyroid cancer (PTC) and NSCLC. RET fusions have been found in 2.5–73% of sporadic PTC and 1–3% of NSCLC patients.10 RET fusion has also been documented to be a rare event in other solid tumors, including colorectal cancer, breast cancer, and salivary gland cancer, accounting for less than 1% of cases of each of these cancer types.9,11,12 To our knowledge, RET fusion is rare in PSC.5,13 Thus, the efficacies of RET inhibitors have not been well established in RET-rearranged PSC patients.
In this study, the PSC patient harbored KIF5B-RET fusion, which is one of the reported RET fusions in NSCLC. To date, more than ten RET fusions have been reported in NSCLC, including canonical RET fusions like KIF5B-RET and CCDC6-RET, as well as uncommon RET fusions like NCOA4-RET, TRIM33-RET, ZNF477P-RET, have been identified in NSCLC.14 To our knowledge, KIF5B-RET fusion and TUBD1-RET fusion have been documented in PSC.5
Recently, two RET inhibitors selpercatinib and pralsetinib, have been approved by the Food and Drug Administration (FDA) for the treatment of metastatic RET fusion-positive NSCLC based on promising results from ARROW (NCT03037385) and LIBRETTO-001 (NCT03157128) clinical trials.7,8 Given that PSC is a form of NSCLC, the patient was treated with pralsetinib. Despite the patient having a poor ECOG PS of 3, she still showed tumor response to first-line pralsetinib. This finding is supported by a published report showing a partial response to pralsetinib in an advanced PSC patient with KIF5B‑RET fusion and poor performance,13 as well as the SIREN study demonstrating the efficacy of the RET-TKI inhibitor selpercatinib in RET-rearranged NSCLC patients including those with ECOG PS ≥ 2.15 Although RET fusion-positive PSC patients are rare, further evaluation of the efficacy and safety of pralsetinib in RET fusion-positive PSC patients is warranted.
In this study, it was reported that the patient died in the presence of heart failure at another hospital. However, upon reviewing the patient’s medical records from our hospital and considering the lack of medical records from the hospital where the patient died, it cannot be concluded that the patient died from heart failure. Additionally, the presence of heart failure as a side effect in clinical trials was not reported.8,16–18 Therefore, whether heart failure is a pralsetinib-related adverse event should be explored in further studies.
There are some limitations associated with the present work. Firstly, the study only involved one patient, and more evidence from clinical trials is necessary to evaluate the efficacy and safety of pralsetinib in RET fusion-positive PSC patients. Secondly, further in vitro and in vivo studies are needed to investigate whether KIF5B-RET is an oncogenic driver in PSC.
Our work provides clinical evidence that RET fusion-positive PSC patients respond to pralsetinib. This case also highlights the need for further research and clinical trials to evaluate the efficacy of RET inhibitors in the treatment of PSC. Identifying effective therapeutic strategies for this rare and aggressive form of lung cancer is crucial in improving patient outcomes and prognosis.
Ethics Statement
The study was reviewed and approved by the Ethic Committee of the Shanghai Changhai Hospital. The patient provided written informed consent to participate in this study.
Consent to Publish
Consent has been obtained from the patient for the publication of the case.
Acknowledgment
The authors thank the patient and her family, medical and research staff who participated in this study. We would like to express our gratitude to Haiwei Du and Yiting Chen from Burning Rock Biotech for their valuable assistance in data interpretation and medical writing support.
Funding
This study was supported by the project of Shanghai Science and Technology Committee Scientific Research Project (Grant number 19411950402).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yendamuri S, Caty L, Pine M, et al. outcomes of sarcomatoid carcinoma of the lung: a surveillance, epidemiology, and end results database analysis. Surgery. 2012;152(3):397–402. doi:10.1016/j.surg.2012.05.007
2. Shum E, Stuart M, Borczuk A, Wang F, Cheng H, Halmos B. Recent advances in the management of pulmonary sarcomatoid carcinoma. Expert Rev Respir Med. 2016;10(4):407–416. doi:10.1586/17476348.2016.1157475
3. Vieira T, Girard N, Ung M, et al. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol. 2013;8(12):1574–1577. doi:10.1097/01.JTO.0000437008.00554.90
4. Schrock AB, Li SD, Frampton GM, et al. pulmonary sarcomatoid carcinomas commonly harbor either potentially targetable genomic alterations or high tumor mutational burden as observed by comprehensive genomic profiling. J Thorac Oncol. 2017;12(6):932–942. doi:10.1016/j.jtho.2017.03.005
5. Liang X, Li Q, Xu B, et al. Mutation landscape and tumor mutation burden analysis of Chinese patients with pulmonary sarcomatoid carcinomas. Int J Clin Oncol. 2019;24(9):1061–1068. doi:10.1007/s10147-019-01454-6
6. Qin J, Chen B, Li C, Yan J, Lu H. Genetic heterogeneity and predictive biomarker for pulmonary sarcomatoid carcinomas. Cancer Genet. 2021;250–251:12–19. doi:10.1016/j.cancergen.2020.11.004
7. Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of Selpercatinib in RET Fusion–Positive Non–Small-Cell Lung Cancer. N Engl J Med. 2020;383(9):813–824. doi:10.1056/NEJMoa2005653
8. Gainor JF, Curigliano G, Kim DW, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, Phase 1/2 study. Lancet Oncol. 2021;22(7):959–969. doi:10.1016/S1470-2045(21)00247-3
9. Li AY, McCusker MG, Russo A, et al. RET fusions in solid tumors. Cancer Treat Rev. 2019;81:101911. doi:10.1016/j.ctrv.2019.101911
10. Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42(2):581–588. doi:10.1016/0092-8674(85)90115-1
11. Santos C, Sanz-Pamplona R, Salazar R. RET-fusions: a novel paradigm in colorectal cancer. Ann Oncol. 2018;29(6):1340–1343. doi:10.1093/annonc/mdy132
12. Paratala BS, Chung JH, Williams CB, et al. RET rearrangements are actionable alterations in breast cancer. Nat Commun. 2018;9(1):4821. doi:10.1038/s41467-018-07341-4
13. Wu Y, Yan Z, Pan J, et al. Partial response to pralsetinib in an advanced pulmonary sarcomatoid carcinoma patient harboring a KIF5B-RET rearrangement: a case report. World J Surg Oncol. 2022;20(1):386. doi:10.1186/s12957-022-02848-z
14. Subbiah V, Gainor JF, Rahal R, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 2018;8(7):836–849. doi:10.1158/2159-8290.CD-18-0338
15. Illini O, Hochmair MJ, Fabikan H, et al. Selpercatinib in RET fusion-positive non-small-cell lung cancer (SIREN): a retrospective analysis of patients treated through an access program. Ther Adv Med Oncol. 2021;13:17588359211019675. doi:10.1177/17588359211019675
16. Subbiah V, Hu MI, Wirth LJ, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, Phase 1/2 study. Lancet Diabetes Endocrinol. 2021;9(8):491–501. doi:10.1016/S2213-8587(21)00120-0
17. Griesinger F, Curigliano G, Thomas M, et al. Safety and efficacy of pralsetinib in RET fusion-positive non-small-cell lung cancer including as first-line therapy: update from the ARROW trial. Ann Oncol. 2022;33(11):1168–1178. doi:10.1016/j.annonc.2022.08.002
18. Subbiah V, Cassier PA, Siena S, et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat Med. 2022;28(8):1640–1645. doi:10.1038/s41591-022-01931-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.