Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
A Longitudinal Study of AFP Trajectories and Clinical Outcomes in Intermediate-Stage Hepatocellular Carcinoma After Hepatectomy
Authors Yang H , Lu L , Guo W , Gong B, Wang X, Chen Y , Chen X
Received 19 August 2023
Accepted for publication 7 December 2023
Published 25 January 2024 Volume 2024:11 Pages 219—228
DOI https://doi.org/10.2147/JHC.S432011
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Hongyi Yang,1,* Linbin Lu,1,* Wanting Guo,2 Baocuo Gong,2 Xuewen Wang,1 Yaying Chen,1 Xiong Chen1
1Department of Oncology, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, People’s Republic of China; 2Department of Oncology, The 900th Hospital of Joint Logistic Support Force, People’s Liberation Army (PLA), Fuzong Clinical College of Fujian Medical University, Fuzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiong Chen, Tel +1-216-612-2438, Fax +86-591-24937089, Email [email protected]
Background: Previous studies have shown that the alpha-fetoprotein (AFP) response has been a key tumour marker in hepatocellular carcinoma (HCC), but its definition remains controversial. Recently, a new study has explored and defined the AFP serological response and used it to explain the subclass of intermediate-stage hepatocellular carcinoma (IM-HCC) with “sharp-falling” AFP change after transarterial chemoembolization (TACE). It may be a new and simple tool for assessing the prognosis of patients. This study aims to explore a simplified AFP trajectory and its impact on overall survival (OS) and disease-free survival (DFS) for IM-HCC after hepatectomy.
Patients and Methods: Between January 2007 and May 2012, data from the Sun Yat-sen University Cancer Center was examined in this longitudinal, retrospective cohort study. A generalized additive model was applied to distinguish potential AFP dynamic trajectories. The Kaplan-Meier method was applied to analyze OS and DFS, and multivariate Cox models were used to calculate adjusted hazard ratios (aHRs) and 95% CIs for overall survival.
Results: 144 patients who had IM-HCC with at least three AFP repeat measurements were included in the study. Three similar trajectories are displayed using the generalized additive model: low-stable (35.4%; n = 51), high-rising (36.1%; n = 52), and sharp-falling (28.5%; n = 41). Compared with the low-stable class, the aHRs for death were 2.84 (1.50, 5.41) and 0.59 (0.25, 1.40) for the high-rising and sharp-falling classes, adjusted by age and log AFP. Simplified AFP trajectory had higher relative importance than sex, intrahepatic tumor number, Child-Pugh class, and baseline AFP.
Conclusion: The simplified AFP trajectory is a promising biomarker for IM-HCC patients undergoing hepatectomy. In the future, it should be verified by a larger population containing various stages of HCC.
Keywords: hepatocellular carcinoma, hepatectomy, AFP trajectory, AFP serological response, overall survival
Introduction
Primary liver cancer ranks as the sixth most commonly diagnosed cancer worldwide and is the third leading cause of cancer-related deaths, and hepatocellular carcinoma (HCC) comprises roughly 75–85% of all primary liver cancers. In China, hepatitis B virus (HBV) infection is a major risk factor for HCC development.1 Alpha-fetoprotein (AFP) serves as a widely used biomarker for the surveillance, diagnosis, and prognostic prediction of HCC.2 In recent years, several studies have reported that AFP level changes may predict treatment outcomes in patients undergoing chemoembolization, antiangiogenic therapy, and liver resection for hepatocellular carcinoma.2–5
Intermediate-stage hepatocellular carcinoma (IM-HCC) is defined as individuals with asymptomatic multicentric tumors (> 3) lacking vascular invasion or extrahepatic dissemination and having Child-Pugh A and B scores.6 This patient population exhibits significant heterogeneity in terms of tumor load, age, liver function, and comorbidities. While the BCLC staging system recommends transarterial chemoembolization (TACE) for intermediate HCC patients,7 hepatic resection (HR) may be a more effective treatment option in carefully selected patients with preserved liver function, including those with portal hypertension or multiple tumor nodules.8 Notably, a recent meta-analysis of 18 high-quality studies demonstrated significantly better overall survival (OS) with HR compared to TACE for stage B and C HCC patients.9
Post-treatment AFP response has gained recognition as a valuable tool for evaluating treatment efficacy, prognosticating tumor outcomes, and monitoring postoperative recurrence. Previous studies have shown that AFP response (AR = lgAFP7/lgAFP0; AFP0: serum a-fetoprotein before hepatectomy; AFP7: serum a-fetoprotein 1 week after hepatectomy) is an independent factor of OS and recurrence-free survival (RFS) after surgical resection.10 Another study stratified patients into three distinct groups based on the rate of change of AFP and predicted clinical outcomes accordingly.11 However, these studies were limited by the absence of a standardized definition for serum AFP response, encompassing inconsistent measurement time intervals preoperatively and postoperatively, as well as the rate of postoperative serum AFP change. Lu et al identified the AFP dynamic changing trajectories through the latent class growth mixed model and divided the patients with IM-HCC treated with TACE into three groups, and the results showed that AFP trajectory was a good predictor of prognosis.12 However, there are currently no studies on the AFP trajectory after HR for IM-HCC. In light of these research gaps, the objective of this longitudinal, retrospective cohort study is to ascertain the latent trajectories of AFP in individuals with IM-HCC following HR and assess its influence on clinical outcomes.
Materials and Methods
Patients and Samples
From January 2007 to May 2012, a derivation cohort of 979 patients diagnosed with HCC at Sun Yat-sen University Cancer Center was retrospectively reviewed. In previous articles, specifics concerning this cohort study have been discussed.13,14 As is shown in Figure 1, in this cohort, 835 patients (85.3%) were eliminated due to treatment refusal, first-line TACE treatment, and less than three follow-up records, and 144 patients were involved in the final analysis.
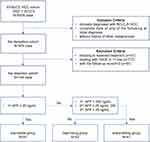 |
Figure 1 Flow chart of the cohort. HCC=hepatocellular carcinoma; TACE=transarterial chemoembolization; BCLC= Barcelona Clinic Liver Cancer. |
The diagnosis of HCC was established based on a combination of serum viral tests for hepatitis B (HBV) or C (HCV), serum AFP levels, and the utilization of two imaging modalities (contrast-enhanced ultrasonography, double-phase helical computed tomography, or magnetic resonance) to identify the characteristic vascular pattern. This pattern includes arterial enhancement surpassing the surrounding liver (wash-in) and hypodensity or hyposignal intensity relative to the surrounding liver (wash-out) during the venous phase. Surgical samples were examined histopathologically to confirm all HCC diagnoses.
The retrospective study received approval from the Department of Clinical Research at Sun Yat-sen University Cancer Center (2017-FXY-129) and complied with the Declaration of Helsinki, and patient data were anonymized to ensure privacy. Informed consent requirements were waived given the study’s retrospective design.
Hepatic Resection
HR was performed in patients with IM-HCC based on computed tomography measurements that determined the appropriate residual liver volume. For patients without cirrhosis, a minimum of 30% of the remaining liver volume was considered sufficient post-resection. However, in the presence of severe fatty liver disease, cirrhosis, or chronic hepatitis, a residual liver volume greater than 50% was deemed necessary. Notably, patients with intermediate or advanced cirrhosis or impaired liver function (Child-Pugh C) were not considered candidates for HR. Experienced liver surgeons performed the surgery.
Serum AFP
Serum AFP levels were quantified using the Roche Cobas E602 system (Roche Diagnostics GmbH, Mannheim, Germany) and an electrochemiluminescence immunoassay following standardized protocols.12,15 Based on previous research, an AFP threshold of 25 ng/mL was established for differentiating between AFP-positive and AFP-negative patients.13,15 Preoperative serum AFP refers to the AFP value obtained closest to the time of hepatectomy within the initial follow-up record. Postoperative serum AFP encompassed the AFP value recorded before any subsequent treatment during each follow-up record subsequent to HR. Duplicate AFP tests were excluded from each follow-up record. AFP serological response was defined as a high preoperative AFP value (1st AFP > 200 ng/mL) declining rapidly below 25 ng/mL in the second or third follow-up records within four months.
Follow-Up
Patients underwent regular monitoring during the initial two years at intervals of two to three months to assess the achievement of complete remission. Subsequently, the monitoring frequency was gradually reduced to every 3–6 months after a two-year remission. At the subsequent visit, blood cell tests, liver function tests, alpha-fetoprotein measures, and computed tomography or magnetic resonance were done. Baseline covariates such as age, gender, largest tumor size, intrahepatic tumor number, Child-Pugh class, and preoperative serum AFP were collected. These data were obtained prior to HR, without any treatment, at the first follow-up record. The primary endpoint of the study was OS, measured from the date of surgery to death from any cause.
Statistical Analysis
A generalized additive model was employed to identify three distinct trajectories of AFP change. Continuous variables were presented as mean (± standard deviation), while categorical variables were reported as numbers and percentages. The comparison of categorical variables was performed using Chi-square or Fisher’s exact tests, whereas the means of continuous variables were compared using Student’s t-tests. Statistical significance was set at a p-value of less than 0.05. Survival analysis was conducted using the Kaplan-Meier method, and differences in survival between groups were assessed using the Log rank test. Cox proportional hazard models were employed to investigate the relationships between AFP trajectories and clinical outcomes. The relative importance of each parameter in relation to survival risk was assessed using the X2 from Harrell’s rms R package.
Results
Clinicopathologic Characteristics
The analysis cohort for this retrospective study consisted of patients (n = 144) who met the inclusion criteria and had IM-HCC following hepatectomy. The enrollment process is presented in Figure 1, while Table 1 displays the clinical and demographic-pathological characteristics of the three patient groups. Of the included patients, 133 (92.4%) were males, and 11 (7.6%) were females. Significant differences were observed in terms of age and preoperative serum AFP levels among the three groups. However, no significant differences were found in gender, largest tumor size, intrahepatic lesion number and Child-Pugh class.
 |
Table 1 Baseline Characteristics of Patients Stratified by Trajectory Classes of AFP |
Curve Fitting of AFP Trajectories
Figure 2 shows three trajectories of serum AFP fitted by the generalized additivity model. Low-stable (35.4%; n = 51), high-rising (36.1%; n = 52), and sharp-falling (28.5%; n = 41) trajectories were classified as three separate trajectories. We observed that AFP levels persisted between 0 and 25 ng/mL before and after HR in the low-stable group. The sharp-falling group exhibited a rapid decline in AFP levels from an initially elevated preoperative level (>200 ng/mL) to the range of 0–25 ng/mL within four months of HR, after which the levels remained stable, defining the AFP serological response curve. In the high-rising group, AFP levels initially declined but subsequently gradually increased to a higher level.
 |
Figure 2 Trajectories of serum AFP in intermediate-stage HCC patients after HR. Red dashed line = AFP value equaled to 25 ng/mL. Shadows = 95% confidence intervals. AFP=a-fetoprotein. |
The Impact of Simplified AFP Trajectory on Clinical Outcomes
Among the 144 patients in the resection cohort, 50 died at the last follow-up, with 15 in the low-stable group, 27 in the high-rising group, and 8 in the sharp-falling group. The OS was then calculated for each trajectory group. Survival curves according to Kaplan-Meier demonstrated that patients in the sharp-falling group exhibited significantly superior OS rates at 1, 3, and 5 years (sharp-falling: 97.5%, 83.6%, and 74.1% vs low-stable: 96.0%, 79.7%, and 66.7% vs high-rising: 70.2%, 47.5%, and 27.8%, respectively) (P < 0.0001; Figure 3). We next estimated the DFS survival for each trajectory group. As demonstrated in Figure 4, the 1-year and 3-year DFS rates in the low-stable group were 78.2% and 62.7%, which was significantly higher than the high-rising group (25.5%, 23.2%), but lower than the sharp-falling group (87.6%, 80.1%) (P < 0.0001; Figure 4).
 |
Figure 3 Kaplan-Meier curves of overall survival in patients with IM-HCC after HR. |
 |
Figure 4 Kaplan-Meier curves of disease-free survival in patients with IM-HCC after HR. |
Table 2 displays the magnitude of the AFP trajectory’s impact on clinical outcomes. In the unadjusted model, relative to the low-stable class, the risk of death was higher in the high-rising class (HR: 3.00, 95% CI: 1.59, 5.68) and lower in the sharp-falling class (HR: 0.65, 95% CI: 0.28, 1.54). After adjusting for age and AFP, the risk of death was reduced by 16% in the high-rising group (HR: 2.84, 95% CI: 1.50, 5.41) and 6% in the sharp-falling group (HR: 0.59, 95% CI: 0.25, 1.40).
 |
Table 2 Trajectory Classes of AFP and Multivariate HRs of Overall Survival with 95% CIs |
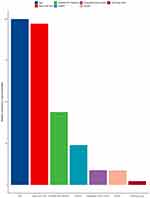
Moreover, the relative importance of various risk factors for OS was analyzed, including age, sex, simplified AFP trajectory, major tumor size, intrahepatic tumor number, Child-Pugh class, and baseline AFP (Figure 5). We could find that age and major tumor size are the two factors that have the greatest impact on OS, followed by AFP trajectory class.
Discussion
With advances in postoperative management and the popularization of liver resection techniques, perioperative mortality rates have significantly decreased. Liver resection is regarded as the most efficacious approach for patients with resectable tumors and preserved liver function.8,16 Even if surgical resection is successful, more than 90% of patients die within 5 years due to intrahepatic recurrent HCC tumors (HCCs). Therefore, the evaluation of surgical outcomes was crucial. Moreover, the ideal assessment tool should be reproducible, objective, inexpensive, and easy to use. Recent studies have highlighted the association between changes in AFP levels before and after hepatectomy and improved prognosis.10,11,17 Nonetheless, discrepancies exist among these studies regarding the definition of AFP changes, encompassing both magnitude and time interval. Lu et al identified the AFP dynamic changing trajectories through the latent class growth mixed model. Specifically, a model of cubic parameters with three classes provided the optimal fit. So they divided the patients with IM-HCC treated with TACE into three groups, and the results showed that AFP trajectory was a good predictor of prognosis.12 Another study used latent class trajectory models to identify trajectories of AFP change rates over time in unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab and obtained similar results.18 In our study, we employed the generalized additive model to delineate three distinct AFP trajectories in HCC patients undergoing hepatic resection, establishing significant correlations between AFP trajectory groups and clinical outcomes, which is consistent with previous findings. Furthermore, the relative importance of each covariate was calculated, revealing that AFP trajectories serve as significant parameters for predicting OS.
Survival analysis among the three groups revealed a significant difference in prognoses, with the sharp-falling group exhibiting better outcomes compared to the low-stable and high-rising groups, consistent with previous research. Shen et al conducted a study involving HCC patients beyond the Milan criteria, stratifying them based on changes in AFP levels following hepatectomy. Their findings indicated that patients with less than 50% reduction in AFP levels after hepatectomy had a poorer prognosis compared to those with normal or greater than 50% reduction in AFP levels.17 Similarly, another study investigated the association between trajectories of serum AFP and clinical outcomes in IM-HCC patients undergoing TACE. The sharp-falling group, characterized by the AFP serological response curve, demonstrated improved OS and RFS.12 Comparable results were observed in a study involving patients with HCC treated with atezolizumab and bevacizumab.19 These collective findings provide further support for the notion of a serological response to AFP, which may indicate a specific underlying pathophysiological process.
In this retrospective study of 144 patients with HCC, we identified significant differences in survival rates among the different trajectory groups. Specifically, the high-rising group exhibited notably lower survival rates compared to the other groups. Recent studies have referred to patients with high post-AFP levels as “non-responders”, suggesting incomplete surgical resection or the presence of intra- or extrahepatic occult metastases.20,21 Aberrant postoperative AFP levels may contribute to residual cancer cell growth by modulating oncological signaling pathways, such as the cAMP-PKA pathway.22 In contrast, the sharp-falling group displayed postoperative AFP levels below 25 ng/mL, which may indicate complete tumor removal. Previous research showed that this part of the population has a lower tumor burden, less vascular invasion, and a higher degree of differentiation. Ultimately, we conducted an analysis to assess the relative contribution of each parameter in predicting clinical outcomes. Interestingly, the results revealed that AFP trajectory patterns had a stronger predictive value than preoperative AFP levels alone. Therefore, considering the dynamic changes between preoperative and postoperative AFP levels might offer more accurate prognostic insights than relying solely on preoperative or postoperative AFP levels. It is noteworthy that the hazard ratio of death between the high-rising and sharp-falling groups reached approximately 4.8-fold, highlighting the potential significance of these findings for both clinical practice and scientific research and offering promising avenues for future investigations.
This study is subject to several limitations that need to be acknowledged. Firstly, it is important to note that this investigation is retrospective in nature, which introduces the potential for selection bias and limits the establishment of causality. Future studies employing a prospective design would be beneficial in overcoming these limitations. Secondly, it is worth mentioning that a substantial proportion of the patients included in this study had a history of chronic HBV infection. Therefore, caution should be exercised when generalizing the findings of this study to patients with HCC resulting from other etiologies, such as HCV infection or alcoholic liver disease. It is crucial to explore the impact of different etiologies on the outcomes of interest in separate investigations. Lastly, to confirm and validate the findings of this study, further research is warranted, particularly in the form of large-scale population studies and prospective randomized controlled trials. Such studies would help to strengthen the evidence base and provide more robust conclusions.
Conclusion
In conclusion, the simplified AFP trajectory is a promising biomarker for IM-HCC undergoing hepatectomy. In the future, it should be verified by a larger-scale population containing various stages of HCC.
Acknowledgments
We gratefully acknowledge the statistical support from the Empower U team of the Department of Epidemiology and Biostatistics, X&Y Solutions Inc., in Boston.
Funding
This study was funded by the Natural Science Foundation of Fujian Province (Nos.2021J01281 and 2020J011147), the Science and Technology Innovation Joint Foundation of Fujian Province (Nos2017Y9125), Central Guidance on Local Science and Technology Development Fund of Fujian Province (Nos 2023L3028) and Research Initiation Foundation of Mengchao Hepatobiliary Hospital of Fujian Medical University(Nos QDZJ-2022-001).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Liu G, Ouyang Q, Xia F, et al. Alpha-fetoprotein response following transarterial chemoembolization indicates improved survival for intermediate-stage hepatocellular carcinoma. HPB (Oxford). 2019;21(1):107–113. doi:10.1016/j.hpb.2018.06.1800
3. Liu B, Shang X, Shi JY, et al. Early alpha-fetoprotein response is associated with survival in patients with HBV-related hepatocellular carcinoma receiving lenvatinib. Front Oncol. 2022;12:807189.
4. Mishra G, Dev A, Paul E, et al. Prognostic role of alpha-fetoprotein in patients with hepatocellular carcinoma treated with repeat transarterial chemoembolisation. BMC Cancer. 2020;20(1):483. doi:10.1186/s12885-020-06806-4
5. Yang SL, Liu LP, Yang S, et al. Preoperative serum alpha-fetoprotein and prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg. 2016;103(6):716–724. doi:10.1002/bjs.10093
6. Romano F, Chiarelli M, Garancini M, et al. Rethinking the Barcelona clinic liver cancer guidelines: intermediate stage and Child-Pugh B patients are suitable for surgery? World J Gastroenterol. 2021;27(21):2784–2794. doi:10.3748/wjg.v27.i21.2784
7. Yamamoto M, Kobayashi T, Hashimoto M, et al. Significance of liver resection for intermediate stage hepatocellular carcinoma according to subclassification. BMC Cancer. 2021;21(1):668. doi:10.1186/s12885-021-08421-3
8. Prince D, Liu K, Xu W, et al. Management of patients with intermediate stage hepatocellular carcinoma. Ther Adv Med Oncol. 2020;12:1758835920970840. doi:10.1177/1758835920970840
9. Hyun MH, Lee Y-S, Kim JH, et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: a meta-analysis of high-quality studies. Hepatology. 2018;68(3):977–993. doi:10.1002/hep.29883
10. Li XL, Zhu V, Cai H, et al. Postoperative alpha-fetoprotein response predicts tumor recurrence and survival after hepatectomy for hepatocellular carcinoma: a propensity score matching analysis. Surgery. 2019;165(6):1161–1167.
11. Rungsakulkij N, Suragul W, Mingphruedhi S, et al. Prognostic role of alpha-fetoprotein response after hepatocellular carcinoma resection. World J Clin Cases. 2018;6(6):110–120. doi:10.12998/wjcc.v6.i6.110
12. Lu L, Shen L, Wu Z, et al. Trajectories of serum α-fetoprotein and intermediate-stage hepatocellular carcinoma outcomes after transarterial chemoembolization: a longitudinal, retrospective, multicentre, cohort study. EClinicalMedicine. 2022;47:101391. doi:10.1016/j.eclinm.2022.101391
13. Shen L, Zeng Q, Guo P, et al. Dynamically prognosticating patients with hepatocellular carcinoma through survival paths mapping based on time-series data. Nat Commun. 2018;9(1):2230.
14. Lu L, Zheng P, Wu Z, et al. Hepatic resection versus transarterial chemoembolization for intermediate-stage hepatocellular carcinoma: a cohort study. Front Oncol. 2021;11:618937. doi:10.3389/fonc.2021.618937
15. Pan YX, Sun XQ, Hu ZL, et al. Prognostic values of alpha-fetoprotein and des-gamma-carboxyprothrombin in hepatocellular carcinoma in china: an analysis of 4792 patients. J Hepatocell Carcinoma. 2021;8:657–670. doi:10.2147/JHC.S316223
16. Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260(2):329–340. doi:10.1097/SLA.0000000000000236
17. Shen JY, Li C, Wen TF, et al. Alpha fetoprotein changes predict hepatocellular carcinoma survival beyond the Milan criteria after hepatectomy. J Surg Res. 2017;209:102–111. doi:10.1016/j.jss.2016.10.005
18. Lu L, Zheng P, Pan Y, et al. Trajectories of α-fetoprotein and unresectable hepatocellular carcinoma outcomes receiving atezolizumab plus bevacizumab: a secondary analysis of IMbrave150 study. Br J Cancer. 2023;129(4):620–625. doi:10.1038/s41416-023-02334-7
19. Zhu AX, Dayyani F, Yen CJ, et al. Alpha-fetoprotein as a potential surrogate biomarker for atezolizumab + bevacizumab treatment of hepatocellular carcinoma. Clin Cancer Res. 2022;28(16):3537–3545. doi:10.1158/1078-0432.CCR-21-3275
20. Zhang Q, Shang L, Zang Y, et al. α-Fetoprotein is a potential survival predictor in hepatocellular carcinoma patients with hepatitis B selected for liver transplantation. Eur J Gastroenterol Hepatol. 2014;26(5):544–552. doi:10.1097/MEG.0000000000000029
21. Kim HS, Park JW, Jang JS, et al. Prognostic values of alpha-fetoprotein and protein induced by vitamin K absence or antagonist-II in hepatitis B virus-related hepatocellular carcinoma: a prospective study. J Clin Gastroenterol. 2009;43(5):482–488. doi:10.1097/MCG.0b013e318182015a
22. Li MS, Li PF, He SP, et al. The promoting molecular mechanism of alpha-fetoprotein on the growth of human hepatoma Bel7402 cell line. World J Gastroenterol. 2002;8(3):469–475. doi:10.3748/wjg.v8.i3.469
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.